Abstract
Background/Aims
The distribution of age of onset of essential tremor (ET) is unclear, with discrepancies in the literature. Some data suggest a bimodal distribution and other data 1 late-life peak. We studied age of ET onset in 2 distinct settings: a population-based study and a tertiary referral center.
Methods
Age of onset data were collected.
Results
In the population, there was only a small peak at the age of ≤30 years (14.1% of cases) but a clear peak in later life (85.9% of cases). In the tertiary referral center, a bimodal distribution was apparent with 1 large peak (42.2% of cases) at the age of ≤40 years and the second large peak (57.8% of cases) in later life. Familial cases accounted for only 52.6% of young-onset cases from the population, yet 82.7% from the tertiary center.
Discussion
In the population-based study, a peak in later life was clearly present but a young-onset peak was barely discernable, comprising few cases. By contrast, in a tertiary referral center, age of onset was clearly bimodal. While age of ET onset is often said to be bimodal, this may be due to the preferential referral to tertiary centers of patients with young-onset, familial ET.
Key Words: Essential tremor, age of onset; Epidemiology, essential tremor; Bimodal
Introduction
Among the fundamental issues in neurological epidemiology are disease frequency (prevalence and incidence rates), age of onset and gender distribution, and risk factors and etiology [1]. Data on age of onset can provide initial insights into the developing and aging brain's susceptibility to particular diseases.
The age of onset of essential tremor (ET) is often said to have a bimodal distribution, with peaks occurring during the second or third decades and seventh or eighth decades of life [2,3,4,5]. For neurological disorders, such bimodality is relatively uncommon. Furthermore, this bimodal distribution of age of onset, which has mainly been observed in clinic-derived patient samples, seems at odds with data from epidemiological studies. The incidence of ET increases with age [6, 7], with no peak in incidence during early life [6], and the prevalence of ET also increases with age [8, 9], with no abundance of cases during the initial decades of life [8]. As a result, the distribution of age of onset of ET is not clear, with apparent discrepancies in the literature. To date, there has not been a data-driven study with a primary focus on the age of onset of ET.
The goal of the current study was to examine and compare the age of tremor onset in ET using patients sampled from different settings including a tertiary referral center in the USA and a population-based epidemiological study in Turkey. In each setting, the method of case evaluation and diagnostic criteria was identical. The data generated from these analyses were also compared to the data in the literature to formulate a consistent statement about age of onset of ET.
Methods
Two ET case samples were used, including 1 from a tertiary referral center and 1 population-based sample. Both studies defined ET based on the presence of moderate amplitude action tremor of the arms or head tremor in the absence of alternative diagnoses; diagnostic criteria have been published in detail for each study [10,11,12,13]. All cases signed written informed consent at the time of enrollment.
Description of the Sample from a Tertiary Referral Center
All ET cases were enrolled in a study of the environmental epidemiology of ET [10, 11]. As described previously [10, 11], these ET cases were adult patients (aged ≥18 years) seen at the Neurological Institute of New York, Columbia University Medical Center. They were identified from a computerized database listing names and diagnoses of all patients billed within the past 3 years supplemented by a computerized database at the Center for Parkinson's Disease and other Movement Disorders, Columbia University Medical Center, which listed names and diagnoses of patients seen within the past 10 years. Each patient had received a diagnosis of ET from their treating neurologist at the Institute. All ET patients were selected for enrollment. Office records were reviewed and patients with diagnoses or physical signs of dystonia, Parkinson's disease or spinocerebellar ataxia were excluded [10, 11]. After enrollment, each patient was examined using a standardized tremor evaluation and a neurological examination to assess signs of parkinsonism and other movement disorders. The tremor examination included 1 test for postural tremor and 5 tests for kinetic tremor performed with each hand (12 tests total). Based on the examination, a study neurologist then independently assigned a diagnosis of ET using published diagnostic criteria, which required the presence of moderate or greater amplitude kinetic tremor during >3 tests or a head tremor (as in the Turkish study below) [10, 11]. Demographic data and information on age of onset and family history were collected. The age of tremor onset was known in 249 of 261 ET cases.
Description of the Population-Based Sample in Turkey
A population-based study of the prevalence of ET was conducted in Mersin, an administrative province on the Mediterranean coast of Turkey (area = 776,000 km2, population of 386,777 individuals >40 years of age) [12, 13]. As described previously [12, 13], the target study population consisted of 2,500 adults who represented 0.65% of the Mersin population >40 years old. The epidemiological survey used door-to-door interviews and examinations. Four study neurologists performed the evaluations; each evaluation was conducted by 2 of the 4. The neurologists visited the 2,500 residents in their homes between July and December, 2002. Each resident was examined using a standardized tremor examination and a neurological evaluation to assess signs of parkinsonism and other movement disorders. The tremor examination included 1 test for postural tremor and 5 for kinetic tremor performed with each hand (12 tests total). Based on examination, each neurologist independently assigned a diagnosis of ET using published diagnostic criteria, which required the presence of moderate or greater amplitude kinetic tremor during >3 tests or a head tremor (as in the tertiary referral center) [12, 13]. As described previously [12, 13], there were 89 prevalent ET cases. Demographic data and information on age of onset and family history were collected as well. The age of tremor onset was known in 71 of 89 ET cases.
Statistical Analyses
All analyses were performed in SPSS (version 13.0). Student's t tests and χ2 tests were used. Bimodality was assessed by examining the distribution of age of onset with a histogram; additionally, we used a Student t test to assess whether the means of the identified peaks differed. In each study, a positive family history was defined as the presence of ≥1 first-degree relative who was reported to have ET. Second-degree relatives were not included in this definition because the high population prevalence of ET would have limited the ability to make meaningful comparisons between studies using such a definition.
Results
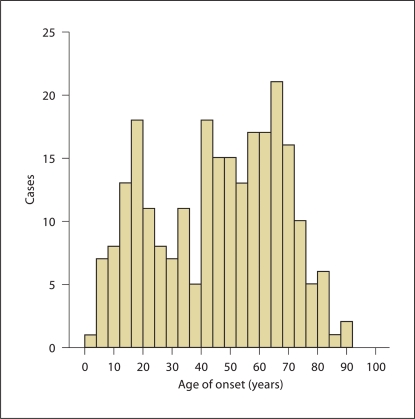
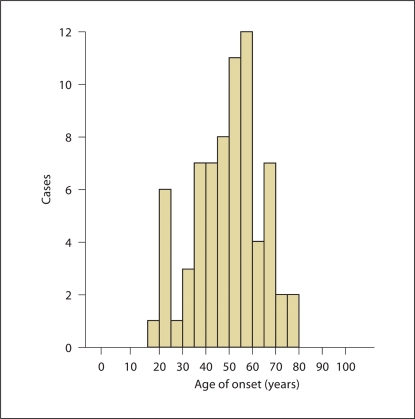
The ET cases from the population-based study in Turkey were younger than the ET cases from the tertiary referral center (t = 4.07, p < 0.001, table 1). Although the mean ages of tremor onset were similar in the 2 studies (t = 1.21, p = 0.23), the distributions (i.e., age of onset by decade) differed between the 2 studies (χ2 = 21.53, p = 0.006). The distribution of age of tremor onset is shown for each of the ET case samples (fig. 1, 2). In the tertiary referral center, a bimodal distribution was readily apparent (fig. 1); there was 1 large peak at the age of ≤40 years [105 (42.2%) of 249 cases] and a second large peak after the age of 40 years [144 (57.8%) of 249 cases]. The means of the 2 peaks differed (20.3 ± 9.4 vs. 58.3 ± 12.6 years, p < 0.001). There were 232 ET cases aged ≥40 years, and the age of onset was known in 221 of these. In an analysis restricted to these 221, a bimodal distribution remained apparent; there was 1 large peak at the age of ≤40 years [77 (34.8%) of 221 cases] and a second large peak after the age of 40 years [144 (65.2%) of 221 cases]. In the population-based sample from Turkey, a very small peak at the age of ≤30 years was barely discernable [10 (14.1%) of 71 cases] and there was a clear second peak after the age of 30 years [61 (85.9%) of 71 cases]; the means of the 2 peaks differed (20.9 ± 3.0 vs. 51.5 ± 11.6 years, p < 0.001; fig. 2).
Table 1.
Current age and age of tremor onset in 2 ET case samples
| Tertiary referral center | Population: Turkey | Statistical significance | |
|---|---|---|---|
| Patients | 249 | 71 | |
| Current mean age ± SD, years | 66.4 ± 16.1 [18–95] | 58.0 ± 12.2 [40–83] | t = 4.07, p < 0.001 |
| Age of tremor onset, years | 45.1 ± 21.7 [3–90] | 48.4 ± 14.6 [17–78] | t = 1.21, p = 0.23 |
| Age of tremor onset by decade | χ2 = 21.53, p = 0.006 | ||
| 0–10 years | 15 (6.0) | 0 (0.0) | |
| 11–20 years | 36 (14.5) | 6 (8.5) | |
| 21–30 years | 22 (8.8) | 4 (5.6) | |
| 31–40 years | 32 (12.9) | 9 (12.7) | |
| 41–50 years | 32 (12.9) | 22 (31.0) | |
| 51–60 years | 44 (17.7) | 16 (22.5) | |
| 61–70 years | 43 (17.3) | 12 (16.9) | |
| 71–80 years | 21 (8.4) | 2 (2.8) | |
| 81 years and older | 4 (1.6) | 0 (0.0) | |
| Proportion of ET cases with a positive family history of ETa | 161 (65.7) of 245 | 31 (49.2) of 63 | χ2 = 5.82, p = 0.016 |
Figures in square brackets are ranges and values in parentheses represent percentages.
Excluding cases with no family history information.
Fig. 1.
The distribution of age of onset in the ET case sample from the tertiary referral center. A bimodal distribution was apparent; there was 1 large peak prior to the age of 40 years [105 (42.2%) of 249 cases] and a second large peak after the age of 40 years [144 (57.8%) of 249 cases].
Fig. 2.
In the population-based sample from Turkey, a small peak was discernable prior to the age of 30 years [10 (14.1%) of 71 cases] and a second larger peak after the age of 30 years [61 (85.9%) of 71 cases].
Approximately two thirds of the cases from the tertiary referral center had a positive family history of ET; in the population-based study, this proportion was smaller but still comprised nearly one half of ET cases (χ2 = 5.82, p = 0.016, table 1). A large proportion of the young-onset cases (defined as ≤40 years) had the familial form of ET. Indeed, familial cases accounted for 86 (82.7%) of the 104 young-onset cases with family history data from the tertiary referral center and 10 (52.6%) of 17 young-onset cases with family history data from the population-based sample in Turkey.
Discussion
In review articles [2, 5], the age of onset of ET is often said to be bimodally distributed. For neurological disorders, however, such bimodality is relatively uncommon; furthermore, this bimodal distribution of age of onset seems at odds with limited data from epidemiological studies [6, 8]. Summary statistics (e.g., mean, range) on age of onset are sometimes reported in ET case series, although the distributions themselves are rarely presented [3,4,5]. Indeed, until now, there has not been a study whose analyses were primarily focused on the age of onset of ET, nor has there been a study that assessed age of onset in different settings using the same evaluation and diagnostic tools. We studied age of ET onset in 2 distinct settings: a population-based epidemiological study and a tertiary referral center. We found in the population-based study, while a late-life peak was clearly discernable, a young-onset peak could barely be identified, comprising very few cases. By contrast, in a sample of cases from a tertiary referral center, the age of onset exhibited a clear bimodal distribution; a young-onset peak prior to the age of 30 or 40 years was sizable, comprising nearly 1 in 2 ET cases and a second major peak occurred in later decades of life. This difference between clinic-based and population-based samples may be due to the preferential referral to tertiary centers of patients with young-onset, familial forms of ET. In a population-based sample, the early-life peak was more difficult to discern, comprised mainly of a small group of familial cases.
We previously demonstrated that clinic cases were 4.73 times more likely to report an affected relative than were community-based cases [14]. In each of our case samples, the majority of the young-onset cases had the familial form of ET; this was most marked in the tertiary referral center but still apparent in the population-based sample (82.7 vs. 52.6%, Fisher's p = 0.07). Also, we have previously presented data that suggest that younger-onset ET is more likely to be familial [15]. Similarly, in other movement disorders (e.g., Parkinson's disease), a high proportion of young-onset cases have a familial form of the disease [16]. Having an underlying susceptibility gene for a disorder may result in the earlier clinical manifestation of symptoms and signs. Alternatively, persons with familial ET may notice their tremor and seek medical advice at an earlier age than those with a nonfamilial form of the disease.
Similar to what was observed in the current study, previous clinic-based samples of ET cases have noted the presence of large young-onset peaks [3,4,5]. By contrast, in epidemiological studies, a young-onset peak has been more difficult to appreciate. The one incidence study of ET that enrolled persons of all ages did not report a bimodal distribution of newly diagnosed ET [6]. In that study, the incidence of ET per 100,000 increased with age: 2.3 (0–19 years of age), 5.4 (20–39), 13.9 (40–49), 34.6 (50–59), 58.6 (60–69), 76.6 (70–79) and 84.3 (≥80), with no peak in incidence during early life. However, incidence data were presented in 20-year age strata and it is conceivable that presentation of age of onset data in smaller age intervals (e.g. yearly intervals) could have revealed a small early-life peak. Prevalence studies in general have not sampled younger age groups so that data are scanty; however, available data suggest that young-onset ET is rare [8]. Furthermore, prevalence studies report age of onset in large (e.g., ≥20-year) age strata and data on current age rather than age of onset. Hence, one cannot exclude the possibility of a small young-onset peak from published epidemiological data.
Our samples were from a tertiary referral center and an epidemiological study in Turkey. The former excluded prevalent cases who were <18 years of age and the latter <40 years of age. While it is apparent that young ages of tremor onset were observed in both samples (e.g., 3 years of age in the tertiary referral center and 17 in the study in Turkey), the exclusions of young prevalent cases may have resulted in an underascertainment of young-onset cases, particularly in the sample from Turkey. Although population-based studies demonstrate that prevalent cases who are young comprise a small proportion of all ET cases (e.g., <2.5% were <40 years of age in 1 study [8]), this source of bias cannot be excluded.
A limitation of this study is that it is difficult to validate reported age of tremor onset. However, we have demonstrated in a previous study that the reported age of onset is reliable [17]. The study had several strengths. We used a large dataset from a tertiary referral center and also utilized a case sample from a population-based epidemiological study. In both settings, ET was evaluated and diagnosed identically. The current study was unique in that it focused primarily on age of onset, presenting data on the primary variable of interest (age of onset) as well as other important factors (e.g., family history).
Acknowledgements
Supported byR01 NS39422, P30 ES09089 and RR00645 (General Clinical Research Center) (NIH, Bethesda, Md., USA).
References
- 1.Cudkowicz ME. Epidemiological methods in neurology. In: Batchelor T, Cudkowicz ME, editors. Principles of Neuroepidemiology. Boston: Butterworth-Heinemann; 2001. [Google Scholar]
- 2.Benito-León J, Louis ED. Essential tremor: emerging views of a common disorder. Nat Clin Pract Neurol. 2006;2:666–678. doi: 10.1038/ncpneuro0347. [DOI] [PubMed] [Google Scholar]
- 3.Lou JS, Jankovic J. Essential tremor: clinical correlates in 350 patients. Neurology. 1991;41:234–238. doi: 10.1212/wnl.41.2_part_1.234. [DOI] [PubMed] [Google Scholar]
- 4.Koller WC, Busenbark K, Miner K, Essential Tremor Study Group The relationship of essential tremor to other movement disorders: report on 678 patients. Ann Neurol. 1994;35:717–723. doi: 10.1002/ana.410350613. [DOI] [PubMed] [Google Scholar]
- 5.Brin MF, Koller W. Epidemiology and genetics of essential tremor. Mov Disord. 1998;13(suppl 3):55–63. doi: 10.1002/mds.870131310. [DOI] [PubMed] [Google Scholar]
- 6.Rajput AH, Offord KP, Beard CM, Kurland LT. Essential tremor in Rochester, Minnesota: a 45-year study. J Neurol Neurosurg Psychiatry. 1984;47:466–470. doi: 10.1136/jnnp.47.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benito-León J, Bermejo-Pareja F, Louis ED. Incidence of essential tremor in three elderly populations of central Spain. Neurology. 2005;64:1721–1725. doi: 10.1212/01.WNL.0000161852.70374.01. [DOI] [PubMed] [Google Scholar]
- 8.Hornabrook RW, Nagurney JT. Essential tremor in Papua New Guinea. Brain. 1976;99:659–672. doi: 10.1093/brain/99.4.659. [DOI] [PubMed] [Google Scholar]
- 9.Benito-León J, Bermejo-Pareja F, Morales JM, et al. Prevalence of essential tremor in three elderly populations of central Spain. Mov Disord. 2003;18:389–394. doi: 10.1002/mds.10376. [DOI] [PubMed] [Google Scholar]
- 10.Louis ED, Zheng W, Applegate L, Shi L, Factor-Litvak P. Blood hormone concentrations and dietary protein consumption in essential tremor. Neurology. 2005;65:391–396. doi: 10.1212/01.wnl.0000172352.88359.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis ED, Applegate L, Graziano J, Parides M, Slavkovich V, Bhat H. Interaction between blood lead concentration and delta-amino-levulinic acid dehydratase gene polymorphisms increases the odds of essential tremor. Mov Disord. 2005;20:1170–1177. doi: 10.1002/mds.20565. [DOI] [PubMed] [Google Scholar]
- 12.Dogu O, Sevim S, Camdeviren H, et al. Prevalence of essential tremor: door-to-door neurological exams in Mersin Province, Turkey. Neurology. 2003;61:1804–1807. doi: 10.1212/01.wnl.0000099075.19951.8c. [DOI] [PubMed] [Google Scholar]
- 13.Dogu O, Sevim S, Louis ED, Kaleagasi H, Aral M. Reduced body mass index in essential tremor: a population-based study in Mersin Province, Turkey. Arch Neurol. 2004;61:386–389. doi: 10.1001/archneur.61.3.386. [DOI] [PubMed] [Google Scholar]
- 14.Louis ED, Barnes LF, Ford B, Ottman R. Family history information on essential tremor: potential biases related to the source of the cases. Mov Disord. 2001;16:320–324. doi: 10.1002/mds.1040. [DOI] [PubMed] [Google Scholar]
- 15.Louis ED, Ford B, Frucht S, Barnes LF, Tang M-X, Ottman R. Risk of tremor and impairment from tremor in relatives of patients with essential tremor: a community-based family study. Ann Neurol. 2001;49:761–769. doi: 10.1002/ana.1022. [DOI] [PubMed] [Google Scholar]
- 16.Klein C. Implications of genetics on the diagnosis and care of patients with Parkinson disease. Arch Neurol. 2006;63:328–334. doi: 10.1001/archneur.63.3.328. [DOI] [PubMed] [Google Scholar]
- 17.Louis ED, Schonberger R, Parides M, Ford B, Barnes L. Test-retest reliability of patient information on age of onset of essential tremor. Mov Disord. 2000;15:738–741. doi: 10.1002/1531-8257(200007)15:4<738::aid-mds1024>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]