Abstract
Background
Dietary fat intake is associated with coronary heart disease risk, but the relationship between fat intake and ischemic stroke risk remains unclear. We hypothesized that total dietary fat as part of a Western diet is associated with increased risk of ischemic stroke.
Methods
As part of the prospective Northern Manhattan Study, 3,183 stroke-free community residents over 40 years of age underwent evaluation of their medical history and had their diet assessed by a food-frequency survey. Cox proportional hazard models calculated risk of incident ischemic stroke.
Results
The mean age of participants was 69 years, 63% were women, 21% were white, 24% black and 52% Hispanic. During a mean of 5.5 years of follow-up, 142 ischemic strokes occurred. After adjusting for potential confounders, risk of ischemic stroke was higher in the upper quintile of total fat intake compared to the lowest quintile (HR 1.6, 95% CI 1.0–2.7). Total fat intake >65 g was associated with increased risk of ischemic stroke (HR 1.6, 95% CI 1.2–2.3). Risk was attenuated after controlling for caloric intake.
Conclusions
The results suggest that increased daily total fat intake, especially above 65 g, significantly increases risk of ischemic stroke.
Key Words: Diet, Fat intake, Neuroepidemiology, Ischemic stroke, Prospective cohort study
Introduction
Dietary fat intake is a risk factor for ischemic heart disease. Multiple observational and interventional studies have shown that diets high in saturated [1, 2] and trans-saturated fat [3] increase LDL cholesterol, decrease HDL cholesterol, and increase the risk of myocardial infarction [4,5,6], while diets high in polyunsaturated and monounsaturated fat have the opposite effect [1]. Largely to limit saturated fat intake, the US Department of Health guidelines recommend that for adults total fat should constitute only 20–35% (max. 65–70 g) of daily caloric intake, and monounsaturated and polyunsaturated fat be substituted for saturated and trans-saturated fat [7].
There is no consensus on the relationship between dietary fat intake and ischemic stroke. Studies have reported that high intake of saturated fat is associated with decreased [8,9,10], increased [11] and neutral [12] stroke risk. Further, there is little agreement on the effect of other fat subtypes, dietary cholesterol and total fat intake. Finally, dietary fat guidelines have recently been relaxed, from 30 to 35% of total caloric intake [7]. The aim of this prospective cohort study was to examine the relationship between total fat intake and ischemic stroke risk in a multi-ethnic urban population.
Methods
Participants
The Northern Manhattan Study (NOMAS) is a prospective cohort study of stroke risk factors in a multi-ethnic population. All participants reside in northern Manhattan, New York, which consists of the region north of 145th Street, south of 218th Street, bordered on the west by the Hudson River, and on the east by the Harlem River. In 1990, when the study began, nearly 260,000 people lived in the community, with 40% over the age of 39, and a race-ethnic mixture consisting of 20% black, 63% Hispanic and 15% white residents.
The methods of participant recruitment and enrolment have been described previously [13, 14]. Participants were enrolled if they were: (1) free of previous stroke; (2) age >40 years, and (3) resident in northern Manhattan for over 3 months in a household with a telephone. Subjects were identified by random digit utilizing dual frame sampling and interviews were conducted using trained bilingual interviewers. The telephone response rate was 91% (9% refused to be screened), and 87% of those eligible indicated willingness to participate. Subjects were recruited from the telephone sample to have an in-person baseline interview and assessment. The enrolment response rate was 75% with an overall response rate of 68% (telephone response × enrolment response). After exclusion of ineligible candidates, a cohort of 3,298 was enrolled between 1993 and 2001. Of these, 3,183 individuals completed the baseline diet survey (97%). The Columbia University Medical Center institutional review board approved the study, and written consent was obtained from participants.
Baseline Evaluation
Participants completed a comprehensive baseline in-person assessment of diet, sociodemographic data, risk factors and medical history [15]. Race/ethnicity was classified by self-identification. Medical history was assessed using questions adapted from the Behavioral Risk Factor Surveillance System from the Centers for Disease Control and Prevention [16]. Standard techniques were used to measure blood pressure, height and weight and to calculate BMI [15]. Fasting blood specimens were analyzed at the Core Laboratory of the Irving Center for clinical research on a Hitachi 912 automated spectrometer (Hitachi, Ohio, USA). Hypertension was defined as either systolic blood pressure levels ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg or history of hypertension. Diabetes mellitus was defined as a history of diabetes or a fasting blood glucose ≥126 mg/dl (7.0 mmol/l). Cardiac disease was defined based on a patient's self-reported history of angina, myocardial infarction, atrial fibrillation or valvular heart disease [15]. Smoking was categorized as past, current or never. Moderate alcohol intake was defined as more than 1 drink per month, but not more than 2 drinks per day. Any physical activity was defined by subject report of engaging in any leisure activity (walking for exercise, running, gardening, etc.) over the 10 days prior to enrolment.
Dietary Assessment
Fat intake at baseline was assessed using a modified Block food frequency questionnaire, which listed 207 foods (HHHQ version Full87, Form A, Form B) [17]. Participants were asked to record how often each food was eaten and the portion size. Fat intake was calculated from questionnaire responses using Block Dietsys version 3.0 software [18], which is based on food composition data from the US Department of Agriculture Nutrient Database for StandardReference [19], and NHANES II (the second National Health and Nutrition ExaminationSurvey) [20]. Calculations accounted for cooking and table fats and visibly fatty portions of meat. The validity of the food frequency questionnaire has been evaluated in previous studies [17].
Outcome Measures
Participants were screened annually by telephone interview to detect events, and continuous surveillance of local hospital admission and discharge ICD-9 codes was conducted. In addition, subjects and their families were reminded to notify NOMAS in the event of a stroke, MI or death. When an event was reported, a physician blinded to all baseline information reviewed the participants' medical record and, where appropriate, autopsy reports or death certificates. The primary outcome measure in this analysis was ischemic stroke.
Statistical Analyses
SAS version 8.02 (SAS Institute, Cary, N.C., USA) was used for statistical analyses. The ischemic stroke incidence rate in each quintile of total fat intake was calculated as the number of ischemic stroke events divided by the follow-up time in that quintile. Cox proportional hazards models were used to obtain HRs with 95% CIs for ischemic stroke risk comparing each quintile of fat intake to the lowest quintile (reference group). Models were adjusted for race-ethnicity, gender, age hypertension, diabetes, cardiac disease, age, race/ethnicity, gender, BMI and physical activity levels. We have dichotomized age as >65 versus <65 years because the relationship between age and stroke risk is different in those over and under 65 years. This definition is consistent with our previous work. Additional adjustments were performed to account for the caloric needs of each individual and we also adjusted for total calories and intake of dietary confounders. A second Cox model was used to determine the relative risk of ischemic stroke associated with a total dietary fat intake greater than 65 g. Models were also constructed using the percentage of daily calories obtained from total fat, and from each fat subtype. In subgroup analysis, we removed 151 subjects with improbable caloric intakes (<800 kcal or >4,200 kcal in men, or <500 kcal or >3,500 kcal in women) [21].
Results
Of the 3,183 participants in NOMAS with dietary information, 63% were women, 21% were non-Hispanic white, 24% non-Hispanic black and 52% Hispanic; their mean age was 69.1 ± 10 years. Subjects were followed for a mean of 5.5 years, during which 142 ischemic strokes occurred. The sociodemographic and risk factor profiles of subjects by quintile of fat intake are shown in table 1. Age, sex, education, current smoking, alcohol intake, weight and BMI were related to total fat intake quintile. Mean caloric intake was 1,565 ± 735 kcal/day (median 1,433 kcal/day), and mean fat intake was 61 ± 34 g/day (median 53 g/day). The average participant consumed 21 g (11% of calories) of saturated fat, 23 g (13% of calories) of oleic acid, 10 g (6% of calories) of linoleic acid, and 243 mg of cholesterol daily. The intake of total fat was associated with increased intake of all other nutrients, as well as total calories.
Table 1.
Baseline characteristics of participants by quintile of total fat intake
| Quintile 1 (n = 637) | Quintile 2 (n = 636) | Quintile 3 (n = 637) | Quintile 4 (n = 636) | Quintile 5 (n = 637) | |
|---|---|---|---|---|---|
| Mean ± SD age, years | 70.5 ± 10.0 | 69.6 ± 10.0 | 68.6 ± 10.2 | 69.1 ± 10.4 | 67.9 ± 10.5a |
| Men | 154 (24) | 213 (33) | 248 (39) | 259 (41) | 313 (49)a |
| Race/ethnicity | |||||
| Hispanic | 335 (53) | 347 (56) | 339 (54) | 338 (55) | 310 (50) |
| Non-Hispanic white | 139 (21) | 127 (20) | 145 (23) | 133 (21) | 126 (20) |
| Non-Hispanic black | 156 (25) | 149 (24) | 140 (22) | 147 (24) | 185 (30) |
| Completed high school | 288 (45) | 263 (41) | 298 (47) | 296 (47) | 320 (50) |
| Former smoker | 223 (35) | 242 (38) | 239 (38) | 241 (38) | 259 (41) |
| Current smoker | 65 (10) | 85 (13) | 91 (14) | 115 (18) | 131 (21)a |
| Moderate alcohol (>1 drink per month and =2 drinks/day) | 172 (27) | 188 (30) | 213 (33) | 222 (35) | 235 (37)b |
| Mean BMI, kg/m2 | 27.6 ± 5.1 | 27.9 ± 5.4 | 27.7 ± 5.6 | 27.4 ± 5.4 | 28.7 ± 6.3b |
| Mean weight, lbs | 156 ± 32 | 160 ± 33 | 161 ± 34 | 160 ± 34 | 172 ± 41a |
| Any physical activity | 374 (59) | 368 (58) | 380 (60) | 391 (62) | 382 (60) |
| Ischemic heart disease | 167 (26) | 143 (22) | 146 (23) | 137 (22) | 162 (26) |
| Diabetes | 132 (21) | 141 (22) | 126 (20) | 145 (23) | 133 (21) |
| Hypertension | 486 (76) | 474 (75) | 449 (70) | 459 (72) | 473 (74) |
Figures in parentheses are percentages.
p for significant trend across row <0.0001.
p for significant trend across row <0.001.
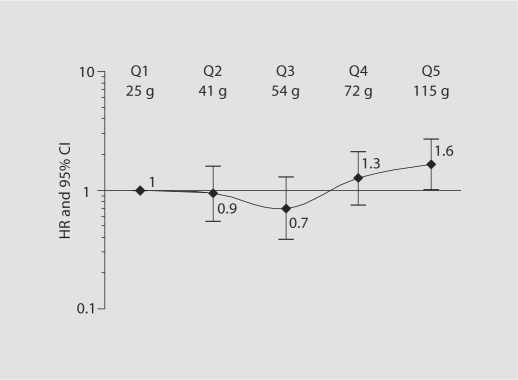
The relationship between total daily fat intake in quintiles and ischemic stroke is shown in figure 1. The ischemic stroke risk for those in the highest quintile of fat intake was higher than for those in the lowest quintile, both in unadjusted analyses and after adjusting for age (<65 vs. >65 years), race/ethnicity (non-Hispanic black, non-Hispanic white, Hispanic), sex, education (completed high school vs. not), hypertension, diabetes, coronary artery disease, moderate alcohol consumption, current smoking, previous smoking, any physical activity and BMI (HR 1.6, 95% CI 1.0–2.9). A test for trend to assess whether there was a significant dose response relationship did not find one. The magnitude of the risk after further adjusting for total calories was similar (HR = 1.6, 95% CI 0.6–3.9) although not significant. Similarly, when fat as a percentage of total daily calories was examined, those who obtained 45% or more of their calories from fat showed a trend toward an increased risk of ischemic stroke (HR 1.4, 95% CI 0.8–2.4, p = 0.08).
Fig. 1.
Risk of ischemic stroke (adjusted hazard ratio, 95% confidence intervals) by quintiles of absolute total fat intake. Mean total daily fat intake (in grams) is shown for each quartile. Hazard ratios are adjusted for all of the following: age (<65 vs. >65 years), race/ethnicity (non-Hispanic black, non-Hispanic white, Hispanic), sex, education (completed high school vs. not), BMI, hypertension, diabetes, coronary artery disease, moderate alcohol consumption, current smoking, previous smoking, physical activity. HR = Hazard ratio; Q = quartile.
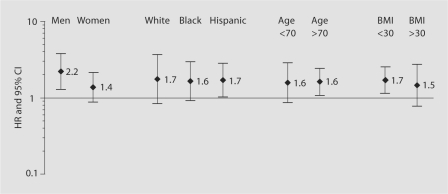
When we examined total fat consumption as dichotomized as ≥65 g versus <65 g, we found that those who consumed >65 g of fat daily had a higher risk of stroke (table 2). This relationship remained significant after adjustment for age, race/ethnicity, sex, education, hypertension, diabetes, coronary artery disease, moderate alcohol consumption, current smoking, previous smoking, any physical activity and BMI. After adjustment for total calories, and other dietary confounders, the significance was attenuated. No significant interactions were found for the relationship with total fat intake and stroke by age, sex, race/ethnicity or BMI (fig. 2).
Table 2.
Risk of ischemic stroke by total daily fat intake (<65 vs. ≥65 g)
| <65 g (n = 2,029) | =65 g (n = 1,154) | |
|---|---|---|
| Cases of ischemic stroke | 79 | 63 |
| Person-years of follow-up | 10,947 | 5,963 |
| Stroke rate per 1,000 person-years | 7.2 | 10.6 |
| Hazard ratio | ||
| Unadjusted | 1.0 (ref.) | 1.5 (1.1–2.1) |
| + Sociodemographics | 1.0 (ref.) | 1.5 (1.1–2.1) |
| + Sociodemographics, risk factors, BMI, PA | 1.0 (ref.) | 1.6 (1.2–2.3) |
| + Sociodemographics, risk factors, BMI, PA, dietary confounders, calories | 1.0 (ref.) | 1.7 (1.0–2.9) |
Figures in parentheses are 95% CI. Sociodemographics = age (<65 vs. =65 years), race/ethnicity (non-Hispanic black, non-Hispanic white, Hispanic), sex, education (completed high school vs. not); risk factors = hypertension, diabetes, coronary artery disease, moderate alcohol, current smoking and previous smoking; BMI and PA = body mass index (as a continuous variable) and any leisure physical activity over the past 10 days; dietary confounders = sodium, potassium, fruit/vegetable calcium, fiber, vitamin E; calories = total calories (in quintiles); ref. = reference.
Fig. 2.
Risk of ischemic stroke (adjusted hazard ratios and 95% confidence intervals) by total fat intake above or below 65 g for population subgroups. Adjusted for age (<65 vs. >65 years), race/ethnicity (non-Hispanic black, non-Hispanic white, Hispanic), sex, education (completed high school vs. not), hypertension, diabetes mellitus, coronary artery disease, moderate alcohol, current smoking and previous smoking, BMI (continuous variable), and any leisure physical activity over the last 10 days.
We also examined the relationship between fat subtype and ischemic stroke. Participants were divided into quintiles by intake of fat subtype (taken as a percentage of total calories), and dietary cholesterol. There was a trend toward an increased risk among those in the top quintile of saturated fat (HR 1.7, 95% CI 0.8–2.3), but not for monounsaturated fat, polyunsaturated fat or dietary cholesterol.
Discussion
Our study supports a significant relationship between the highest quintile of total dietary fat intake and the risk of ischemic stroke in a multi-ethnic urban population which did not differ by race/ethnicity. Additionally, we found that daily intake of ≥65 g of fat (approximately 37% of calories for the average study participant in this study) was associated with a 60% higher ischemic stroke risk, even after adjustment for vascular risk factors. However, after adjustment for caloric intake, the significance associated with increased total fat intake was attenuated, although the risk effect remained similar. This attenuation may be related to over-adjustment in a model with a large number of co-variates, or to the relatively low total caloric intake in the overall cohort. Further, adjusting by total caloric intake is a methodology used by some but not all studies performing dietary analysis.
Previous studies of dietary fat intake and ischemic stroke have produced mixed results. High saturated fat intake [11], high intakes of fish [22, 23], fish-derived polyunsaturated fats [24], and high serum polyunsaturated fats [25, 26] have been associated with decreased ischemic stroke risk. Other studies reported that dietary intakes of animal fat, saturated fat, monounsaturated fat and cholesterol were positively related to subclinical vascular disease, including carotid wall thickness [23, 27].
A number of prospective cohort studies have reported that higher intakes of saturated fat are associated with decreased stroke risk [8,9,10]. Most of these studies, however, were performed in Japanese populations that consume relatively low amounts of total fat (median 47 g daily), protein and cholesterol [9, 10], and which have a higher rate of stroke and particularly hemorrhagic stroke than Western populations. Indeed, the very lowest levels of intake of some fat subtypes are associated with a higher stroke risk, but these findings are probably not applicable to the US population, in which daily mean fat intake is approximately 68 g among women and 95 g among men [28]. A few US studies have also reported that increasing saturated fat, cholesterol and monounsaturated fat intake may be associated with lower rates of ischemic stroke [8], although these results are controversial [29,30,31]. Neither the Health Professionals Study or Nurses Health Study report a deleterious effect on ischemic stroke with high total fat intake, although total fat intake was associated with intraparenchymal hemorrhage [2, 12].
Prospective dietary information examining the relationship between vascular risk and dietary intake, including fat, is limited among minority populations, especially Hispanics. Indeed, few studies have utilized prospective data to examine the relationship between total fat intake and ischemic stroke in different racial/ethnic groups [32, 33]. Our work suggests that fat intake is high among all three racial/ethnic groups in this urban community as we report that over 36% of participants consumed more than 65 g of fat per day, with whites (30%), blacks (37%) and Hispanics (37%) consuming similar high amounts.
The strengths of the NOMAS study include our large, multi-ethnic population-based sample, rigorous follow-up with valid assessment of ischemic stroke outcomes, and a detailed baseline assessment including measurement of height and weight. The food frequency survey is valid, as evidenced by the appropriate caloric intakes we observed for our population. Our observed mean and median total calorie intakes are slightly low, but they are consistent with intakes expected in an elderly, sedentary, predominantly female population [34].
Limitations of the study include an estimation of fat intake using a single food frequency questionnaire covering consumption over the prior 12 months. This type of data collection could result in dietary misclassification. Previous work has shown a reasonable correlation between the results of the Block on repeat administration, and compared to a 7-day food diary [7, 17]. Another limitation of this study was the inability to examine the effect of trans-saturated fat on ischemic stroke due to lack of specific data on this fat subtype. Additionally, we had limited data available on ischemic stroke subtypes as well as small numbers of hemorrhagic stroke and therefore we could not estimate these risks.
Data on dietary fat and ischemic stroke risk are less established than those on cardiac risk [35]. Our findings provide additional evidence suggesting that a high dietary fat intake is significantly associated with elevated risk of ischemic stroke.
Acknowledgements and Funding
This work is supported by grants from the National Institute of Neurological Disorders and Stroke (R01 NS 29993 and T32 NS 07153) and was presented in part at the International Stroke Conference, New Orleans, February 2005.
References
- 1.Ginsberg H, Kris-Etherton P, Dennis B, Elmer PJ, Ershow A, Lefevre M, Pearson T, Roheim P, Ramakrishnan R, Reed R, Stewart K, Stewart P, Phillips K, Anderson N. Effects of reducing dietary saturated fatty acids on plasma lipids and lipoproteins in healthy subjects: the Delta Study, Protocol 1. Arterioscler Thromb Vasc Biol. 1998;18:441–449. doi: 10.1161/01.atv.18.3.441. [DOI] [PubMed] [Google Scholar]
- 2.Walden C, Retzlaff B, Buck B, Wallick S, McCann B, Knopp R. Differential effect of National Cholesterol Education Program (NCEP) Step II diet on HDL cholesterol, its subfractions, and apoprotein A-I in hypercholesterolemic women and men after 1 year: the beFIT study. Arterioscler Thromb Vasc Biol. 2000;20:1580–1587. doi: 10.1161/01.atv.20.6.1580. [DOI] [PubMed] [Google Scholar]
- 3.Judd J, Clevidence B, Muesing R, Wittes J, Sunkin M, Podczasy J. Dietary trans fatty acids: effects on plasma lipids and lipoproteins of healthy men and women. Am J Clin Nutr. 1994;59:861–868. doi: 10.1093/ajcn/59.4.861. [DOI] [PubMed] [Google Scholar]
- 4.Amos CI, Krushkal J, Thiel TJ, Young A, Zhu DK, Boerwinkle E, de Andrade M. Comparison of model-free linkage mapping strategies for the study of a complex trait. Genet Epidemiol. 1997;14:743–748. doi: 10.1002/(SICI)1098-2272(1997)14:6<743::AID-GEPI30>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 5.Ascherio A, Rimm E, Giovannucci E, Spiegelman D, Stampfer M, Willett W. Dietary fat and risk of coronary heart disease in men: cohort follow up study in the United States. BMJ. 1996;313:84–90. doi: 10.1136/bmj.313.7049.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clifton P, Keogh J, Noakes M. Trans fatty acids in adipose tissue and the food supply are associated with myocardial infarction. J Nutr. 2004;134:874–879. doi: 10.1093/jn/134.4.874. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services, U.S. Department of Agriculture . Dietary Guidelines for Americans. Washington: US Government Printing Office; 2000. p. 6. [Google Scholar]
- 8.Gillman M, Cupples L, Millen B, Ellison R, Wolf P. Inverse association of dietary fat with development of ischemic stroke in men. JAMA. 1997;278:2145–2150. [PubMed] [Google Scholar]
- 9.Sauvaget C, Nagano J, Hayashi M, Yamada M. Animal protein, animal fat and cholesterol intakes and the risk of cerebral infarction mortality in the adult health study. Stroke. 2004;35:1531–1537. doi: 10.1161/01.STR.0000130426.52064.09. [DOI] [PubMed] [Google Scholar]
- 10.Seino F, Date C, Nakayama T, Yoshiike N, Yokoyama T, Yamaguchi M, Tanaka H. Dietary lipids and incidence of cerebral infarction in a Japanese rural community. J Nutr Sci Vitaminol (Tokyo) 1997;43:83–99. doi: 10.3177/jnsv.43.83. [DOI] [PubMed] [Google Scholar]
- 11.Matsui T, Arai H, Yuzuriha T, Yao H, Miura M, Hashimoto S, Higuchi S, Matsushita S, Morikawa M, Kato A, Sasaki H. Elevated plasma homocysteine levels and risk of silent brain infarction in elderly people. Stroke. 2001;32:1116–1119. doi: 10.1161/01.str.32.5.1116. [DOI] [PubMed] [Google Scholar]
- 12.Suk SH, Sacco RL, Boden-Albala B, Cheun JF, Pittman JG, Elkind MS, Paik MC. Abdominal obesity and risk of ischemic stroke: the Northern Manhattan Stroke Study. Stroke. 2003 doi: 10.1161/01.STR.0000075294.98582.2F. [DOI] [PubMed] [Google Scholar]
- 13.Boden-Albala B, Sacco RL, Lee HS, Grahame-Clarke C, Rundek T, Elkind MVE, Giardina EG, DiTullio MR, Homma S, Paik MC. Metabolic syndrome and ischemic stroke risk. Stroke. 2008;39:30–35. doi: 10.1161/STROKEAHA.107.496588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkind MS, Cheng J, Boden-Albala B, Paik MC, Sacco RL. Elevated white blood cell count and carotid plaque thickness: the Northern Manhattan Stroke Study. Stroke. 2001;32:842–849. doi: 10.1161/01.str.32.4.842. [DOI] [PubMed] [Google Scholar]
- 15.Elkind M, Cheng J, Boden-Albala B, Rundek T, Thomas J, Chen H, Rabbani LE, Sacco RL. Tumor necrosis factor receptor levels are associated with carotid atherosclerosis. Stroke. 2002;33:31–38. doi: 10.1161/hs0102.100531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gentry EM, Kalsbeek WD, Hogelin GC, Jones JT, Gaines KL, Forman MR, Marks JS, Trowbridge FL, et al. The behavioral risk factor surveys. II. Design, methods, and estimates from combined state data. Am J Prev Med. 1985;1:9–14. [PubMed] [Google Scholar]
- 17.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 18.Block Dietary Data Systems: Dietsys + analysis software, version 56, 1999.
- 19.U.S. Department of Agriculture . USDA nutrient database for standard reference, release 12. Riverdale, MD: Agricultural Research Service; 1998. [Google Scholar]
- 20.National Center for Health Statistics . National Health and Nutrition Examination Survey II. Hyattsville: MD; 1982. [Google Scholar]
- 21.Willet W. Nutritional Epidemiology. ed 2. New York: Oxford University Press; 1998. [Google Scholar]
- 22.Keli S, Feskens E, Kromhout D. The Zutphen Study. Stroke. 1994;25:328–332. doi: 10.1161/01.str.25.2.328. [DOI] [PubMed] [Google Scholar]
- 23.Ellison RC, Myers RH, Zhang Y, Djousse L, Knox S, Williams RR. Effects of similarities in lifestyle habits on familial aggregation of high density lipoprotein and low density lipoprotein cholesterol: the NHLBI Family Heart Study. Am J Epidemiol. 1999;150:910–918. doi: 10.1093/oxfordjournals.aje.a010099. [DOI] [PubMed] [Google Scholar]
- 24.Ricci S, Celani M, Righetti E, Caruso A, De Medio G, Trovarelli G, Romoli S, Stragliotto E, Spizzichino L. Fatty acid dietary intake and the risk of ischaemic stroke: a multicentre case-control study. J Neurol. 1997;244:360–364. doi: 10.1007/s004150050102. [DOI] [PubMed] [Google Scholar]
- 25.Iso H, Sato S, Umemura U, Kudo M, Koike K, Kitamura A, Imano H, Okamura T, Naito Y, Shimamoto T. Linoleic acid, other fatty acids, and the risk of stroke. Stroke. 2002;33:2086–2093. doi: 10.1161/01.str.0000023890.25066.50. [DOI] [PubMed] [Google Scholar]
- 26.Simon J, Fong J, Bernert JTJ, Browner W. Serum fatty acids and the risk of stroke. Stroke. 1995;26:778–782. doi: 10.1161/01.str.26.5.778. [DOI] [PubMed] [Google Scholar]
- 27.Bikkina M, Levy D, Evans JC, Larson MG, Benjamin EJ, Wolf PA, Castelli WP. Left ventricular mass and risk of stroke in an elderly cohort: the Framingham Heart Study. JAMA. 1994;272:33–36. [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention Trends in intake of energy and macronutrients: United States, 1971–2000. MMWR. 2004;53:80–82. [PubMed] [Google Scholar]
- 29.Brunner E, Shipley MJ, Blane D, Smith GD, Marmot MG. When does cardiovascular risk start? Past and present socioeconomic circumstances and risk factors in adulthood. J Epidemiol Community Health. 1999;53:757–764. doi: 10.1136/jech.53.12.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ornish D. Dietary fat and ischemic stroke [Letter] JAMA. 1998;279:1172. [PubMed] [Google Scholar]
- 31.Stein H. Dietary fat and ischemic stroke [Letter] JAMA. 1998;279:1172. [PubMed] [Google Scholar]
- 32.Gans K, Burkholder G, Risica P, Lasater T. Baseline fat-related dietary behaviors of white, Hispanic, and black participants in a cholesterol screening and education project in New England. J Am Dietetic Assoc. 2003;103:699–706. doi: 10.1053/jada.2003.50135. [DOI] [PubMed] [Google Scholar]
- 33.Bermudez O, Falcon L, Tucker K. Intake and food sources of macronutrients among older Hispanic adults: association with ethnicity, acculturation, and length of residence in the United States. J Am Dietetic Assoc. 2000;100:665–673. doi: 10.1016/s0002-8223(00)00195-4. [DOI] [PubMed] [Google Scholar]
- 34.Wakimoto P, Block G. Dietary intake, dietary patterns, and changes with age: an epidemiological perspective. J Gerontol A Biol Sci Med Sci. 2001;56:65–80. doi: 10.1093/gerona/56.suppl_2.65. [DOI] [PubMed] [Google Scholar]
- 35.Sacco R, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB: Gorelick P, Halperin J, Harbaugh R, Johnston SC, Katzan I, Kelly-Hayes M, Kenton EJ, Marks M, Schwamm LH, Tomsick Tl. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke. Stroke. 2006;37:577–617. doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]