Abstract
Background
Cysteinyl leukotrienes are recognized to act via receptors (CysLTRs) expressed on cell surface plasma membranes. Agents that block CysLT1R, are therapeutics for allergic disorders. Eosinophils contain multiple preformed proteins stored within their intracellular granules. Cell-free eosinophil granules are present extracellularly as intact membrane-bound organelles in sites associated with eosinophil infiltration, including asthma, rhinitis and urticaria, but have unknown functional capabilities.
Objective
We evaluated the expression of CysLTRs on eosinophil granule membranes and their functional roles in eliciting protein secretion from within eosinophil granules.
Methods
We studied secretory responses of human eosinophil granules isolated by subcellular fractionation. Granules were stimulated with cysteinyl leukotrienes and eosinophil cationic protein and cytokines were measured in the supernatants. Receptor expression on granule membranes and eosinophils was evaluated by flow cytometry and western blot.
Results
We report that receptors for cysteinyl leukotrienes, CysLT1R, CysLT2R and the purinergic P2Y12 receptor (P2Y12R), are expressed on eosinophil granule membranes. Leukotriene (LT) C4 and extracellularly generated LTD4 and LTE4 stimulated isolated eosinophil granules to secrete eosinophil cationic protein. MRS 2395, a P2Y12R antagonist, inhibited cysteinyl leukotrienes-induced eosinophil cationic protein release. Montelukast, likely not solely as an inhibitor of CysLT1R, inhibited eosinophil cationic protein release elicited by LTC4 and LTD4 as well as by LTE4.
Conclusion
These studies identify previously unrecognized sites of localization, the membranes of intracellular eosinophil granule organelles, and function for cysteinyl leukotriene-responsive receptors that mediate cysteinyl leukotriene-stimulated secretion from within eosinophil granules, including those present extracellularly.
Clinical implications
Cysteinyl leukotrienes elicit cell-free eosinophil granule secretion suggesting new roles, amenable to therapeutic interventions, for these lipid mediators in eosinophil-associated diseases.
Keywords: granules, cysteinyl leukotriene, eosinophil, allergy, asthma, montelukast
INTRODUCTION
Cysteinyl leukotrienes (cys-LTs) constitute an important class of potent, pro-inflammatory mediators that are synthesized from membrane-derived arachidonic acid via the 5-lipoxygenase pathway leading to the formation of LTA4 that is converted into LTC4 by the action of LTC4 synthase.1 Intracellular LTC4 is actively transported extracellularly, where it is enzymatically converted sequentially to LTD4 and then to LTE4.1 Cys-LTs are cell-membrane impermeant and are recognized to mediate their actions by engaging two heptahelical G protein-coupled receptors (GPCRs), designated CysLT1R and CysLT2R, that are expressed on cell surface plasma membranes.1,2 The rank order of affinities of cys-LTs for human CysLT1R and CysLT2R, based on transfected cells, is LTD4≫LTC4=LTE4 and LTC4=LTD4>LTE4, respectively.3,4
Eosinophils, prominent leukocytes in allergic inflammation and anthelminthic responses,5 are characterized by an abundance of intracellular granules that contain preformed proteins including distinct cationic proteins, such as eosinophil cationic protein (ECP), and a wide range of preformed cytokines, chemokines and growth factors.6,7 Human eosinophils are major sources of cys-LTs and express both CysLT1R and CysLT2R.8 Cys-LTs and their receptors have critical roles in allergic diseases and represent important therapeutic targets for the control of asthma and other pathophysiological conditions.9,10 Medications, including those recognized to inhibit ligand-binding to CysLT1R, such as montelukast, are used in the management of asthma and related allergic diseases.1
In addition to its conventional plasma membrane expression, CysLT1R has been immunolocalized to nuclei in colorectal adenocarcinoma cells11 and in a human mast cell line,12 although the functions of nuclear CysLT1Rs have not been defined. We have recognized that some cytokine and chemokine receptors are richly present on eosinophil granules;13 and we have recently demonstrated that eosinophil granules, upon extrusion from eosinophils, respond to a stimulating cytokine, interferon-γ, and a chemokine, eotaxin-1 (CCL11), via cognate granule membrane-expressed receptors, to activate intragranular signaling pathways that elicit granule protein secretion.14 Intact membrane-bound eosinophil granules have long been recognized to be present extracellularly in tissues and secretions in many human eosinophil-enriched disorders, including asthma, rhinitis, urticaria, atopic dermatitis, eosinophilic esophagitis and helminth infections.15–21 The capacity of cell-free human eosinophil granules to act via receptor-mediated responses to polypeptide agonists and secrete granule-derived cytokines and cationic proteins has indicated that cell-free eosinophil granules may be functionally significant.14 In the present study we have evaluated whether receptors for cys-LTs, in addition to their conventional plasma and nuclear membrane localizations, are expressed and functional on the surface membranes of cell-free human eosinophil granules. We investigated the efficacy of intracellular LTC4 and extracellular LTD4 and LTE4 as potential agonists of eosinophil granule secretion and the capacity of montelukast to inhibit cys-LT-elicited eosinophil granule secretion.
METHODS
Eosinophil purification and subcellular fractionation
Eosinophils were purified from the blood of healthy and atopic donors as previously described.14,22 Experiments were approved by the Beth Israel Deaconess Medical Center Committee on Clinical Investigation, and informed consent was obtained from all subjects. Subcellular fractionation and eosinophil granule isolation was performed as described.14,22 Briefly, eosinophils were disrupted by nitrogen cavitation (600 psi, 10 min) and post-nuclear supernatants were ultracentrifuged (100,000 g, 1 h at 4°C) in linear isotonic Optiprep (Axis-Shield, Oslo, Norway) gradients (0–45%). Purity of isolated granules free of plasma membranes or other contaminating structures was rigorously ascertained as previously documented.14,22
Stimulation of isolated eosinophil granules
Subcellular fractions containing isolated granules were mixed with RPMI + 0.1% ovalbumin (without phenol red) (Sigma, St. Louis, MO, USA) followed by centrifugation (2,500 g, 10 min). Granule pellets were resuspended in 250 μl of the same medium. Treatments with montelukast (0.1 and 1 μM, Merck) and MRS 2395 (2,2-dimethyl-propionic acid 3-(2-chloro-6-methylaminopurin-9-yl)-2-(2,2-dimethyl-propionyloxymethyl)-propyl ester) (1 and 10 μM, Sigma), a selective P2Y12R antagonist, were performed for 15 min prior to stimulation with LTC4, LTD4, or LTE4 for 30 min at 37°C. Thereafter, following centrifugation at 4°C (2,500 g, 10 min), granule supernatants were collected and stored at −80°C. Drugs were diluted in DMSO at a final concentration <0.01%, which had no effect on granule secretion.
Assays of granule-secreted proteins
ECP levels in eosinophil granule supernatants were analyzed by a quantitative ECP ELISA kit (Medical & Biological Labs, Nakaku Nagoya, Japan) according to the manufacturer’s instructions. Stimulated ECP secretion represents ECP levels from stimulated samples minus ECP levels from unstimulated samples. Cytokines, IL-4, IL-6, IFN-γ, IL-10, Il-12 (p70), IL-13 and TNF-α, were quantified using multiplex assays (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Flow cytometry of isolated granules and eosinophils
Isolated granules or eosinophils were incubated for 1 h with primary antibody (Ab) or primary Ab premixed with blocking peptide. Then the granules were washed and incubated with the respective FITC-conjugated secondary Abs for 15 min on ice in the absence of granule fixation. After staining, granules were fixed in buffer containing 2% paraformaldehyde without methanol (Electron Microscopy Sciences, Fort Washington, PA, USA) for 5 min. Control or non-immune Abs were included for all. Analyses were performed on a FACScan with CELLQUEST software (BD Biosciences, San Jose, CA, USA).
Mouse anti-human P2Y12R polyclonal Ab (1:100) was purchased from Abnova Corporation (Taipei, Taiwan). Goat polyclonal Ab against a peptide mapping the N-terminus domain of CysLT1R (N-20) (5 μg/ml) and the blocking peptide (20 μg/ml) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit polyclonal Abs against a peptide mapping the C-terminus of the CysLT1R (5 μg/ml) and the N-terminus of the CysLT2R (5 μg/ml) and the blocking peptide (20 μg/ml) were purchased from Cayman Chemical (Ann Arbor, MI, USA). FITC-conjugated F(ab′)2 goat anti-mouse, donkey anti-goat and goat anti-rabbit IgGs were used as secondary antibodies (1:100). Mouse, goat and rabbit normal IgGs were used as control Abs (Jackson Immuno Research Inc. West Grove, PA, USA).
Western blotting
Granules and eosinophils were lysed in LDS sample reducing buffer (Nupage, Invitrogen, Carlsbad, CA, USA) and boiled for 5 min. Samples were loaded on 10% Bis-Tris gels (Invitrogen) and run using 3-(n-morpholino) propanesulfonic acid MOPS running buffer. Gels were transferred to nitrocellulose membranes (Thermo Fisher Scientific, San Jose, CA, USA), blocked with 5% milk for at least 1 h and probed with rabbit anti-P2Y12R polyclonal Ab (1:400, Alomone Labs, Jerusalem, Israel) or the Ab premixed with the blocking peptide overnight. Anti-rabbit Ab conjugated to HRP (1:15000, Jackson Immuno Research) was used as secondary Ab. Membranes were developed with West Femto chemiluminescence kits (Thermo Fisher Scientific).
Statistical analysis
Secreted ECP levels, means of duplicates ± SD, are ECP levels from stimulated granules minus ECP levels from unstimulated granules. Data are shown for one experiment representative of three. Quantities of unstimulated and stimulated ECP secreted varied amongst replicate experiments, but the patterns of release and statistical differences were consistent in each of the replicate experiments. Results were analyzed by one-way ANOVA, followed by the Newman-Keuls test. P values < 0.05 were considered significant (two tailed test).
RESULTS
Extracellular eosinophil granules express on their membranes amino-terminal, ligand-binding domains for cys-LTs receptors
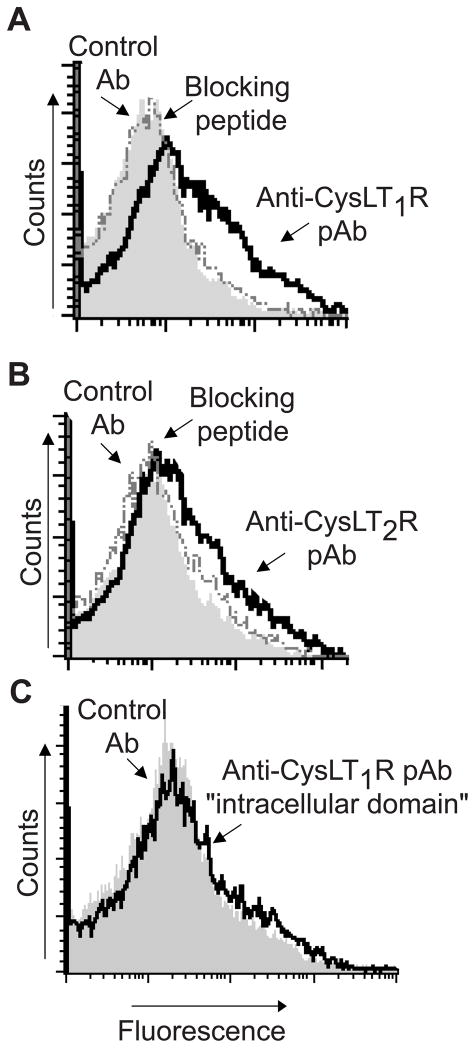
To evaluate whether secretory responses of eosinophil granules, as intracellularly resident or extracellularly released organelles, might be mediated by intracrine or paracrine acting cys-LTs, we first analyzed by flow cytometry the expression of CysLT1R and CysLT2R proteins on the surface membranes of isolated human eosinophil granules. Without membrane permeabilization, granules displayed immunoreactivity for both CysLT1R (Fig 1, A) and CysLT2R (Fig 1, B) using polyclonal Abs raised against epitopes present specifically in the nominally “extracellular,” ligand-binding regions of each CysLTR. Specificities of each polyclonal Ab for CysLT1R (Fig 1, A) and CysLT2R (Fig 1, B) were corroborated by complete neutralization of immunostaining through preincubation of the anti-CysLT1R and anti-CysLT2R polyclonal Abs with their respective specific blocking peptide immunogens. In contrast, eosinophil granules exhibited no staining with a polyclonal Ab raised to an “intracellular” carboxyl-terminal domain of CysLT1R (Fig 1, C).
FIG 1.
Isolated granules expressed extracellular domains of the A, CysLT1 receptor (CysLT1R) and B, CysLT2 receptor (CysLT2R), but not C, the carboxy-terminal intracellular domain of CysLT1R. Shaded histograms represent staining with control antibody (Ab). Solid and dashed lines represent staining with polyclonal specific antibodies (pAb) and anti-CysLTR pAbs neutralized by absorption with their respective immunogen peptide, respectively. Data are from one experiment, representative of three.
Extracellular eosinophil granules secrete eosinophil cationic protein (ECP) in response to cysteinyl leukotrienes
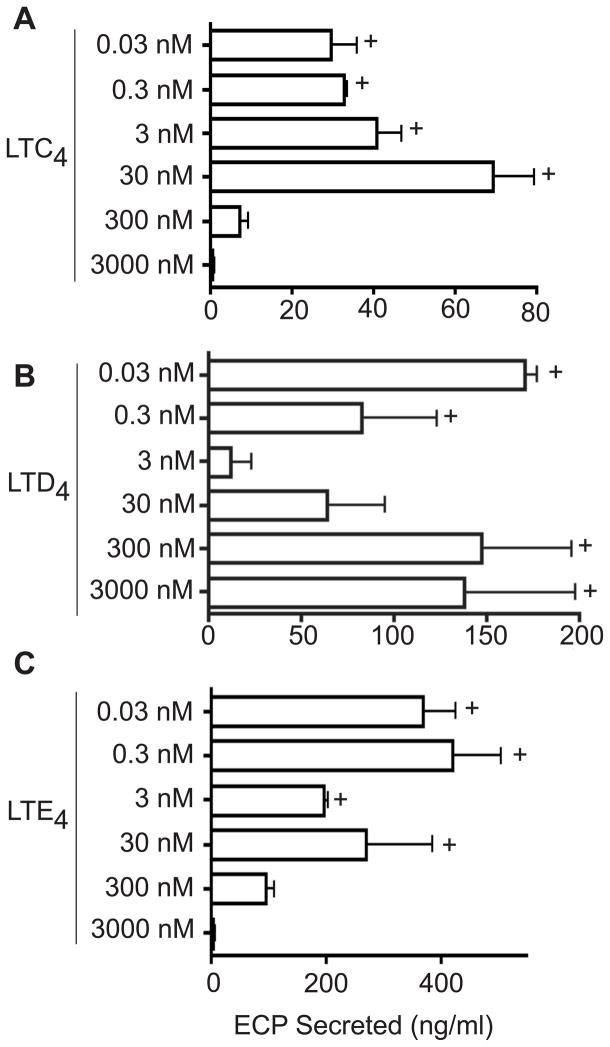
Given our recent finding that a cytokine and a chemokine could elicit receptor-mediated secretion by cell-free eosinophil granules,14 we evaluated whether cys-LTs might elicit functional secretory responses by extracellular human eosinophil granules that express CysLTRs. Isolated eosinophil granules were stimulated with LTC4, LTD4 and LTE4; and all three cys-LTs effectively stimulated secretion of ECP from within eosinophil granules (Fig 2). Notably, dose-responses to the three cys-LTs differed. LTC4 (Fig 2, A) and LTE4 (Fig 2, C) elicited ECP secretion only in lower concentrations (including well below 30 nM), fully compatible with physiologic signaling responses in vivo. Inhibited ECP secretion at higher LTC4 and LTE4 levels of 300 and 3000 nM is consistent with the high-dose inhibition characteristic of GPCRs. Likewise, LTD4 (Fig 2, B) induced granule ECP secretion at significant levels at very low physiologic concentrations (0.3 and 0.03 nM) and not at higher intermediate (3 and 30 nM) concentrations. In contrast, LTD4 also elicited granule ECP secretion at higher concentrations (300 and 3000 nM). This dose-response suggests engagements of two receptors for LTD4 – the first responds to low LTD4 levels and then exhibits higher dose inhibition and a second receptor putatively mediates secretion elicited by much higher concentrations of LTD4. Whereas a cytokine (interferon-γ) and a chemokine (eotaxin-1, CCL11) stimulated selective, differential secretion of specific cytokines, as well as ECP, from eosinophil granules,14 we evaluated whether cys-LTs elicited cytokine secretion from within eosinophil granules. All three cys-LTs failed to induce cytokine secretion of known eosinophil secretable cytokines,23 as measured in granule supernatants using cytokine multiplex assays for IL-4, IL-6, IFN-γ, IL-10, IL-12 (p70), IL-13 and TNF-α. Within the limits of cytokine detection, these findings suggest that cys-LT stimulation of eosinophil granules may provide a means for selective mobilization and secretion of the granule cationic protein, ECP, without concomitant mobilization of eosinophil granule-stored cytokines.
FIG 2.
Isolated granules were stimulated with different concentrations (0.03 – 3,000 nM) of A, LTC4, B, LTD4 and C, LTE4 and eosinophil cationic protein (ECP) levels were measured in the supernatants. Data is shown for one experiment representative of three. + represents significantly increased ECP release (P < 0.05) compared with non-stimulated granules.
A notable finding above was the capacity of low concentrations of LTE4 to elicit granule ECP secretion (Fig 2, C). As a major extracellularly generated cys-LT, the capacity of LTE4 to stimulate eosinophil granule secretory responses could be pertinent to the known, albeit often over-looked, presence of free membrane-bound eosinophil granules in human diseases, including allergic asthma and rhinitis, dermatitis, helminth infections, eosinophilic esophagitis, and urticaria.14 LTE4 is a weak stimulus for both human CysLT1R and CysLT2R.3,4 Moreover, LTE4, in contrast to LTD4, has elicited airways responses in humans not likely based on CysLT1R and CysLT2R.24–26
Cysteinyl leukotriene-induced eosinophil cationic protein (ECP) release is inhibited by MRS 2395 via eosinophil granule-expressed P2Y12 receptors
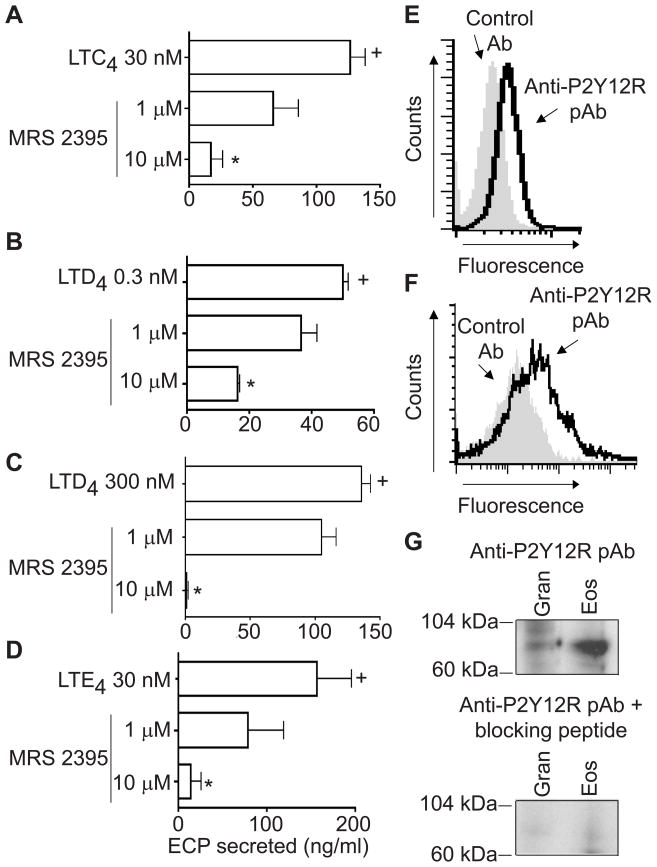
An additional human LTE4 receptor, the purinergic P2Y12R, was identified by in silico and in vitro methods.27 To assess if ECP secretion induced by cys-LTs on eosinophil granules might be mediated by P2Y12R, isolated granules were pretreated with MRS 2395, a P2Y12R antagonist. LTC4 (Fig 3, A), LTD4 (Fig 3, B and C), and LTE4 (Fig 3D) -induced ECP secretion was dose-dependently inhibited by MRS 2395. Nonaka et al in their in silico screening for P2Y12R ligands identified both LTE4 and LTD4 (LTC4 not tested) as potential endogenous ligands for this receptor.27 In our assays, a P2Y12R antagonist effectively inhibited ECP secretion from eosinophil granules induced by all three cys-LTs.
FIG 3.
MRS 2395, a selective P2Y12 receptor (P2Y12R) antagonist, dose-dependently inhibited the eosinophil cationic protein (ECP) release induced by A, LTC4 30 nM, B, LTD4 0.3 nM and C, 300 nM and D, LTE4 30 nM. + and * represent P < 0.05 for ECP released compared with non-stimulated and leukotriene-stimulated granules, respectively. E, Eosinophil and F, isolated granule surface membranes expressed the P2Y12R. Shaded histograms and solid lines represent staining with control Ab and with an anti- P2Y12R specific polyclonal Ab, respectively. G, The expression of the P2Y12R was confirmed on eosinophil and isolated granules by Western blots. Specificity of immunodetection was confirmed by neutralization with the anti-P2Y12R pAb by absorption with its respective immunogen peptide. All data is shown for one experiment representative of three. Gran, granules; Eos, eosinophils.
Human eosinophils express several mRNAs that encode P2X and P2Y receptor subtypes: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y14, P2X1, P2X4 and P2X7.28 However, the expression of the subtype P2Y12R had not been recognized for human eosinophils. To ascertain that the inhibitory effects of MRS 2395 on granules were due to its inhibitory actions on P2Y12R, we investigated the expression of this receptor on eosinophils and isolated granules. By flow cytometry, under membrane non-permeabilizing conditions, eosinophils (Fig 3, E) and isolated, extracellular granules (Fig 3, F) exhibited immunostaining for P2Y12R. Moreover, expression of P2Y12R on isolated granules and eosinophils was confirmed by Western blots (Fig 3, G). The specificity of the polyclonal Ab was ascertained by complete neutralization through preincubation of the anti-P2Y12R polyclonal Ab with its respective specific blocking peptide immunogen.
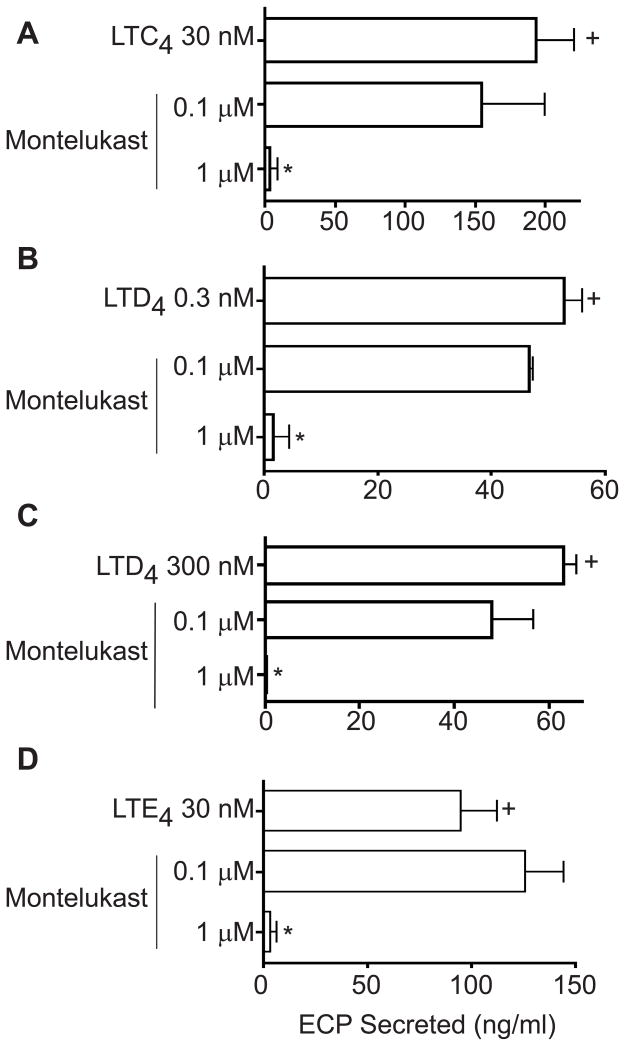
Montelukast inhibits eosinophil cationic protein (ECP) secretion from cysteinyl leukotriene-stimulated eosinophil granules
To evaluate the therapeutic potential of montelukast, currently used clinically based on its actions as a CysLT1R antagonist, we assessed the capacities of montelukast to inhibit LTC4-, LTD4- and LTE4-elicited ECP secretion from human eosinophil granules. Notably, montelukast inhibited eosinophil granule ECP secretion induced by LTC4 (30 nM) (Fig 4, A), LTD4 (0.3 and 300 nM) (Fig 4, B and C), and LTE4 (30 nM) (Fig 4, D).
FIG 4.
Montelukast dose-dependently inhibited eosinophil cationic protein (ECP) secretion induced by A, LTC4 30 nM, B, LTD4 0.3 nM and C, 300 nM and D, LTE4 30 nM. Data is shown for one experiment representative of three. + and * represent P < 0.05 for ECP released compared with non-stimulated granules and leukotriene-stimulated granules, respectively.
DISCUSSION
Our findings of functional receptors for cys-LTs on cell-free extracellular human eosinophil granule membranes, sensitive to inhibition by montelukast and a P2Y12R antagonist, identify novel mechanisms whereby cys-LTs may serve as intracrine and paracrine mediators of eosinophil granule-derived secretion. Cys-LTRs, heretofore, have been widely recognized to localize and function principally at cell plasma membranes.1 To date, recognition of intracellular sites of CysLTRs has been limited to their immunolocalization, without defined functional roles, on nuclei of a human mast cell line12 and colon adenocarcinoma cells.11 We now demonstrate that receptors for cys-LTs are expressed and functional on the membranes of an intracellularly derived organelle, the granules of human eosinophils. With Abs specific to each receptor. the protein receptor components immunologically demonstrated to be present on human eosinophil granules include those for CysLT1R, CysLT2R and the purinergic receptor P2Y12. Notably, each CysLTR protein was expressed with its ligand-binding domain on the outer membranes of human eosinophil granules.
As is true of other GPCRs, there is the potential for individual protein chains of Cys-LTRs to form hetero-dimers or hetero-oligomers, which might influence their pharmacology and function.29 Previously in a human mast cell line, functionally significant heterodimeric combinations of CysLT1R and CysLT2R were demonstrated.12 We document that three protein components of Cys-LT receptors, CysLT1R, CysLT2R and the purinergic receptor P2Y12, are expressed on human eosinophil granules. Whether the three candidate CysLTR protein chains function or interact as homodimers, heterodimers, or oligomers in human eosinophil granules can not be ascertained in these “primary” cells in contrast to transfectable cell lines. Nevertheless, of potential clinical pertinence, whatever the interactions maybe amongst the Cys-LTR protein chains, cell-free human eosinophil granules, long recognized to be present as intact membrane bound structures in tissues and secretions associated with varied allergic (e.g., asthma, rhinitis, urticaria) and other eosinophil-associated diseases,15–21 have the capacity to secrete ECP in response to low and even sub-nanomolar concentrations of the three cys-LTs, including the two extracellularly generated cys-LTs, LTD4 and LTE4.
Cys-LT-elicited ECP secretion was inhibited by MRS 2395, a selective P2Y12R antagonist. Clinically, this receptor is blocked by clopidogrel. Moreover, isolated granule secretory responses to each of the cys-LTs were blocked by montelukast. The 1 μM concentration of montelukast that uniformly inhibited LTC4-, LTD4- and LTE4-elicited granule secretion of ECP is in accord with levels of montelukast achieved in patients receiving this agent.30 Decreases in serum ECP levels have been demonstrated in asthmatic subjects treated with montelukast.31 Montelukast is known as a potent selective CysLT1R antagonist effective as a therapeutic for asthma and other allergic conditions.32 Inhibitory actions of montelukast beyond those based mainly on inhibition of CysLT1R have been reported.33–35 Montelukast inhibits the recently de-orphanized GPR1733 and several purinergic G protein-coupled receptors, suggesting that CysLT1R antagonists possibly interact functionally with signaling pathways of P2Y receptors.34 Besides, on human eosinophils, montelukast was suggested as a regulator of eosinophil protease activity through a leukotriene-independent mechanism.35 In part, because LTE4 is a weak agonist for CysLT1R, montelukast sensitive mechanisms that are operative in stimulating eosinophil granule ECP secretion are likely other than singularly by CysLT1R blockage.
Within eosinophils, synthesis of LTC4 (but not of extracellularly formed LTD4 or LTE4) occurs at perinuclear membranes and cytoplasmic lipid bodies.8,36–38 For granules, as intracellular organelles, the presence of functional membrane-expressed receptors might be indicative of intracrine roles for LTC4 as regulators of granule protein mobilization, sorting and secretion. We previously recognized a role principally for LTC4, and not LTD4 or LTE4, as an intracrine mediator of CCR3 receptor-elicited, vesicular transport-mediated interleukin-4 secretion by human eosinophils.39 Such release of IL-4 via vesicular transport from within eosinophils13 likely involves more complex steps beyond intracellular granule secretion that may be regulated by actions of LTC4. That intracellular LTC4 can activate release of ECP from cys-LT responsive receptors expressed on eosinophil granule membranes helps in further delineating roles for LTC4 as an intracrine mediator of eosinophil secretory responses. For granules, as extruded extracellular “organelles,” the expression of functional CysLTRs on granule membranes capable of responding to the extracellularly formed cys-LTs, LTD4 and LTE4, underscores the secretion-competence of cell-free eosinophil granules. Together, the recognition that CysLTRs are localized on the outer membranes of human eosinophil granules and mediate cys-LT-elicited secretion from within these granules identifies new roles, amenable to therapeutic interventions, for cys-LTs as mediators in eosinophil-associated diseases.
Acknowledgments
This work was supported by the National Institutes of Health Grants AI020241, AI022571 and AI051645 and an investigator-initiated grant from Merck.
Abbreviations used
- Ab
antibody
- cys-LTs
cysteinyl leukotrienes
- CysLTRs
cysteinyl leukotriene receptors
- ECP
eosinophil cationic protein
- GPCRs
G protein-coupled receptors
- LTs
leukotrienes
- P2Y12R
P2Y12 receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peters-Golden M, Henderson WR., Jr Leukotrienes. N Engl J Med. 2007;357:1841–54. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 2.Rovati GE, Capra V. Cysteinyl-leukotriene receptors and cellular signals. Scientific-WorldJournal. 2007;7:1375–92. doi: 10.1100/tsw.2007.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch KR, O’Neill GP, Liu Q, Im DS, Sawyer N, Metters KM, et al. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399:789–93. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- 4.Heise CE, O’Dowd BF, Figueroa DJ, Sawyer N, Nguyen T, Im DS, et al. Characterization of the human cysteinyl leukotriene 2 receptor. J Biol Chem. 2000;275:30531–6. doi: 10.1074/jbc.M003490200. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard C, Rothenberg ME. Biology of the eosinophil. Adv Immunol. 2009;101:81–121. doi: 10.1016/S0065-2776(08)01003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–74. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 7.Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol. 2000;105:651–63. doi: 10.1067/mai.2000.105712. [DOI] [PubMed] [Google Scholar]
- 8.Bandeira-Melo C, Weller PF. Eosinophils and cysteinyl leukotrienes. Prostaglandins Leukot Essent Fatty Acids. 2003;69:135–43. doi: 10.1016/s0952-3278(03)00074-7. [DOI] [PubMed] [Google Scholar]
- 9.Capra V, Thompson MD, Sala A, Cole DE, Folco G, Rovati GE. Cysteinyl-leukotrienes and their receptors in asthma and other inflammatory diseases: critical update and emerging trends. Med Res Rev. 2007;27:469–527. doi: 10.1002/med.20071. [DOI] [PubMed] [Google Scholar]
- 10.Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. J Immunol. 2004;173:1503–10. doi: 10.4049/jimmunol.173.3.1503. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen CK, Campbell JI, Ohd JF, Morgelin M, Riesbeck K, Landberg G, et al. A novel localization of the G-protein-coupled CysLT1 receptor in the nucleus of colorectal adenocarcinoma cells. Cancer Res. 2005;65:732–42. [PubMed] [Google Scholar]
- 12.Jiang Y, Borrelli LA, Kanaoka Y, Bacskai BJ, Boyce JA. CysLT2 receptors interact with CysLT1 receptors and down-modulate cysteinyl leukotriene dependent mitogenic responses of mast cells. Blood. 2007;110:3263–70. doi: 10.1182/blood-2007-07-100453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spencer LA, Melo RC, Perez SA, Bafford SP, Dvorak AM, Weller PF. Cytokine receptor-mediated trafficking of preformed IL-4 in eosinophils identifies an innate immune mechanism of cytokine secretion. Proc Natl Acad Sci U S A. 2006;103:3333–8. doi: 10.1073/pnas.0508946103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neves JS, Perez SA, Spencer LA, Melo RC, Reynolds L, Ghiran I, et al. Eosinophil granules function extracellularly as receptor-mediated secretory organelles. Proc Natl Acad Sci U S A. 2008;105:18478–83. doi: 10.1073/pnas.0804547105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erjefalt JS, Persson CG. New aspects of degranulation and fates of airway mucosal eosinophils. Am J Respir Crit Care Med. 2000;161:2074–85. doi: 10.1164/ajrccm.161.6.9906085. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe K, Misu T, Inoue S, Edamatsu H. Cytolysis of eosinophils in nasal secretions. Ann Otol Rhinol Laryngol. 2003;112:169–73. doi: 10.1177/000348940311200211. [DOI] [PubMed] [Google Scholar]
- 17.Cheng JF, Ott NL, Peterson EA, George TJ, Hukee MJ, Gleich GJ, et al. Dermal eosinophils in atopic dermatitis undergo cytolytic degeneration. J Allergy Clin Immunol. 1997;99:683–92. doi: 10.1016/s0091-6749(97)70031-9. [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez-Pena EJ, Knab J, Buttner DW. Immunoelectron microscopic evidence for release of eosinophil granule matrix protein onto microfilariae of Onchocerca volvulus in the skin after exposure to amocarzine. Parasitol Res. 1998;84:607–15. doi: 10.1007/s004360050459. [DOI] [PubMed] [Google Scholar]
- 19.Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119:206–12. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Toyoda M, Maruyama T, Morohashi M, Bhawan J. Free eosinophil granules in urticaria: a correlation with the duration of wheals. Am J Dermatopathol. 1996;18:49–57. doi: 10.1097/00000372-199602000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Chehade M, Sampson HA, Morotti RA, Magid MS. Esophageal subepithelial fibrosis in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2007;45:319–28. doi: 10.1097/MPG.0b013e31806ab384. [DOI] [PubMed] [Google Scholar]
- 22.Neves JS, Perez SA, Spencer LA, Melo RC, Weller PF. Subcellular fractionation of human eosinophils: isolation of functional specific granules on isoosmotic density gradients. J Immunol Methods. 2009;344:64–72. doi: 10.1016/j.jim.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spencer LA, Szela CT, Perez SA, Kirchhoffer CL, Neves JS, Radke AL, et al. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol. 2009;85:117–23. doi: 10.1189/jlb.0108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee TH, Woszczek G, Farooque SP. Leukotriene E(4): Perspective on the forgotten mediator. J Allergy Clin Immunol. 2009;124:417–21. doi: 10.1016/j.jaci.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Gauvreau GM, Parameswaran KN, Watson RM, O’Byrne PM. Inhaled leukotriene E(4), but not leukotriene D(4), increased airway inflammatory cells in subjects with atopic asthma. Am J Respir Crit Care Med. 2001;164:1495–500. doi: 10.1164/ajrccm.164.8.2102033. [DOI] [PubMed] [Google Scholar]
- 26.Austen KF, Maekawa A, Kanaoka Y, Boyce JA. The leukotriene E(4) puzzle: Finding the missing pieces and revealing the pathobiologic implications. J Allergy Clin Immunol. 2009;124:406–14. doi: 10.1016/j.jaci.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nonaka Y, Hiramoto T, Fujita N. Identification of endogenous surrogate ligands for human P2Y12 receptors by in silico and in vitro methods. Biochem Biophys Res Commun. 2005;337:281–8. doi: 10.1016/j.bbrc.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari D, la Sala A, Panther E, Norgauer J, Di Virgilio F, Idzko M. Activation of human eosinophils via P2 receptors: novel findings and future perspectives. J Leukoc Biol. 2006;79:7–15. doi: 10.1189/jlb.0505286. [DOI] [PubMed] [Google Scholar]
- 29.Milligan G. G protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br J Pharmacol. 2009;158:5–14. doi: 10.1111/j.1476-5381.2009.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friesen CA, Neilan NA, Schurman JV, Taylor DL, Kearns GL, Abdel-Rahman SM. Montelukast in the treatment of duodenal eosinophilia in children with dyspepsia: effect on eosinophil density and activation in relation to pharmacokinetics. BMC Gastroenterol. 2009;9:32. doi: 10.1186/1471-230X-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopriva F, Janostakova A, Jarmila S, Zapalka M, Hajduch M. Montelukast decreases plasma endothelin-1 and serum eosinophil cationic protein levels in paediatric atopic asthma. Clin Drug Investig. 2006;26:351–6. doi: 10.2165/00044011-200626060-00006. [DOI] [PubMed] [Google Scholar]
- 32.Storms W. Update on montelukast and its role in the treatment of asthma, allergic rhinitis and exercise-induced bronchoconstriction. Expert Opin Pharmacother. 2007;8:2173–87. doi: 10.1517/14656566.8.13.2173. [DOI] [PubMed] [Google Scholar]
- 33.Ciana P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, et al. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. Embo J. 2006;25:4615–27. doi: 10.1038/sj.emboj.7601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mamedova L, Capra V, Accomazzo MR, Gao ZG, Ferrario S, Fumagalli M, et al. CysLT1 leukotriene receptor antagonists inhibit the effects of nucleotides acting at P2Y receptors. Biochem Pharmacol. 2005;71:115–25. doi: 10.1016/j.bcp.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langlois A, Ferland C, Tremblay GM, Laviolette M. Montelukast regulates eosinophil protease activity through a leukotriene-independent mechanism. J Allergy Clin Immunol. 2006;118:113–9. doi: 10.1016/j.jaci.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Bozza PT, Yu W, Penrose JF, Morgan ES, Dvorak AM, Weller PF. Eosinophil lipid bodies: specific, inducible intracellular sites for enhanced eicosanoid formation. J Exp Med. 1997;186:909–20. doi: 10.1084/jem.186.6.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brock TG, Anderson JA, Fries FP, Peters-Golden M, Sporn PH. Decreased leukotriene C4 synthesis accompanies adherence-dependent nuclear import of 5-lipoxygenase in human blood eosinophils. J Immunol. 1999;162:1669–76. [PubMed] [Google Scholar]
- 38.Bandeira-Melo C, Phoofolo M, Weller PF. Extranuclear lipid bodies, elicited by CCR3-mediated signaling pathways, are the sites of chemokine-enhanced leukotriene C4 production in eosinophils and basophils. J Biol Chem. 2001;276:22779–87. doi: 10.1074/jbc.M101436200. [DOI] [PubMed] [Google Scholar]
- 39.Bandeira-Melo C, Woods LJ, Phoofolo M, Weller PF. Intracrine cysteinyl leukotriene receptor-mediated signaling of eosinophil vesicular transport-mediated interleukin-4 secretion. J Exp Med. 2002;196:841–50. doi: 10.1084/jem.20020516. [DOI] [PMC free article] [PubMed] [Google Scholar]