Abstract
Ruptures of the anterior cruciate ligament (ACL) are still associated with high rates of long-term complications, even in patients undergoing modern, state-of-the-art replacement. Tissue-engineering approaches have been shown to be of value in improving treatment of ACL ruptures. However, the success of tissue-engineering procedures depends on the choice of an appropriate biomaterial. Decellularized ACL tissue potentially combines the structural composition of the targeted tissue with a reduced risk of graft rejection or disease transmission. In this study, we tested the effectiveness of currently available decellularization methods based on TRITON-X, sodium dodecyl sulfate (SDS), and trypsin. After identifying the most effective decellularization method, the capacity for reseeding with ACL fibroblasts was studied. All decellularization protocols reduced DNA content, with TRITON-X treatment having the greatest effect. Concurrently, decellularization did not affect tissue collagen or total protein content, but did decrease glycosaminoglycan content. TRITONX also resulted the least glycosaminoglycan depletion. Porcine ACL tissue after decellularization with TRITON-X could be successfully reseeded with human ACL fibroblasts as demonstrated by steady DNA content and increasing pro-collagen expression.
Keywords: ACL repair, decellularization, tissue engineering
Ruptures of the anterior cruciate ligament (ACL) are an important health problem due to their high incidence, the immediate limitation of mobility, and increased risk of persisting disability due to subsequent premature osteoarthritis.1-4 Recent studies have shown that even state-of-the-art ACL replacement is associated with an unabated risk of joint degeneration in a startlingly high proportion of patients.1,2,4 To address this problem, tissue-engineered treatments including augmented primary repair and reconstruction have been developed and shown promising results in advancing treatment options in ACL ruptures.5-10
However, the success of tissue engineering for ACL treatment is dictated by the three elements of cells, signals, and biomaterial.11 The optimal biomaterial would be one that simultaneously supports rapid and directed cell proliferation and orchestrated tissue remodeling, and also behaves structurally, i.e., in composition and architecture, like native ACL tissue until it is completely remodeled. Also, it must not go unnoticed that structure and composition of a biomaterial play as much a role in signaling as growth factors or other mediators.11,12 Various materials, especially collagen, have been intensively studied to create biomaterials that mimic the native ACL as closely as possible and are endowed with the abovementioned characteristics. However, regardless of any cross-linking procedure or the most rigorous addition of matrix proteins, the exact structure of ACL cannot yet be rebuilt with the currently available technology.
An interesting approach to this problem is decellularization of native tissues. The use of numerous chemical methods has been studied as an option to obtain structurally and compositionally adequate biomaterials for tissue engineering of heart valves,13 skin,14 blood vessels,15 and nerves.16 Decellularization has a number of potential benefits: the minimization of immunogenic reactions leading to graft rejection, the provision of an optimized biomaterial without competing donor cells, and a reduced risk of disease transmission.17,18 However, it could potentially be harmful to the function of the biomaterial if decellularization altered the biochemical tissue composition or structure. A wide variety of methods, based on detergents and enzymes, have been developed to decellularize tissues and organs.17,19 Probably the most commonly used and best known reagent for cell removal is trypsin, which has successfully been used in orthopedic and cardiovascular applications.17,20,21 However, despite its high effectiveness, trypsin is also disruptive, and exposure times should be well balanced. TRITON-X is a well-studied, non-ionic detergent, that has been employed with success to decellularize heart valves,20 blood vessels,22 as well as tendons23 and ligaments.24 Sodium dodecyl sulfate (SDS), finally, is an ionic detergent with a long-standing record in tissue engineering of decellularized tissues25-27 that has recently also been used for decellularization of connective tissues.24
The primary objective of this study is to compare these previously described methods in tissue decellularization to identify the most effective treatment, i.e., the treatment that removes all cellular constituents most completely without significantly changing the structural composition of the tissue. However, even the most effective decellularization method is useless if it precludes subsequent cellular repopulation of the scaffold. Thus, as secondary objective, after identification of the most effective method for ACL decellularization, we asked whether this treatment had effects on proliferation and biosynthesis of ACL fibroblasts seeded within the decellularized scaffold.
MATERIALS AND METHODS
Experimental Design
Anterior cruciate ligaments were aseptically harvested from eight Yorkshire pigs (six females, two males; mean age 16.8±0.5 months; mean weight 51.2±11.1 kg), which were sacrificed for another IACUC-approved study. Tissue was stored in PBS containing 5% penicillin and streptomycin until processing, but for no longer than 1 week. Samples were decellularized using TRITON-X, SDS, or trypsin, or treated with PBS to serve as controls. Details of protocols are given in Table 1. All decellularization procedures were concluded with DNase (Worthington, Lakewood, NJ) and RNase (Roche, Indianapolis, IN) treatments, and washes. The effectiveness of decellularization was assessed by measurement of DNA content within the tissue after decellularization. Furthermore, collagen and glycosaminoglycan (GAG) content, as well as total protein content, were measured to assess alterations in biochemical composition of the tissue due to decellularization processes.
Table 1.
Decellularization Protocols
| Trypsin | SDS | TRITON-X | Control |
|---|---|---|---|
| 0.1% Trypsin | 0.1% SDS | 0.25% TRITON-X | PBS −/− |
| 0.02% EDTA | PBS −/− | 0.25% NA-deoxycholate | |
| PBS −/− | PBS −/− | ||
| 48h | 24h | 24h at 37°C | |
| wash w/ medium 72h/4°C | |||
| All decellularization procedures were concluded with: Ribonuclease (RNase 100 μg/mL) Deoxyribonuclease (DNase 150 IU/mL) 24h at 37°C wash w/PBS and 0.02% EDTA for 24h at 4°C |
|||
In the second phase of the experiment, porcine ACL tissue samples from the same animals were decellularized using the protocol that combined the highest effectiveness in decellularization with the least effect on the tissue biochemical composition. These constructs were then evaluated for their capacity to serve as a biomaterial for human ACL fibroblasts tissue engineering. Fibroblasts were obtained from explant cultures of ACL biopsies from three adolescent, female patients (15±0 years of age) undergoing arthroscopic ACL reconstruction after recent rupture. Previous studies proved this tissue is a good source of viable ACL fibroblasts, even for extended periods after the initial trauma.28,29 Briefly, ACL tissue was washed three times using −/− PBS (Mediatech, Manassas, VA), minced, plated in 100 mm Petri-dishes, and cultured in DMEM (Mediatech, Manassas, VA) supplemented with 5% FBS (HyClone, South Logan, UT), 100 IU/ml penicillin, 100 mg/ml streptomycin, and 0.25 μg/ml amphotericin B (Mediatech, Herndon, VA). Once cells approached 95% confluence, the explants were removed and the cells were passaged using a standard trypsin/EDTA protocol, as was done for subsequent passaging. Cells from the first and second passage were seeded onto decellularized ACL. Briefly, cells were statically seeded by setting 50 μl of cell suspension containing approximately 500,000 cells on top of each piece of tissue. Cells were allowed to attach to the tissue for 1 h in an incubator set at 378C, 95% rH, and 5% CO2. Subsequently, all seeded samples were carefully transferred to fresh 24-well plates and standard media containing DMEM (Mediatech, Manassas, VA) supplemented with 5% FBS (HyClone, South Logan, UT), 100 IU/ml penicillin, 100 mg/ml streptomycin, 0.25 μg/ml amphotericin B (Mediatech, Herndon, VA), and 250 μM ascorbic acid (Wako Chemicals, Richmond, VA) was gently added. Media was changed twice weekly. Triplicates of ACL tissue samples were obtained for analysis of DNA content and procollagen production at 2, 7, and 14 days of culture. Additionally, the content of soluble collagen released into the cell culture medium was measured at 7, 10, and 14 days. Histological assessment using hematoxylin and eosin staining was used to study cell location within the sample.
Sample Digestion
All samples obtained for DNA, GAG, or procollagen measurements were digested using a previously described papain protocol.30 Briefly, samples were lyophilized, weighed, and 1 mL papain in phosphate buffer (0.125 mg/mL papain) was added. Care was taken to digest no more than 20 mg of sample per milliliter of papain.31 Subsequently, all samples were digested at 60°C for 3 h. All samples dissolved completely and were either directly processed or stored at −80°C.
DNA Measurement in Decellularized and Reseeded Samples
Triplicates of samples were assessed immediately after digestion. The content of DNA in the digest was assessed fluorometrically using the PicoGreen assay (Quant-iT Pico-Green assay, Molecular Probes, Eugene, OR) and adjusted for dry weight and normalized for empty samples.
GAG Measurement in Decellularized Samples
Following a validated protocol, the GAG content of digested samples was measured photometrically at 540 nm using 1,9-dimethyl-methylene blue as color reagent, and regressed on a standard of shark chondroitin sulfate.32 Again GAG content was adjusted for dry weight and normalized for empty samples.
Total Protein and Collagen Content of Decellularized Samples
Samples of decellularized ACL obtained for collagen and total protein measurement were hydrolyzed. Briefly, samples were suspended in constant boiling 6N hydrochloric acid, frozen by immersion in a dry ice-isopropanol slurry, and flushed and sealed under an atmosphere of nitrogen. All samples were thus hydrolyzed for 24 h at 110°C. Amino acid contents of cooled, washed, and dried samples were measured by ninhydrin-based postcolumn derivatization and ion exchange chromatigraphic separation (440 and 570 nm) using an cation/anion exchange column and stringent pH control.33 Hydroxyproline contents were used as a surrogate measure of total collagen content.
Procollagen Measurement in Reseeded Samples
The procollagen content in the digested, reseeded samples was assessed using a commercially available ELISA kit for the procollagen Type I C-peptide (PIP EIA kit, Takara Bio Inc., Shiga, Japan). All results were adjusted for dry weight and normalized for empty samples. A procollagen assay, rather than a collagen assay was used for two reasons. Firstly, procollagen is the original product of the cell and reflects cellular metabolism independent from extracellular protein processing. Secondy, our collagen assay is not sensitive enough to validly measure the small amounts of newly synthesized collagen against the strong background of the ACL.
Histological Assessment of Reseeded Samples
After fixation in neutral buffered formalin, samples were embedded in paraffin. Seven-micrometer-thick section were cut and fixed on glass slides. Representative sections were stained with hematoxylin and eosin to study the location of the reseeded cells in the ACL. Histological assessment was done with a Olympus BX51 microscope using a mounted Olympus DP25 camera and corresponding software (all by Olympus, Center Valley, PA).
Collagen Content of Cell Culture Medium
Exactly 2 ml cell culture medium was collected at each time point, and the identical volume of fresh medium was added to cultures. The collected media samples were stored at −80°C until processing. Collagen content was measured using the SIRCOL kit (Biocolor, Carrickfergus, UK) and normalized using medium from controls of decellularized ACL tissue without cells.
Statistical Evaluation
All results are presented as mean ± SD with 95% confidence intervals, or as percentages with 95% confidence intervals. Parameters of decellularization protocols and recellularization were compared using ANOVA with subgroup analyses using Bonferroni correction for multiple testing, or paired t-tests, as appropriate. A p-value of less than 5% was assumed significant. All calculations were done using intercooled STATA 10 (Stata Corp LP, College Station, TX).
RESULTS
Decellularization of ACL Samples
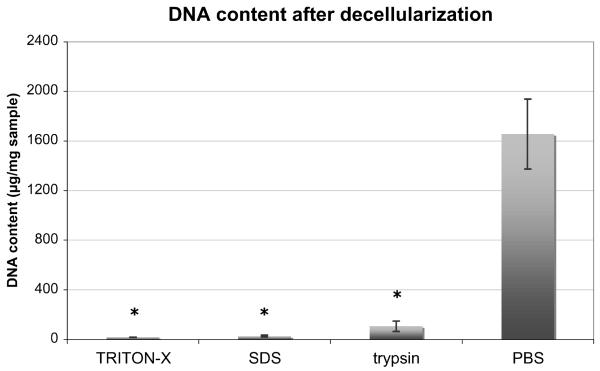
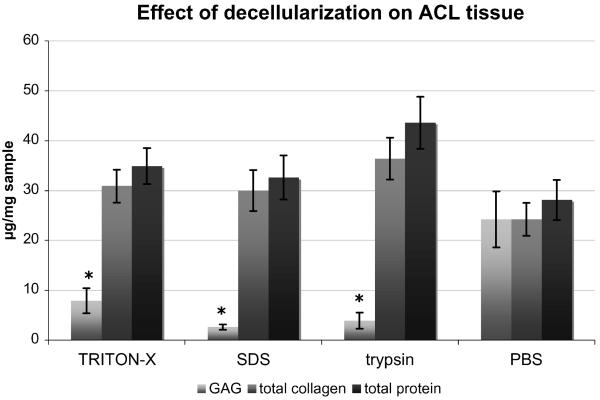
All three protocols showed a high effectiveness in decellularization. All protocols showed significantly less DNA content than controls treated with PBS (p < 0.001). There were no statistically significant differences in DNA content between the treatment groups (Fig. 1). Decellularization affected the biochemical integrity of the tissue only minimally (Fig. 2). There was no significant difference between exposed samples and controls in either collagen (p = 0.15) or total protein content (p = 0.11). However, there was a significant reduction in GAG content after any type of decellularization when compared to native tissue (p < 0.001). The largest decrease of GAG was seen after exposure to SDS with an 89.5% (95%CI: 70.8% to 100%) reduction compared to controls treated with PBS. TRITON-X and trypsin exposure led to GAG reduction by 67.5% (95%CI: 49.4% to 85.5%) and 83.6% (95%CI: 66.1% to 100%), respectively. While there was no difference in GAG content between trypsin and SDS at the 5% level, the content of GAG in TRITON-X samples was much closer to the intact level after decellularization.
Figure 1.
Differences in DNA content after decellularization. While TRITON-X, SDS, and trypsin showed significantly less DNA than controls (*, p < 0.05), there was no statistical difference between the three treatment groups themselves (given as mean ± SEM).
Figure 2.
Effects of decellularization on tissue composition. GAG content was significantly lower in all treated groups when compared to controls (*, p < 0.05), but there were no significant differences in total collagen and total protein contents (given as mean ± SEM).
Reseeding of Decellularized ACL Samples
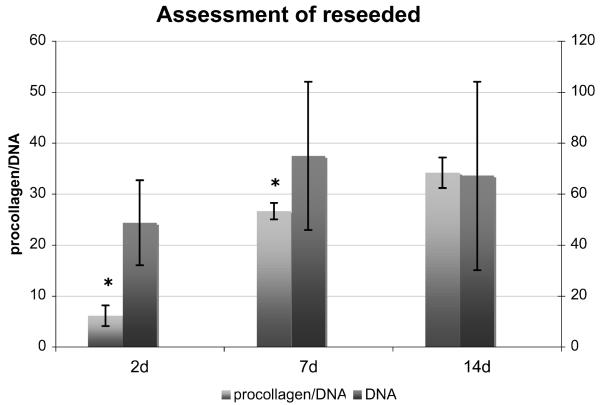
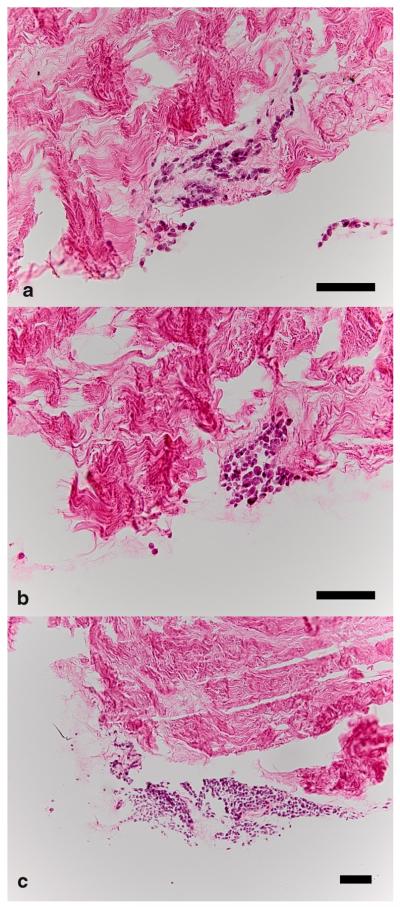
Porcine ACL tissue decellularized with TRITON-X could be successfully reseeded with human ACL fibroblasts. Over 14 days, we found a consistent DNA content (p = 0.30). There was an increase in procollagen content with time (p = 0.03). Briefly, the procollagen content of samples increased by 16% (95%CI: 2% to 29%) per day. After adjusting for DNA content, i.e., measuring procollagen production per DNA instead of per sample, the increase of procollagen with time showed an even higher significance (p = 0.003), yet the relative increase did not change (15% per day, 95%CI: 6% to 25%) (Fig. 3). Histological assessment showed that cells formed clusters on the surface of the biomaterial without much infiltration into the tissue (Fig. 4).
Figure 3.
Parameters of reseeding of grafts decellularized with TRITON-X, which was shown to be the most effective and least destructive decellularization method. There was no significant change in DNA content over time. The content of procollagen per DNA increased significantly (given as mean ± SEM). *, p < 0.05.
Figure 4.
Representative histological specimens. Typically, variably sized clusters of cells with little tissue infiltration were seen in histology. (a) Cluster of cells seen at 7 days at ×20. (b, c) Cell clusters at 14 days at ×20 and ×10. The embedded scale bars represent 100 μm.
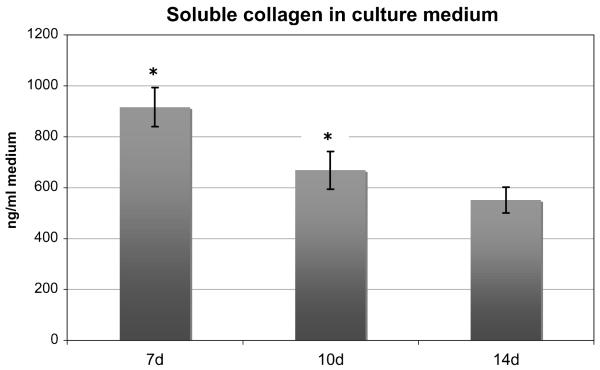
The analysis of the content of soluble collagen in the collected culture medium showed a statistically significant overall decrease over time (p = 0.02). The mean content at 7 days was 916.7 ± 231.5 ng/ml and fell to 668.9 ± 221.3 ng/ml at 10 days, and finally to 552.0 ± 152.3 ng/ml at 14 days (Fig. 5). However, the subgroup analysis of these individual groups with Bonferroni adjustment showed that only the decrease in soluble collagen content from 7 to 10 days was significant (p = 0.05), while the further decrease from 10 to 14 days was not (p = 0.71), suggesting an asymptotic rather than a linear decrease pattern.
Figure 5.
Amount of soluble collagen in the culture medium. Throughout the experiment, this amount decreased significantly (*, p < 0.05) (given as mean ± SEM).
DISCUSSION
The objectives of this study were to find the most effective and least destructive procedure among three ACL decellularization protocols and to assess the capacity of ACL tissue treated with this protocol to serve as a biomaterial for human ACL tissue engineering. The results demonstrated that treatment with TRITON-X combined high effectiveness in decellularization with minimal changes in tissue biochemistry. Also, we could observe that ACL tissue decellularized with TRITON-X could be successfully reseeded with ACL fibroblasts showing persistent DNA content and increasing procollagen production.
It has been shown that implanted grafts undergo an initial phase of cellular necrosis, followed by an inflammatory reaction, and finally tissue remodeling. The course of these events can result in both remodeling as well as tunnel enlargement or frank graft rejection.34,35 It has been hypothesized that decellularized grafts could optimize ACL tissue engineering, and even ACL replacement, since they avoid the abovementioned problems by removing the intrinsic cells and thus the stimulus for inflammation. In 2004, Cartmell and colleagues published a study on decellularization of patellar tendon grafts with Tri(n-butyl)phosphate (TBP) or SDS and found 70%–90% reduction of intrinsic cells and similar biomechanics despite morphological changes in the tissue.23 Woods et al. studied the effectiveness of TRITON-X combined with either SDS or TBP and found the former to be most effective in decellularization, but also most destructive in terms of glycosaminoglycan and collagen depletion of samples and increase in tensile stiffness.24 Unfortunately, this study used TRITON-X as a baseline treatment in all groups, thus rendering a direct comparison of SDS, TRITON-X, and TBP impossible. Also, this study showed that SDS-treated samples were only poorly repopulated by fibroblasts, and Gratzer et al., in 2006, demonstrated this was due to such matrix alterations as mentioned above and not remnants of SDS.36
What stands out from these studies is the need for a systematic, direct comparison of those decellularization methods that have been developed in the literature. The effectiveness of such a procedure needs to be measured by the three parameters: completeness of cell removal, preservation of the structural and compositional nature of the tissue, and the capacity for repopulation by fibroblasts. Of note, since the biomaterial is meant to primarily act as a scaffold for cells, its biomechanical properties are not as important as they would be in a tissue-engineered whole ACL graft.24 Our findings showed that all treatments are highly effective in decellularization, and given the absolute values and fairly narrow confidence intervals of remaining DNA, there is no reason to suspect a different result in a larger study. We used DNA measurement to determine decellularization effectiveness, since this is a very sensitive method to test for the presence of cells. We did not assess decellularization histologically, since such assessment might yield a false negative result due to oversight of remaining cells in the physiologically already rather hypocellular ACL. Furthermore, findings concerning structural alterations due to decellularization showed no significant changes in the contents of collagen or total protein, but did show a reduction in glycosaminoglycan content, with the potential of a complete depletion of GAG by SDS or trypsin, which is in accordance with findings from previous studies.15,36 For GAG content, however, the relatively wide confidence intervals would allow for considerably different results in future studies. Of the protocols tested in this study, TRITON-X had the least detrimental effect on GAG content. In summary, we interpreted these findings as a recommendation for the use of TRITON-X to decellularize ACL tissue effectively and with the least adverse effects.
Reseeding of the decellularized ACL with human fibroblasts was successful, and our measurements showed persistent DNA contents and increasing procollagen measures over time, depicting the typical behavior of highly differentiated cells with high biosynthetic activity but low mitotic rates. Lower seeding density could lead to higher rates of mitosis, which might be beneficial in a defect healing environment. Yet it is important to consider that orchestrated biosynthesis is more important than rapid cell growth, since the latter would lead to a functionally and mechanically inferior scar tissue.37-39 Our histological specimens showed that the cells formed clusters on the surface of the biomaterial, as is often seen in static seeding.40,41 Deeper infiltration into the tissue might be seen at later follow-ups, but since our study was designed to test the feasibility of reseeding in general and not the behavior of the reseeded cells, data on time points later than 2 weeks are not available. Another reason for poor infiltration is the rather high density of the material. In fact, a previous study showed that ultrasonic modification of decellularized tendon increases recellularization.42 Finally, we interpret the decreasing content of free soluble collagen in the culture medium despite increasing procollagen production as an indirect proof of ongoing incorporation of newly synthesized collagen into the extracellular matrix. It should be remembered that procollagen contents should only be seen as a surrogate of collagen, but, as pointed out earlier, we decided to use procollagen rather than collagen measurement because we wanted to study cellular behavior independent from extracellular processes.
Our study has some shortcomings. Firstly, the question emerges whether it is necessary to decellularize tissue for ACL tissue engineering at all. Disease transmission with ACL allografts seems almost negligible,43 and studies have suggested that enzymatic removal of cell surface epitopes reduces immunogenicity of xenografts.44 However, as mentioned above, it has been shown that the presence of intrinsic cells, and their necrosis after graft harvest and implantation, can delay host cell infiltration and affect ligamentization.34,35,45 Although the specifics of donor–host cell interaction, both beneficial and detrimental, remain widely unknown, it is rather unlikely that the implantation of allogenic or even xenogenic cells will have any benefit to the host. Future studies comparing these methodologically different, yet technically equivalent, methods such as decellularization or epitope removal will be needed to definitively answer this question. Secondly, there is currently no reason to choose xenogenic over allogenic tissue for ACL tissue engineering other than availability. However, a xenogenic material is more easily and abundantly available, which would prove most valuable in a clinical application. Also, it is likely that the findings obtained from porcine tissue are also valid for human ACL. Lastly, there might be additional confounding factors we did not include in our analysis, yet the fairly narrow confidence intervals and findings consistent with the literature support the precision and validity of our findings, while the size of our sample discourages further sub-grouping.46 In summary, given the lack of prior comparative studies, the character of our study is more hypothesis-building in nature than hypothesis-testing.
In conclusion, our findings commend decellularization with TRITON-X over the use of SDS or trypsin, measured by effectiveness of cell removal and minimization of biochemical alterations. Also, porcine ACL tissue decellularized with TRITON -X can be successfully reseeded with human fibroblasts, which produce procollagen in increasing quantities. Future studies addressing the in vivo efficacy of decellularized ACL and other available scaffolds would be of great interest.
ACKNOWLEDGMENTS
This study was supported by the NIH-NIAMS grant R01 AR052772.
Footnotes
Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements) that might pose a conflict of interest in connection with the submitted article.
REFERENCES
- 1.Liden M, Sernert N, Rostgard-Christensen L, et al. Osteoarthritic changes after anterior cruciate ligament reconstruction using bone-patellar tendon-bone or hamstring tendon autografts: a retrospective, 7-year radiographic and clinical follow-up study. Arthroscopy. 2008;24:899–908. doi: 10.1016/j.arthro.2008.04.066. [DOI] [PubMed] [Google Scholar]
- 2.Neuman P, Englund M, Kostogiannis I, et al. Prevalence of tibiofemoral osteoarthritis 15 years after nonoperative treatment of anterior cruciate ligament injury: a prospective cohort study. Am J Sports Med. 2008;36:1717–1725. doi: 10.1177/0363546508316770. [DOI] [PubMed] [Google Scholar]
- 3.Jones AP, Sidhom S, Sefton G. Long-term clinical review (10–20 years) after reconstruction of the anterior cruciate ligament using the Leeds-Keio synthetic ligament. J Long-Term Eff Med Implants. 2007;17:59–69. doi: 10.1615/jlongtermeffmedimplants.v17.i1.90. [DOI] [PubMed] [Google Scholar]
- 4.Kessler MA, Behrend H, Henz S, et al. Function, osteoarthritis and activity after ACL-rupture: 11 years follow-up results of conservative versus reconstructive treatment. Knee Surg Sports Traumatol Arthrosc. 2008;16:442–448. doi: 10.1007/s00167-008-0498-x. [DOI] [PubMed] [Google Scholar]
- 5.Murray MM, Spindler KP, Abreu E, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 6.Murray MM, Spindler KP, Ballard P, et al. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007–1017. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- 7.Murray MM, Spindler KP, Devin C, et al. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res. 2006;24:820–830. doi: 10.1002/jor.20073. [DOI] [PubMed] [Google Scholar]
- 8.Spindler KP, Murray MM, Carey JL, et al. The use of platelets to affect functional healing of an anterior cruciate ligament (ACL) autograft in a caprine ACL reconstruction model. J Orthop Res. 2009;27:631–638. doi: 10.1002/jor.20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fufa D, Shealy B, Jacobson M, et al. Activation of platelet-rich plasma using soluble type I collagen. J Oral Maxillofac Surg. 2008;66:684–690. doi: 10.1016/j.joms.2007.06.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleming BC, Spindler KP, Palmer M, et al. Collagen-platelet composites improve the biomechanical properties of healing ACL grafts in a porcine model. Am J Sports Med. 2009 doi: 10.1177/0363546509332257. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell E. Tissue engineering in perspective. In: Lanza RP, Vacanti J, editors. Principles of tissue engineering. 2nd ed. Academic Press; San Diego: 2000. pp. xxxv–xli. [Google Scholar]
- 12.Hubbell JA. Matrix effects. Academic Press; San Diego: 2007. pp. 297–308. [Google Scholar]
- 13.Rieder E, Seebacher G, Kasimir MT, et al. Tissue engineering of heart valves: decellularized porcine and human valve scaffolds differ importantly in residual potential to attract monocytic cells. Circulation. 2005;111:2792–2797. doi: 10.1161/CIRCULATIONAHA.104.473629. [DOI] [PubMed] [Google Scholar]
- 14.Chen RN, Ho HO, Tsai YT, et al. Process development of an acellular dermal matrix (ADM) for biomedical applications. Biomaterials. 2004;25:2679–2686. doi: 10.1016/j.biomaterials.2003.09.070. [DOI] [PubMed] [Google Scholar]
- 15.Courtman DW, Pereira CA, Kashef V, et al. Development of a pericardial acellular matrix biomaterial: biochemical and mechanical effects of cell extraction. J Biomed Mater Res. 1994;28:655–666. doi: 10.1002/jbm.820280602. [DOI] [PubMed] [Google Scholar]
- 16.Dumont CE, Hentz VR. Enhancement of axon growth by detergent-extracted nerve grafts. Transplantation. 1997;63:1210–1215. doi: 10.1097/00007890-199705150-00004. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt CE, Baier JM. Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials. 2000;21:2215–2231. doi: 10.1016/s0142-9612(00)00148-4. [DOI] [PubMed] [Google Scholar]
- 19.Badylak SF. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol. 2004;12:367–377. doi: 10.1016/j.trim.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Grauss RW, Hazekamp MG, Oppenhuizen F, et al. Histological evaluation of decellularised porcine aortic valves: matrix changes due to different decellularisation methods. Eur J Cardiothorac Surg. 2005;27:566–571. doi: 10.1016/j.ejcts.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 21.Schenke-Layland K, Vasilevski O, Opitz F, et al. Impact of decellularization of xenogeneic tissue on extracellular matrix integrity for tissue engineering of heart valves. J Struct Biol. 2003;143:201–208. doi: 10.1016/j.jsb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Dahl S, Koh J, Prabhakar V, et al. Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant. 2003;21:659–666. [PubMed] [Google Scholar]
- 23.Cartmell JS, Dunn MG. Development of cell-seeded patellar tendon allografts for anterior cruciate ligament reconstruction. Tissue Eng. 2004;10:1065–1075. doi: 10.1089/ten.2004.10.1065. [DOI] [PubMed] [Google Scholar]
- 24.Woods T, Gratzer PF. Effectiveness of three extraction techniques in the development of a decellularized bone-anterior cruciate ligament-bone graft. Biomaterials. 2005;26:7339–7349. doi: 10.1016/j.biomaterials.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 25.Rieder E, Kasimir MT, Silberhumer G, et al. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J Thorac Cardiovasc Surg. 2004;127:399–405. doi: 10.1016/j.jtcvs.2003.06.017. [DOI] [PubMed] [Google Scholar]
- 26.Chen R, Ho H, Tsai Y, et al. Process development of an acellular dermal matrix (ADM) for biomedical applications. Biomaterials. 2004;25:2679–2686. doi: 10.1016/j.biomaterials.2003.09.070. [DOI] [PubMed] [Google Scholar]
- 27.Hudson T, Liu S, Schmidt C. Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng. 2004;10:1346–1358. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- 28.Murray MM, Martin SD, Martin TL, et al. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg [Am] 2000;82:1387–1397. doi: 10.2106/00004623-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Murray MM, Spector M. The migration of cells from the ruptured human anterior cruciate ligament into collagen-glycosaminoglycan regeneration templates in vitro. Biomaterials. 2001;22:2393–2402. doi: 10.1016/s0142-9612(00)00426-9. [DOI] [PubMed] [Google Scholar]
- 30.Hoemann CD. Molecular and biochemical assays of cartilage components. In: Ceuninck F, Pastoureau P, editors. Cartilage and osteoarthritis. Humana Press; Totowa, NJ: 2004. pp. 127–156. [DOI] [PubMed] [Google Scholar]
- 31.Hoemann CD, Sun J, Chrzanowski V, et al. A multi-valent assay to detect glycosaminoglycan, protein, collagen, RNA, and DNA content in milligram samples of cartilage or hydrogel-based repair cartilage. Anal Biochem. 2002;300:1–10. doi: 10.1006/abio.2001.5436. [DOI] [PubMed] [Google Scholar]
- 32.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 33.Smith J. Postcolumn amino acid analysis. In: Smith BJ, editor. Protein sequencing protocols. Humana Press; Totowa, NJ: 1997. [Google Scholar]
- 34.Arnoczky SP, Tarvin GB, Marshall JL. Anterior cruciate ligament replacement using patellar tendon. An evaluation of graft revascularization in the dog. J Bone Joint Surg [Am] 1982;64:217–224. [PubMed] [Google Scholar]
- 35.Delay BS, McGrath BE, Mindell ER. Observations on a retrieved patellar tendon autograft used to reconstruct the anterior cruciate ligament. A case report. J Bone Joint Surg [Am] 2002;84-A:1433–1438. doi: 10.2106/00004623-200208000-00020. [DOI] [PubMed] [Google Scholar]
- 36.Gratzer PF, Harrison RD, Woods T. Matrix alteration and not residual sodium dodecyl sulfate cytotoxicity affects the cellular repopulation of a decellularized matrix. Tissue Eng. 2006;12:2975–2983. doi: 10.1089/ten.2006.12.2975. [DOI] [PubMed] [Google Scholar]
- 37.Forslund C, Aspenberg P. Improved healing of transected rabbit Achilles tendon after a single injection of cartilage-derived morphogenetic protein-2. Am J Sports Med. 2003;31:555–559. doi: 10.1177/03635465030310041301. [DOI] [PubMed] [Google Scholar]
- 38.Rodeo SA, Potter HG, Kawamura S, et al. Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors. J Bone Joint Surg [Am] 2007;89:2485–2497. doi: 10.2106/JBJS.C.01627. [DOI] [PubMed] [Google Scholar]
- 39.Spindler KP, Murray MM, Detwiler KB, et al. The biomechanical response to doses of TGF-beta 2 in the healing rabbit medial collateral ligament. J Orthop Res. 2003;21:245–249. doi: 10.1016/S0736-0266(02)00145-6. [DOI] [PubMed] [Google Scholar]
- 40.Freed LE, Vunjak-Novakovic G, Langer R. Cultivation of cell-polymer cartilage implants in bioreactors. J Cell Biochem. 1993;51:257–264. doi: 10.1002/jcb.240510304. [DOI] [PubMed] [Google Scholar]
- 41.Vunjak-Novakovic G, Obradovic B, Martin I, et al. Dynamic cell seeding of polymer scaffolds for cartilage tissue engineering. Biotechnol Prog. 1998;14:193–202. doi: 10.1021/bp970120j. [DOI] [PubMed] [Google Scholar]
- 42.Ingram J, Korrosis S, Fisher J, et al. Ultrasonic modification of acellular tendon to enhance recellularisation. Eur Cells Mater. 2005;10:14. [Google Scholar]
- 43.Tomford WW. Transmission of disease through transplantation of musculoskeletal allografts. J Bone Joint Surg [Am] 1995;77:1742–1754. doi: 10.2106/00004623-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 44.Stone KR, Abdel-Motal UM, Walgenbach AW, et al. Replacement of human anterior cruciate ligaments with pig ligaments: a model for anti-non-gal antibody response in long-term xenotransplantation. Transplantation. 2007;83:211–219. doi: 10.1097/01.tp.0000250598.29377.13. [DOI] [PubMed] [Google Scholar]
- 45.Tohyama H, Yasuda K. Extrinsic cell infiltration and revascularization accelerate mechanical deterioration of the patellar tendon after fibroblast necrosis. J Biomech Eng. 2000;122:594–599. doi: 10.1115/1.1319659. [DOI] [PubMed] [Google Scholar]
- 46.Vavken P, Culen G, Dorotka R. Management of confounding in controlled orthopaedic trials: a cross-sectional study. Clin Orthop Relat Res. 2008;466:985–989. doi: 10.1007/s11999-007-0098-y. [DOI] [PMC free article] [PubMed] [Google Scholar]