Abstract
A method was developed to obtain phage-display ligands that bind to a select population of cells in histological specimens of freshly harvested solid human cancers. It combines phage-display panning with laser capture microdissection (LCM). This method allows selection of phage ligands bound to subpopulations of specific cells contained in tumor tissue on histological sections. Naïve phage scFv library was incubated directly on a histological section of human breast cancer that was snap frozen immediately after surgical resection. Tumor and stromal cells were captured by LCM and bound phages were recovered by bacterial infection. Individual phage clones selected after panning were evaluated for their binding ability by immunofluorescence staining on tumor tissue from the same patient. One phage-display antibody clone selected on tumor stroma showed selective binding on tumor stroma but did not bind to malignant cell population. The expressed scFv of this clone showed no significant binding to normal tissue, or 13 other breast cancers, or 4 colon cancer samples. Using the same method, phage display antibody clones were selected on tumor cells which showed binding to tumor cells and normal tissue. This method is applicable for selection of ligands to virtually any portion of a histological specimen amenable to LCM. This may speed the process of generating ligands to any subset of cells or noncellular feature present on histological specimens.
Keywords: Phage display, Laser Capture Microdissection (LCM), Biopanning, Tumor
1. Introduction
Solid tumors such as breast cancer are complex disorganized tissues. Developing specific antibodies against tumor elements (tumor cell, tumor stroma, and tumor blood vessel) is important in tumor diagnosis and therapy. Herceptin is a successful example in breast cancer treatment as it specifically targets ErbB2. Bevacizumab, a recombinant humanized monoclonal antibody that binds vascular endothelial growth factor, inhibits tumor growth by targeting tumor blood vessels (Ranieri et al., 2006; Jain, 2008). Other components of solid tumors, such as stromal cells, also appear to be potential targets for tumor therapy. There is increasing evidence that tumor growth is dependent upon tumor microenvironments (Yauch et al., 2008; Pietras et al., 2008).
Phage-display technology has been used extensively to generate bioactive ligands to a variety of molecular targets. The most common selection strategy is to pan the naïve phage library against anchored purified target antigens or cells. This allows convenient separation steps of bound and unbound phage. Efforts to select against targets that are in a more natural state include intravenous injection of naïve phage and recovery of bound phage from tumor targets. This has been performed successfully in murine models (Arap et al., 1998; Krag et al., 2002). Our lab has successfully performed panning in human cancer patients (Krag et al., 2006). Selection of ligands to tumors in human cancer patients has the advantage of clinical relevance. However, it does not allow precise control of which cellular compartment in the tumor is being targeted.
Molecular features of freshly harvested tumor tissue are largely preserved by rapid freezing. Laser capture microdissection (LCM) is used to select a pure population of specificcellsfroma histological specimen (Bonner et al., 1997). We have combined LCM of freshly harvested tumor tissue with phage panning to obtain ligands against specific tumor cell compartments. We present here a functional method for panning on histological specimens with a naïve phage library and LCM selection of cells. We provide examples of scFvs that bind selectively to the stromal compartment and tumor cells of human breast cancer.
2. Material and methods
2.1. Phage-display library
Tomlinson I single-chain variable fragment (scFv) library, cloned in ampicillin-resistant phagemid vector pIT2, was obtained from MRC, HGMP Resource Centre (Hinxton, Cambridge, UK). The library size is ~1.47×108 different scFv fragments. All functional scFvs in the library bind to Protein A and L, and they also have C-Myc and His6 tags for detection and purification.
2.2. Human tissue and slides
Human breast cancer tissues were obtained from cancer patients immediately following therapeutic surgical resection using a protocol approved by the University of Vermont Committees on Human Research. After tumor resection, the tissues were snap frozen and embedded in Optimal Cutting Temperature (OCT) compound (Tissue-Tek, Sakura Finetek U. S.A., Inc., Torrance, California), and stored at −80 °C. Frozen sections (6 μm thick) were cut in a Minotome™ microtomecryostat (IEC Division of Damon Corporation, Massachusetts), mounted onto clean uncoated glass slides and PEN membrane slides (P.A.L.M. Microlaser Technologies AG, Bernried, Germany), and then stored at −80 °C until further processing. A portion of the tumor was formalin fixed, and paraffin embedded for slide preparation.
2.3. Phage panning on tumor stroma and tumor cell specimens with laser capture microdissection
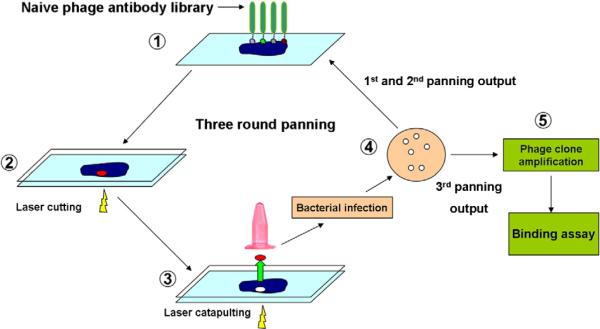
Breast cancer tissue frozen sections mounted on PALM membrane slides were fixed in 2% paraformaldehyde in PBS at room temperature for 15 min. After rinsing 3 times in PBS, slides were stained with hematoxylin for 10 s and rinsed in tap water for 5 min. Slides were then rinsed in PBS for 5 min. 1×1012TU phage library in 200 μl binding buffer (1×PBS with 0.05% Tween-20, 10% goat serum and 0.2% casein blocker) were put directly on the tissue section for 2 h in a moist box at room temperature (Fig. 1 step-1). After incubation, membrane slides were rinsed 10 times in PBST (1×PBS containing 0.1% tween-20) and once in PBS to remove unbound phage. In order to keep the tissue and bound phage moist, a blank membrane from a PALM slide was used to cover the exposed surface of the specimen (Fig. 1, step-2). The slide was placed on the laser capture microscope stage (PALM MicroLaser Systems, Germany) and the cells to be microdissected were identified. The chosen stroma and tumor cells were cut by UV laser (Fig. 1 step-2). The cut tissues were then catapulted to a collection tube containing 60 μl PBS (Fig. 1, step-3). A total of 100 tissue sections were catapulted, resulting in removal of approximately 0.5 mm2 of tumor stroma or tumor island. Phage bound to the catapulted tumor stroma and tumor cells were recovered by infection to the TG-1 host strain. The infected cells were selected on a TYE-ampicillin plate (Fig. 1 step-4). The colonies were counted to determine the number of phage recovered after LCM. For tumor stroma cells, a total of 3 rounds of panning were performed. The first and second panning outputs were pooled and 1×1012 TU of amplified phage were used for the third round of stroma panning. Individual colonies were randomly selected from the third panning output for stroma-binding assay. Only one round of panning was performed on tumor cells as the phage output was small enough to allow the tumor-binding assessment of all the clones.
Fig. 1.
Schematic presentation of phage panning on tissues sections with LCM. Naive phage library was directly incubated with tissue sections mounted on PALM membrane slides (1). After rinsing steps, a blank PALM membrane covered slides to keep tissue moist (2). Tissue was cut (2) and captured (3) by laser beams. The collected tissue pieces were directly used to infect host stain, and infected cells were selected on TYE-ampicillin plate (4). Panning outputs were pooled and amplified for the next round of panning. Three rounds of panning were performed and individual colonies were randomly selected from the 3rd panning output for binding assay (5).
2.4. Tumor-binding analysis of phage clones by immunofluorescence (IF) microscopy
Frozen sections were prepared from the same tumor used for panning, and then mounted on 12-mm round coverslips. The coverslips were placed in 24-well plates (Thermo Fisher Scientific, USA). Selected monoclones were amplified and normalized by chemititration (Shukla and Krag, 2005a). 1×1012 TU selected phage monoclones in 200ul PBS were incubated with the tissue sections in 24-well plates at room temperature and shaken for 2 h. After incubation, slides were rinsed 10 times in PBST (PBS containing 0.1% tween-20) and once in PBS. Mouse anti-M13 antibody (GE Healthcare, Piscataway, New Jersey) and goat anti-mouse IgG-AlexaFlour 488 (Molecular Probes, Invitrogen, California) were used to label the phage. The slides were observed under fluorescence microscopy. Naïve phage library and KM13 helper phage were used as negative controls.
2.5. Tumor stroma binding analysis of phage clones by immunohistochemistry (IHC)
Clones that demonstrated stromal binding on frozen tissue sections were evaluated for binding to formalin-fixed and paraffin-embedded tumor tissue samples prepared from the same tumor specimen. Briefly, deparaffinized tissue sections were incubated with phage clones for 2 h at room temperature, washed with PBS, and then incubated with horseradish peroxidase (HRP)-conjugated anti-M13 antibody (Amersham) for 1 h. Binding was detected using diaminobenzidine (DAB) as a substrate (Sigma-Aldrich).
2.6. Production and purification of soluble scFv antibody
Soluble scFv proteins were produced by infection in E coli HB2151 by the method described earlier (Golchin and Aitken, 2008). Briefly, E coli HB2151 (OD 0.4) was incubated with an aliquot of the selected phage clone at 37 °C for 1 h, then plated onto TYE agar containing 100 μg/mL ampicillin and 1% (v/v) glucose for overnight growth at 30 °C. Individual colonies were amplified and saved in 20% glycerol for soluble scFv production. The HB2151 phage overnight culture was diluted 1:100 into 200 ml 2×YT containing 100 μg/mL ampicillin and 0.1% glucose. The bacterial culture was shaken at 37 °C until the culture density reached to 0.9 OD600. The expression of the scFvs was then induced by the addition of 25 ml 2×TY containing 100 μg/mL ampicillin and 9 mM IPTG. Incubation continued for 20 h at 30 °C. The bacterial culture was centrifuged at 3000 rpm for 15 min and the supernatant was used for ELISA evaluation and scFv purification.
For ELISA, the microtitre plates coated with protein-A (0.5 μg/well, Southern Biotech, Birmingham, Alabama) were incubated with the culture supernatant for 1 h at room temperature. Soluble scFv was detected using a HisProbe-HRP antibody (Pierce Rockford, Illinois). For purification of scFv in supernatants, centrifuge columns packed with protein-L resin (Pierce) were used. Soluble scFv proteins were eluted with 0.1 M glycine at pH 3.0 and immediately neutralized by 1 M Tris buffer, pH 9.0. Eluted fractions were analyzed by SDS-PAGE. The fractions found to contain purified scFv were pooled, concentrated 100-fold (Amicon Ultra-4 centrifugal filter, Millipore, UK), and stored at −20 °C in PBS with 20% glycerol.
2.7. Binding analysis of scFv antibodies to normal human tissues and tumor tissues by immunofluorescence (IF) microscopy
The specific binding of purified scFvs to tumor was evaluated on breast cancer tissues and a panel of normal human tissues. The scFvs at 4 μg/ml were incubated with tissue sections for 1 h at room temperature. Slides were rinsed 3 times in PBS and incubated with Alexa 488 conjugated Anti-His antibody (Upstate Temecula, California). The slides were observed under fluorescence microscopy. scFvs derived from a non-binding phage clone were used as a negative control.
2.8. Soluble scFv competition experiment
To confirm the binding ability of soluble scFv 07-2931 to tumor stroma, its inhibitory effect on its representative phage clone binding was examined. Frozen sections of the breast cancer tissue were incubated with soluble 07-2931 scFv (10 μg/ml and 1 μg/ml) for 60 min at room temperature. Then 1 × 1013 TU/ml of phage clone 07-2931 was added for 2 h at room temperature. Slides were rinsed 10 times in PBST (containing 0.1% tween-20 PBS) and once with PBS. Mouse anti-M13 antibody (Amersham) and Goat anti-mouse IgG-AlexaFlour 488 (Molecular Probes) were used to label the phage. The slides were observed under fluorescence microscopy. A non-binding soluble scFv selected from the same library was used as control at the same concentration.
2.9. DNA sequencing and alignment
The vector DNA of each selected clone was purified using QIAprep spin miniprep kit (Qiagen, Inc., Chatsworth, California) according to manufacturer's instructions. The pIII sequencing primer, 5-CCC TCA TAG TTA GCG TAA CG-3, was used for sequencing the DNA insert. The sequence reactions were carried out using BigDye Ver.1 Dye Terminator kit (PE Biosystems) by the Vermont Cancer Center DNA Analysis Facility. The amino acid sequences of variable regions of scFv clones were compared with current available sequences in the Protein Data Bank (PDB) and NCBI non-redundant protein sequences (NR) database.
3. Results
3.1. Optimal conditions for panning on histological specimens with LCM
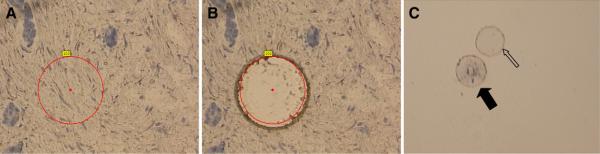
Preliminary LCM experiments demonstrated that phage lost viability due to dryness that occurred following standard LCM protocols (Sun et al., 2009). To prevent drying, a membrane of a blank PALM slide was placed on the panned slide to cover the tissue and bound phage. The added membrane created a very thin chamber on the surface of the slide. This protected phage from drying out and did not interfere with the laser cutting and capture process. Fig. 2 shows LCM cutting and catapulting of specimens with the overlying membrane. Selected tumor stroma tissue was circled (Fig. 2A). Fig. 2B shows the tissue hole left after sample capture. Fig. 2C shows the catapulted sample (larger solid arrow) and the overlying membrane (smaller hollow arrow) in the collection buffer. The overlying membrane spontaneously separated from the tissue which allowed phage to contact bacteria for infection. Using the overlying membrane resulted in no decrease in phage viability observed for up to 6 h.
Fig. 2.
LCM process of tumor stroma tissue. Tumor stroma was circled (A), then cut and captured by laser beam (B). Tissue was deposited in collecting tube with PBS buffer (C). The overlying membrane (C smaller hollow arrow) spontaneously separated from the sample (C larger solid arrow) when placed in buffer (C).
3.2. Selection of tumor stroma and tumor cell binding clones
Using the panning methods described above, three rounds of panning were performed on breast cancer tumor stroma cells. The number of clones recovered increased with sequential panning. The output from Round 3 was about 100 phage clones per tumor stroma cell. One round panning was performed on tumor cells from another breast cancer specimen. One hundred-fifty phage clones were recovered and directly screened by IF stain on frozen sections from the same specimen.
3.3. Demonstration of tumor stroma and tumor cell binding clones
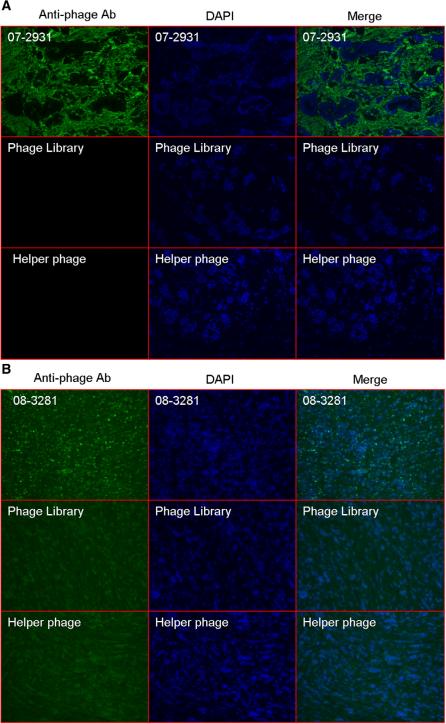
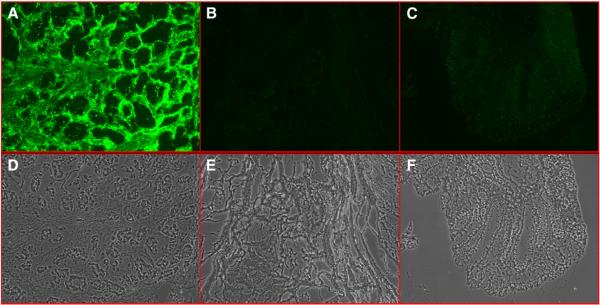
All steps involved with evaluation of individual clones, including incubation and rinsing, were successfully accomplished with IF staining in 24-well plates. We evaluated 150 randomly picked phage clones from each group. For the tumor stroma panning group, 20% (31 out of 150) of the clones showed strong selective binding to tumor stroma and no identifiable binding to tumor cells (Fig. 3A). DNA sequence data of the 31 positive clones from tumor stroma binders showed they were the same clone (07-2931). No antibodies with a CDR sequence similar to clone 07-2931 have been reported in the PDB or NR databases. For tumor cells panning group, 4% (6 out of 150) of the tested clones showed selective binding to tumor cells. Fig. 3B show the IF stain results of one of these binders (clone 08-3281). The negative control phage from naïve phage library and KM13 helper phage did not show any binding to tumor tissue. DNA sequence data of 6 tumor cell binding clones showed they were unique clones.
Fig. 3.
Immunofluorescence analysis of phage clone 07-2931 (A) and clone 08-3281 (B) binding to human breast cancer tissue. Breast cancer frozen sections derived from the same clinic patient for panning were incubated with phage clone while same quantity phage library and no-insert helper phage were usedas control. The phage was visualized by fluorescence using mouse-anti-M13 phage antibody followed by Alex-488-conjugated goat anti-mouse antibody. DAPI was used for nuclear counterstaining and tissue structure. Immunofluorence (IF) stain with phage clone (first row of A and B) show selective signals (green) located on tumor stroma (A) and tumor cells (B). As controls, Naive scFv phage library stain (second row) and M13 helper phage (third row) show no signals on tumor tissue.
3.4. Binding profile of phage clone 07-2931 on paraffin slides
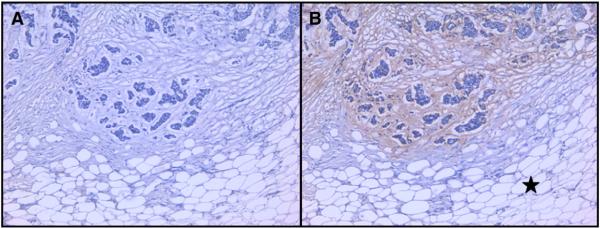
IHC evaluation of phage clone (07-2931) was performed on paraffin-fixed samples from the same breast cancer that was used for panning. This clone showed strong binding to tumor stroma and no binding to fat tissue or fibrous tissue outside the tumor island area (Fig. 4), while the phage library showed no staining on tumor and connective tissues.
Fig. 4.
Immunohistochemistry (IHC) stain with phage clone 07-2931 on breast cancer tissue paraffin slides. Two adjacent cut sections of breast cancer from same patient specimen were used. (A) negative control with naïve phage library shows no binding. (B) Clone 07-2931 bound to tumor stroma around cancer cell islands but not fat connective tissue (see black star in B) outside the tumor nest.
3.5. Binding profile of soluble scFv 07-2931 and 08-3281
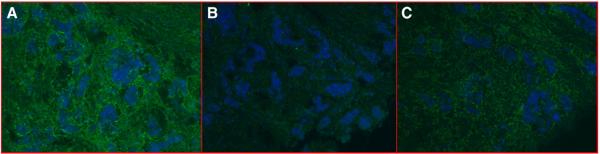
IF staining with purified scFv 07-2931 and 08-3281 showed selective binding to tumor stroma (Fig. 5A) and tumor cells. Further screening on a panel of normal frozen human tissue showed that 07-2931 had no significant binding to most normal tissues studied, including breast (Fig. 5B), colon (Fig. 5C), ovary, uterus, skin, liver, kidney, lung, or spleen. No significant staining was also observed on 4 additional colon cancer and 13 breast cancer specimens. We conclude that scFv 07-2931 binds breast cancer tumor stroma with significant specificity to this individual tumor specimen. It has very low cross-reactivity to normal human tissue, colon cancer, or other breast cancer specimens. Compared with 07-2931, scFv 08-3281 had relatively high cross reactivity to normal uterus tissue, which means scFv 08-3281 may bind to normal antigens expressed in breast cancer tumor tissue.
Fig. 5.
Immunofluorescence analysis of soluble scFv 07-2931 (A, B, C) to human breast cancer tissue and normal human tissue. Image A shows strong binding of scFv 07-2931 to breast cancer tumor stroma. Lack of binding is demonstrated on normal breast tissue (B) and colon (C). The lower row is the set of corresponding phase contrast images of above picture.
3.6. Soluble scFv 07-2931 competes with binding of phage clone 07-2931
To confirm that bindings of soluble scFv 07-2931 and phage-display 07-2931 were to the same epitope, scFv 07-2931 was added to compete with the binding of phage clone 07-2931 on breast cancer tumor stroma. Fig. 6 shows phage clone binding (A), and inhibition of binding by soluble scFv at 10 μg/ml (B), and 1 μg/ml (C), while the unrelated scFv control has no effect on phage 07-2931 binding to tumor stroma.
Fig. 6.
Competitive inhibition of phage clone 07-2931 binding by soluble scFv 07-2931. Specimen A shows binding of phage clone 07-2931. Specimen B was preincubated with scFv 07-2931 10 μg/ml and shows inhibition of phage clone 07-2931 binding. Specimen C was preincubated with scFv 07-2931 1 μg/ml and shows intermediate inhibition of phage clone 07-2931 binding.
4. Discussion
Our long term goal is to develop bioactive ligands using phage-display technology to treat cancer. It is important that targets used for ligand selection are clinically relevant. Panning directly on histological specimens or disaggregated cells from preserved tissue has successfully yielded ligands to normal human tissues, such as thymic stroma (Van Ewijk et al., 1997) skeletal muscle (Tanaka et al., 2002), and breast cancer (Jakobsen et al., 2007; Shukla and Krag, 2005b). LCM is a method of accurately selecting pure populations of cells from tissue specimens and offers further accuracy of specific populations of cells present in a solid tumor (Bonner et al., 1997). This approach has wide applicability since tumor tissue in commonly available for most cancer patients.
We observed that standard LCM conditions resulted in substantially decreased phage infectivity. Simulating the conditions on the microscope stage but not performing the catapulting step identified lack of moisture as a critical variable (Sun et al., 2009). Creating a sandwich with the PALM slide membrane allowed moisture to be retained and still allowed successful catapulting. This became an enabling step allowing application of panning on highly selected cells on a histological specimen.
Other strategies have been developed to overcome decreased phage viability associated with the capture step. For example, recovery was performed by PCR amplification and re-cloning into a phage-display vector (Tanaka et al., 2002; Ruan et al., 2006; Yao et al., 2005). Another strategy involved freeze drying to protect the phage (Lu et al., 2004). Multiple tumor fragments were incubated with phage, frozen, and then cut sections mounted on LCM slides. The specimen was then freeze dried and subjected to LCM. Finally phage were directly recovered with bacteria. A third strategy was to use LCM to collect large number of cells and then pan on the LCM recovered cells in immunotubes (Kubo et al., 2008). The method we describe here, which is applicable for the PALM system, is relatively straightforward. Phage panning, recovery, and amplification steps use routine protocols and do not require unusual steps to accomplish selection of desirable clones.
Demonstration of the feasibility of our method was performed on breast cancer specimens with selection of relatively abundant nonmalignant stromal tissue. Phage yield was excellent and about 100 phage were recovered per cell. In these experiments a total area of about 0.5 mm2 was removed. However, it is likely that considerably fewer cells could be removed and still obtain binding phage. This is important for selection on cells that are in low abundance in a tumor specimen. For example, endothelial cells in tumor blood vessels are excellent candidate targets but are low in number relative to other cellular elements in the tumor.
Fluorescence imaging was used to evaluate clone binding. A number of modifications were made to the imaging protocol that shortened the time for clone evaluation. The main feature was to move nearly all phage incubation and staining steps to a 24 well format. Histological tumor specimens were placed on round cover slips that could be accommodated into the wells on a 24 well plate. Phage incubation, rinsing, and fluorescent labeling steps were performed without removing the specimen from the well. In addition to decreased processing time, the quality of the staining was improved. This appears to be due to the full immersion of the specimen in fluid and rocking during incubation steps. This eliminated the edge effect seen when incubations were performed on glass slides with a grease pen border for fluid retention.
Panning tumor specimens and separately recovering phage bound to tumor stroma and tumor cells successfully yielded clones with good binding to the target cells. The goal of developing this method was primarily to demonstrate differential binding to specific cellular elements present within a solid tumor. This goal was met and clones that bound to tumor stroma and no other tumor elements, and clones that bound to malignant cells and no other tumor elements were identified. Significant efforts to optimize specificity beyond differential binding to tumor elements were not made. Other subtractive methods commonly performed, such as subtraction of the library to normal nontumor cells, will be expected to enhance specificity.
In conclusion, a phage panning method is described which allows panning on pure populations of cells present in a histological solid tumor specimen. A strategy that allows increased throughput of image-based clone evaluation using a 24 well format is also described. Examples of scFv with good sensitivity and specificity to tumor elements are presented. This method should facilitate wide application of phage display to highly select populations of cells or other microscopic structures present on a histological specimen.
Acknowledgements
This study was supported by the Department of Defense Congressionally Directed Medical Research Program U.S. Army grant W81XWH-05-1-0237, and in part by SD Ireland Professorship of Oncology for Research and Vermont Cancer Center core grant P30CA022435.
Abbreviations
- LCM
laser capture microdissection
- IPTG
isopropyl-β-D-1-thiogalactopyranoside
- mAb
monoclonal antibody
- scFv
single chain antibody variable fragments
- PBS
phosphate buffered-saline
- ELISA
Enzyme-Linked ImmunoSorbent Assay
References
- Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279(5349):377. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- Bonner RF. Laser capture microdissection: molecular analysis of tissue. Science. 1997;278(5342):1481. doi: 10.1126/science.278.5342.1481. [DOI] [PubMed] [Google Scholar]
- Golchin M, Aitken R. Isolation by phage display of recombinant antibodies able to block adherence of Escherichia coli mediated by the K99 colonisation factor. Vet. Immunol. Immunopathol. 2008;121(3–4):321. doi: 10.1016/j.vetimm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Jain RK. Taming vessels to treat cancer. Sci. Am. 2008;298(1):56. doi: 10.1038/scientificamerican0108-56. [DOI] [PubMed] [Google Scholar]
- Jakobsen CG, et al. Phage display derived human monoclonal antibodies isolated by binding to the surface of live primary breast cancer cells recognize GRP78. Cancer Res. 2007;67(19):9507. doi: 10.1158/0008-5472.CAN-06-4686. [DOI] [PubMed] [Google Scholar]
- Krag DN, et al. Phage-displayed random peptide libraries in mice: toxicity after serial panning. Cancer Chemother. Pharmacol. 2002;50(4):325. doi: 10.1007/s00280-002-0489-4. [DOI] [PubMed] [Google Scholar]
- Krag DN, et al. Selection of tumor-binding ligands in cancer patients with phage display libraries. Cancer Res. 2006;66(15):7724. doi: 10.1158/0008-5472.CAN-05-4441. [DOI] [PubMed] [Google Scholar]
- Kubo N, et al. Identification of oligopeptide binding to colon cancer cells separated from patients using laser capture microdissection. J. Drug Target. 2008;16(5):396. doi: 10.1080/10611860802088796. [DOI] [PubMed] [Google Scholar]
- Lu H, Jin D, Kapila YL. Application of laser capture microdissection to phage display peptide library screening. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endo. 2004;98(6):692. doi: 10.1016/j.tripleo.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Pietras K, et al. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5(1):e19. doi: 10.1371/journal.pmed.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranieri G, et al. Vascular endothelial growth factor (VEGF) as a target of bevacizumab in cancer: from the biology to the clinic. Curr. Med. Chem. 2006;13(16):1845. doi: 10.2174/092986706777585059. [DOI] [PubMed] [Google Scholar]
- Ruan W, et al. Identification of clinically significant tumor antigens by selecting phage antibody library on tumor cells in situ using laser capture microdissection. Mol. Cell. Proteomics. 2006;5(12):2364. doi: 10.1074/mcp.M600246-MCP200. [DOI] [PubMed] [Google Scholar]
- Shukla GS, Krag DN. A sensitive and rapid chemiluminescence ELISA for filamentous bacteriophages. J. Immunoassay. Immunochem. 2005a;26(2):89. doi: 10.1081/ias-200051990. [DOI] [PubMed] [Google Scholar]
- Shukla GS, Krag DN. Phage display selection for cell-specific ligands: development of a screening procedure suitable for small tumor specimens. J. Drug Target. 2005b;13(1):7. doi: 10.1080/10611860400020464. [DOI] [PubMed] [Google Scholar]
- Sun Y, et al. Biopanning phage-display libraries on small tissue sections captured by laser capture microdissection. J. Biotech. Res. 2009;1(2):55. [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, et al. In situ phage screening. A method for identification of subnanogram tissue components in situ. J. Biol. Chem. 2002;277(33):30382. doi: 10.1074/jbc.M203547200. [DOI] [PubMed] [Google Scholar]
- Van Ewijk W, et al. Subtractive isolation of phage-displayed single-chain antibodies to thymic stromal cells by using intact thymic fragments. Proc. Natl. Acad. Sci. U. S. A. 1997;94(8):3903. doi: 10.1073/pnas.94.8.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao VJ, et al. Targeting pancreatic islets with phage display assisted by laser pressure catapult microdissection. Am. J. Pathol. 2005;166(2):625. doi: 10.1016/S0002-9440(10)62283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch RL, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455(7211):406. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]