Abstract
Background
The study was conducted to examine the impact of oral contraceptives (OCs) on serum antimullerian hormone (AMH) levels by obesity status in reproductive-age women.
Study design
Ovulatory women, ages 18–35 years, of normal (< 25 kg/m2; n = 10) and obese (> 30 kg/m2; n = 10) body mass index (BMI) received a low-dose OC (20 mcg ethinyl estradiol/100 mcg levonorgestrel) for two cycles. Serum samples obtained at several time points during active pill use and hormone-free intervals were analyzed for AMH, FSH, LH, estradiol and inhibin B.
Results
AMH levels did not differ by OC cycle day in either BMI group. On average, AMH levels were 34% lower in the obese group (2.9±2.1 versus 4.4±1.8ng/ml, p<0.05). Modeling to determine differences in AMH throughout the cycle based on obesity status demonstrated significiantly lower levels (p<0.05), whereras serum AMH, FSH, LH, estradiol and inhibin B levels revealed no correlations when all time points were included.
Conclusions
In reproductive-aged women, serum AMH levels do not appear to fluctuate during OC use but AMH levels are significantly lower in obese women. Lower levels do not appear to be due to differences in gonadotropin levels or ovarian activity.
Keywords: Antimullerian hormone, oral contraceptives, obesity
1. Introduction
Antimullerian hormone (AMH) is becoming widely accepted as a clinical marker of ovarian reserve, the quantity of oocytes remaining. Levels are thought to be the earliest marker of a decline in ovarian reserve [1], to be a good predictor of poor ovarian response to controlled ovarian hyperstimulation [2], and to display minimal inter- and intra-cycle variability [3,4]. Although the AMH assay has been approved for research use only, physicians are checking levels for clinical use. Fertility centers are not uncommonly using AMH as their preferred measure of ovarian reserve.
AMH has the potential to be a convenient measure of ovarian reserve in women on oral contraceptives (OCs). An effective measure of ovarian reserve in women on OCs would be beneficial for screening oocyte donors and women in their thirties, delaying pregnancy but concerned about future fertility. Recent studies have shown that AMH levels do not appear to change after prolonged OC use [5]. However, it is unknown, on which OC cycle day to best measure ovarian reserve, especially if AMH levels were to fluctuate on a day-to-day basis with OCs use. In theory, AMH levels could fluctuate with follicular development [6]. We hypothesized that AMH levels could fluctuate while women are on OCs, depending on the degree of follicular development.
Additionally, the validity of AMH levels in obese women may also hamper its clinical use. Recent studies have shown AMH levels to be lower in perimenopausal obese women compared to perimenopausal non-obese women [7,8]. Differences in AMH were not mirrored by changes in other markers of ovarian reserve such as follicle-stimulating hormone (FSH) and antral follicle count [8]. One could argue, however, that this discrepancy may be due to the fact that AMH is an earlier marker of ovarian aging in perimenopausal women and that differences in antral follicle count and FSH were not yet observable in this cohort.
It is unknown if differences in AMH in obese and normal BMI women will be observed in a younger, reproductive cohort, in which ovarian aging would be less likely to have occurred. We hypothesized that in a younger cohort, AMH levels would be lower in obese women compared to normal weight women. In this study, we sought to determine if AMH levels fluctuate in OCs users, to determine if AMH levels differ between obese and normal BMI reproductive-age women on OCs, and to compare changes in AMH during an OC cycle to changes in gonadotropins and granulosa cell hormone products (estradiol and inhibin B).
2. Materials and methods
In this exploratory study, regularly menstruating women, ages 18–35 years, of normal (<25 kg/m2, N=10) and obese (>30kg/m2, N=10) body mass index (BMI) were recruited for a study of OCs and obesity. Evidence of normal ovulatory cycles (luteal phase progesterone ≥ 3 ng/mL serum) was obtained prior to enrollment. Baseline measurements of height and weight were obtained for calculation of BMI. Body composition (% body fat) was determined by air displacement plethysmography. Subjects subsequently initiated a lowdose OC (20 mcg ethinyl estradiol/100 mcg levonorgestrel) on menstrual cycle day 1 and continued for two cycles. Serum was subsequently obtained after 21 days of active pills, at the end of the 7-day hormone-free interval, and twice weekly thereafter in the second cycle until the end of the active pills for a total of 7 samples per subject (n = 140 samples). FSH, luteinizing hormone (LH), inhibin B, and estradiol levels were quantified in the serum samples by sensitive and specific immunoassays. FSH and LH were measured using the automated Immulite chemiluminescent assay system (Siemens Medical Solutions, Los Angeles, CA). The average interassay CVs were 10.0% and 7.5%, respectively. Estradiol was measured using an ultra-sensitive competitive binding radioimmunoassay (Diagnostic Systems Laboratories, Webster, TX). Inhibin B was measured by ELISA using an enzymatically amplified two-site, two-step, sandwich-type ELISA (Diagnostic Systems Laboratories, Webster, TX) with an assay sensitivity of 7 pg/mL; the average interassay CV was 6.9%. AMH was measured for each subject at all time points using a commercially available ELISA (Diagnostic Systems Laboratories, Webster, TX); the assay sensitivity is 0.1 ng/mL and the average interassay CV was 6.5%.
Informed consent was obtained from all subjects under an OHSU Institutional Review Board (IRB) approved protocol [9]. Additional consent was not obtained for this secondary analysis of AMH and inhibin B, as the IRB felt that the analysis fell within the scope of the original research protocol.
Demographics were compared between the obese and normal groups using a two-sample Student’s t test. Results are presented as the mean ± standard deviation. As AMH, FSH, and estradiol were not normally distributed, they were log-transformed prior to analysis. After log transformation, AMH levels were compared across and between the obese and normal BMI groups using linear regression with a cluster term to adjust for repeat measures within an individual. Subsequently, general estimating equation (GEE) models were created with an interaction term (cycle day × obesity status) to analyze the change in AMH levels across the OC cycle by obesity status, with a variable for cycle day to analyze the independent effect of cycle day on AMH levels, and with a variable for obesity status to analyze the independent effect of obesity on AMH levels. GEE models allow for analysis of data with repeated measurements on the same subject over time, differing from repeated measure analysis of variance (MANOVA) in that it does not require an equal number of measurements and equal time spacing. Similar analyses were conducted with log-transformed FSH and estradiol and inhibin B. Pair-wise correlations of AMH, inhibin B, the gonadotropins and estradiol were conducted using Pearsons and Spearman correlation statistic, where appropriate. All analyses were conducted using STATA 10.0 (College Station, TX).
3. Results
Mean BMI and percent body fat were significantly different between the obese (37.3±6.0kg/m2 and 50.3±3.5%) and normal (21.9±1.6kg/m2 and 27.6±7.2%) groups (p<0.05). Age did not differ significantly between the two groups (normal 29±6.3 years versus obese 29±4.9 years).
AMH levels were, on average, 34% lower in the obese group (2.9±2.1 versus 4.4±1.8ng/ml, p<0.05). In the normal and obese BMI groups, AMH levels did not differ by OC cycle day (p= 0.88 and p=0.43, respectively, Fig. 1A). Across the cycles, the CV of AMH averaged 16.1% ± 7 in the normal group and 16.1%±4.4 in the obese group. Modeling to determine differences in AMH throughout the OC cycles based on obesity status showed that the obese group had significantly lower AMH levels across the OC cycles (pobesity<0.05) and that change in AMH levels across the OC cycle did not differ by obesity status (pobesity×cycle=0.81, Fig. 1A).
Fig. 1.
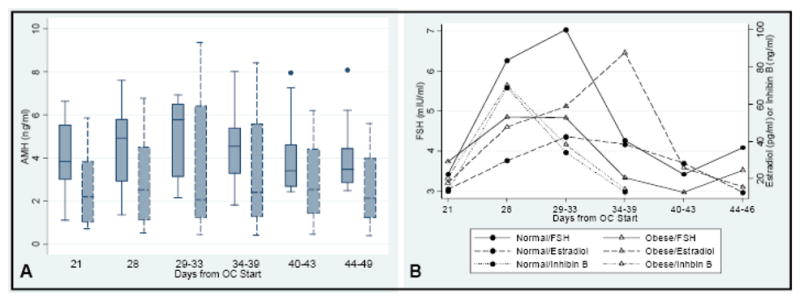
Hormone levels during OC use in obese and normal groups. Days 1–28 occur during the first cycle of OCs (days 22–28 are the hormone free interval) and days 29–49 in the second cycle of OCs. A) AMH levels. Normal weight subjects are shown in solid line box plots and obese in dashed line box plots. Boxes represent the interquartile range (25%–75%), center lines the median, and points outliers. B) FSH, inhibin B, and estradiol levels. FSH levels are depicted with solid lines, inhibin B with dots, and estradiol with dashes. The obese group is depicted with open triangles and the normal group with closed circles. Points depict mean hormone levels.
After adjusting for repeat measures, average FSH, estradiol, and inhibin B levels did not significantly differ between the obese and normal groups while on OCs (P= 0.94, 0.1, 0.7, respectively). However, modeling to determine differences in estradiol levels throughout the OC cycle based on obesity status showed that the obese group had a significantly higher estradiol curve during the first two weeks of the second OC cycle (Pobesity<0.05, see Fig. 1B). In both groups, estradiol levels fluctuated based on cycle day (Pcycle<0.05, Fig. 1B). FSH differed by cycle day (Pcycle<0.05) but not obesity status (Pobesity=0.9, Fig. 1B). Similarly, inhibin B levels differed by cycle day (Pcycle<0.001) but did not differ by obesity status (Pobesity=0.7). No significant correlation was found between gonadotropin, estradiol, or inhibin levels and AMH when all time points were included. Inhibin B levels were the most highly correlated with AMH (r=0.23, p=0.09).
4. Discussion
In summary, AMH levels did not appear to fluctuate significantly during OC use. While on OCs, AMH levels were significantly lower in obese women compared to normal BMI women. These differences did not appear to be due to differences in hypothalamic-pituitary-ovarian activity.
Previous studies have compared menstrual cycle day 3 AMH levels during spontaneous cycles to those during OC use [5,10,11]. One study found that AMH levels along with FSH levels were suppressed in the OC cycle [10]; the other two found no significant difference in AMH levels [5,11]. This discrepancy may be due to the study of an infertile population [10] rather than the general population [5,11]. Our findings are consistent with findings in spontaneous cycles. Variations in AMH levels during spontaneous cycles do not appear to be clinically meaningful or greater than intercycle variability despite follicular development and fluctuating gonadotropin levels during the menstrual cycle [11].
Our study size was small (only 20 subjects); however, despite repeated testing across the cycle (7 samples per subject), we failed to see statistically significant fluctuation in values, even on low-dose OCs. Graphically minor fluctuations were seen during the first 2 weeks of active pills. Future larger studies could focus on these time points. However, it will be important to preemptively define what fluctuation size would be deemed clinically meaningful.
We found that AMH levels were lower at every time point in obese OC users. This has been previously seen in a study of normal cycling, late reproductive-age women [7] on day 3 of a spontaneous menstrual cycle. In this current study of significantly younger women (29 years versus 46 years of age), this discrepancy in AMH levels persisted. In the study of Freeman et al. [7], AMH levels were 65% lower, while in our study the levels were 34% lower. Absolute differences, however, were larger in our study with a mean difference of 1.5 ng/mL. Using previous estimates, this difference would translate to menopause occurring 3 years earlier in obese women [12], a phenomenon that has not been observed.
We sought to determine if lower AMH levels in obese OC users could be attributed to escape from hypothalamic-pituitary-ovarian suppression. In fact, the obese group was more likely to show signs of follicular development (exhibited by a higher estradiol curve following a rise in FSH during the first hormone free-interval) in the second cycle of OCs. However, AMH levels did not correlate with gonadotropin or estradiol levels. In addition, the pattern of change in AMH did not mirror changes in FSH, estradiol, or inhibin B in the same cohort of obese subjects. Thus, we hypothesize that differences in AMH levels may not be related to gross changes in hypothalamic, pituitary, or ovarian function.
AMH has been shown to inhibit FSH augmentation of CYP19 expression and aromatase activity [13] in preantral follicles and small antral follicles. AMH levels in larger antral follicles and periovulatory follicles are suppressed [14]. Perhaps high local aromatase activity and estrogen to androgen ratios suppress local AMH production [15], allowing for continued growth of the dominant follicle with the normal decline in FSH. A recent study indirectly supports this theory; aromatase inhibition leads to increased local AMH production [16]. High ovarian aromatase activity in obese women could lead to suppression of AMH production and lower AMH levels. Adipocytokines such as adiponectin may either directly or indirectly alter granulosa cell AMH production through this mechanism. Adiponectin reportedly inhibits aromatase activity in the ovary [17]. Obese women have low levels of adiponectin [18].
Of note, inhibin B levels did fluctuate during the OC cycle in both the obese and normal BMI women. Inhibin B levels rose during the hormone-free interval and declined with the reinitiation of hormonal active pills in both groups. We did not observe lower inhibin B levels in the obese cohort. This finding differs from the findings of Gracia et al. [19] who noted consistently lower inhibin B levels in late reproductive-age, obese women.
In conclusion, AMH levels appear to be significantly lower in obese OC users compared to their normal BMI counterparts. Lower levels do not appear to be due to differences in the hypothalamic-pituitary-ovarian axis activity. The etiology for lower AMH levels in obese women is still unknown. A larger clinical study is indicated to study the relationship between AMH and obesity in reproductive-age women. In the meantime, AMH levels should be interpreted with caution in obese women.
Acknowledgments
Financial Support: HD 01243-03 Women’s Reproductive Health Research Fellow (NICHD K-12), PHS Grant 5 M01 RR000334, 1 R03 HD 053611-01, UNC WRHR HD 050113-02 and the University of North Carolina: University Research Council Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van Rooij IA, Broekmans FJ, Scheffer GJ, et al. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83:979–87. doi: 10.1016/j.fertnstert.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 2.van Rooij IA, Broekmans FJ, te Velde ER, et al. Serum anti-Mullerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17:3065–71. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- 3.Fanchin R, Taieb J, Lozano DH, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Mullerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod. 2005;20:923–7. doi: 10.1093/humrep/deh688. [DOI] [PubMed] [Google Scholar]
- 4.Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER, Broekmans FJ. Anti-Mullerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057–63. doi: 10.1210/jc.2006-0331. [DOI] [PubMed] [Google Scholar]
- 5.Somunkiran A, Yavuz T, Yucel O, Ozdemir I. Anti-Mullerian hormone levels during hormonal contraception in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2007;134:196–201. doi: 10.1016/j.ejogrb.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Visser JF, de Jong FH, Laven JSE, Themmen APN. Anti-Mullerian hormone: a new marker for ovarian function. Reproduc. 2006;131:1–9. doi: 10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]
- 7.Freeman EW, Gracia CR, Sammel MD, Lin H, Lim LC, Strauss JF., 3rd Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertil Steril 2007. 2007;87:101–6. doi: 10.1016/j.fertnstert.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 8.Su HI, Sammel MD, Freeman EW, Lin H, Deblasis T, Gracia CR. Body size affects measures of ovarian reserve in late reproductive age women. Menopause. 2008;15:857–61. doi: 10.1097/gme.0b013e318165981e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelman AB, Carlson NE, Cherala G, et al. Impact of obesity on oral contraceptive pharmacokinetics and hypothalamic-pituitary-ovarian activity. Contraception. 2009;80:119–27. doi: 10.1016/j.contraception.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arbo E, Vetori DV, Jimenez MF, Freitas FM, Lemos N, Cunha-Filho JS. Serum anti-mullerian hormone levels and follicular cohort characteristics after pituitary suppression in the late luteal phase with oral contraceptive pills. Hum Reprod. 2007;22:3192–6. doi: 10.1093/humrep/dem258. [DOI] [PubMed] [Google Scholar]
- 11.Streuli I, Fraisse T, Pillet C, Ibecheole V, Bischof P, de Ziegler D. Serum antimullerian hormone levels remain stable throughout the menstrual cycle and after oral or vaginal administration of synthetic sex steroids. Fertil Steril. 2008;90:395–400. doi: 10.1016/j.fertnstert.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 12.van Disseldorp J, Faddy MJ, Themmen AP, et al. Relationship of serum antimullerian hormone concentration to age at menopause. J Clin Endocrinol Metab. 2008;93:2129–34. doi: 10.1210/jc.2007-2093. [DOI] [PubMed] [Google Scholar]
- 13.Grossman MP, Nakajima ST, Fallat ME, Siow Y. Mullerian-inhibiting substance inhibits cytochrome P450 aromatase activity in human granulosa lutein cell culture. Fertil Steril. 2008;89:1364–70. doi: 10.1016/j.fertnstert.2007.03.066. [DOI] [PubMed] [Google Scholar]
- 14.Weenen C, Laven JS, Von Bergh AR, et al. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 15.Andersen CY, Byskov AG. Estradiol and regulation of anti-Mullerian hormone, inhibin-A, and inhibin-B secretion: analysis of small antral and preovulatory human follicles’ fluid. J Clin Endocrinol Metab. 2006;91:4064–9. doi: 10.1210/jc.2006-1066. [DOI] [PubMed] [Google Scholar]
- 16.Andersen CY, Lossl K. Increased intrafollicular androgen levels affect human granulosa cell secretion of anti-Mullerian hormone and inhibin-B. Fertil Steril. 2008;89:1760–5. doi: 10.1016/j.fertnstert.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Ledoux S, Campos DB, Lopes FL, Dobias-Goff M, Palin MF, Murphy BD. Adiponectin induces periovulatory changes in ovarian follicular cells. Endocrinol. 2006;147:5178–5186. doi: 10.1210/en.2006-0679. [DOI] [PubMed] [Google Scholar]
- 18.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 19.Gracia CR, Freeman EW, Sammel MD, Lin H, Nelson DB. The relationship between obesity and race on inhibin B during the menopause transition. Menopause. 2007;12:559–66. doi: 10.1097/01.gme.0000172268.24949.94. [DOI] [PubMed] [Google Scholar]


