Abstract
Purpose
We determined whether human immunodeficiency virus (HIV) infection affects body cell mass and fat mass wasting among adults with pulmonary tuberculosis (PTB).
Methods
We screened 967 Ugandan adults for PTB and HIV infection in a cross-sectional study. We compared anthropometric and bioelectric impedance analysis (BIA) body composition parameters among HIV-seropositive and HIV-seronegative men and women with or without PTB using a non-parametric test.
Results
We found that poor nutritional status associated with TB differed among men and women. Anthropometric and BIA body composition did not differ between HIV-seropositive and HIV-seronegative patients regardless of gender. Average weight group difference in men comprised of body cell mass and fat mass in equal proportions of 43%. In women, average weight group difference comprised predominantly of fat mass of 73% and body cell mass of 13%. Compared to individuals without TB, patients with TB had lower body mass index, weight, body cell mass, and fat mass regardless of gender and HIV status.
Conclusions
Gender but not HIV status was associated with body composition changes in TB. Tuberculosis appears to be the dominant factor driving the wasting process among co-infected patients.
Keywords: Tuberculosis, HIV, bioelectrical impedance, gender, wasting, body composition
Introduction
Body wasting is a prominent and cardinal feature of tuberculosis (TB) disease (1, 2) and is a marker of disease severity and outcome. In sub-Saharan Africa, a large proportion of patients with TB also have co-infection with human immunodeficiency virus (HIV) (3). Co-infection may worsen the wasting seen in either TB disease or HIV infection alone (4, 5). Wasting in TB is associated with reduced caloric intake due to anorexia or loss of appetite and increase in consumption of calories due to altered metabolism induced by inflammation and immune response (6–8).
Several studies (9–15) in sub-Saharan Africa have shown the impact of dual infection with HIV and TB on nutritional status using anthropometric measurements. However, body composition measured by anthropometry including fat mass and fat-free mass (16) may be associated with observer bias. Furthermore anthropometry may not predict body cell mass, the metabolically active component of the body that may be associated with adverse effects on survival. Therefore, anthropometry provides only limited information about nutritional status in patients. Bioelectrical impedance analysis (BIA) offers a useful alternative to anthropometry because it measures multiple body compartments and provides a more detailed assessment of body composition. Several cross-sectional studies (10, 12, 14, 15, 17) have examined the impact of HIV infection on body composition of adults with TB disease but are limited because they lacked comparison group free of TB disease or HIV infection to show the independent metabolic effects of TB disease and HIV infection. Some studies were limited by small sample sizes (10, 14, 15) while others (6) comprised only men in the study population.
The current cross-sectional study with a large sample size including both men and women was conducted in Kampala, Uganda, to determine whether HIV infection is associated with body cell mass and fat mass wasting among adults with TB disease. We hypothesized that HIV-seropositive patients with TB disease had marked depletion of body cell mass and fat mass stores when compared to HIV-seronegative patients with TB disease.
Materials and methods
We conducted a cross-sectional study to address the objective of the present study. The study population consisted of 445 index TB patients and 522 household contacts without evidence of TB aged 18 or more years selected from Kawempe Division in Kampala, Uganda. Index patients presenting with sputum positive pulmonary tuberculosis to the National Tuberculosis and Leprosy Program at the Tuberculosis Clinic of Upper Mulago Hospital complex and their household contacts were recruited to the study between July 2002 and March 2008. The institutional review boards at Case Western Reserve University and the Ugandan National AIDS Research Subcommittee approved the study, with final approval by the Office for Protection from Research Risk of the National Institutes of Health.
All subjects in the study were given appropriate pre- and post-test HIV counseling and AIDS education. At enrollment, basic demographic information and a medical history were collected, and a standardized physical examination was conducted by a medical officer. Active TB was confirmed by sputum smear microscopy and culture. Patients with active TB were treated with standard four-drug chemotherapy for tuberculosis per guidelines of the Ugandan Ministry of Health. Adults with a previous history of treated pulmonary TB were excluded in the study. Of the 522 adult household contacts who were enrolled in the study, 23 household contacts were excluded, leaving 944 participants in total available for analysis. Of the 23 excluded contacts, 22 contacts had unknown TB or HIV status and one contact was missing height measurement. Household contacts that were excluded had comparable age, sex, weight, height, and body mass index with those who were included in the analysis.
Nutritional status was assessed using anthropometric measurements such as height and weight and BIA Detroit, MI, RJL Systems. All measurements were performed by one trained observer using the same equipment and recommended standard conditions with regard to body position, previous exercise, dietary intake, skin temperature, and voiding of the bladder were taken into consideration in taking BIA measurements (18). All measurements were performed on the day index patients were confirmed to have TB disease while household contacts were measured on a scheduled visit within three weeks following identification of the index patient.
Body-mass index (BMI) was computed using the relationship of weight in kilograms divided by height in meters squared (kg/m2). BIA, is a simple, non-invasive technique, that has been recommended for nutritional studies in the clinical setting (18, 19) and has been shown to be sufficiently precise for clinical investigation of body composition analysis (18–22). Single-frequency BIA was performed at 50 kHz and 800 mA with standard tetrapolar lead placement (23). Before performing measurements on each subject, the BIA instrument was calibrated using the manufacturer’s recalibration device. The resistance and reactance were based on measures of a series circuit (20). BIA measurements were performed in triplicate for each subject. Body weight was determined to the nearest 0.1 kg using a SECA adult balance, and standing height was determined to the nearest 2 mm. Body cell mass and fat-free mass were calculated from BIA measurements using equations that were previously cross-validated in a sample of patients (white, black and Hispanic) with and without HIV infection (20) and have been applied elsewhere in African studies (10, 12, 17). Extracellular mass was calculated as fat-free mass minus body cell mass and fat mass as body weight minus fat-free mass (24).
HIV-1 infection was diagnosed on the basis of a positive enzyme-linked immunosorbent assay for HIV-1 antibodies (Recombigen; Cambridge Biotech, Cambridge, MA). HIV-seropositive participants who were newly identified with HIV were not on antiretroviral therapy at the time of measurement; no patients with pre-existing HIV infection at the time of household evaluation were on antiretroviral therapy.
All study participants in the analysis were categorized into 4 mutually independents groups: HIV-seropositive patients with and without TB disease, HIV-seronegative patients with and without TB disease. Measures of central tendency and variability were compared across 4 mutually exclusive groups using Wilcoxon-Mann Whitney test for average anthropometric and BIA body composition parameters, hemoglobin, and CD4 cell counts. We made comparisons between HIV-seropositive patients with TB disease and HIV-seronegative patients with TB disease and between HIV-seropositive individuals without TB disease and HIV-seronegative individuals without TB disease to understand the effects of HIV infection and TB disease on body cell mass and fat mass. BMI was dichotomized at cutoff of 18.5 kg/m2 and proportions compared among the 4 mutually exclusive groups using chi-square and Fischer’s exact tests. Fischer’s exact test was used where expected counts were less than 5. A p-value of <0.05 was considered significant in all analyses. BMI, < 18.5 kg/m2 was considered consistent with malnutrition (25). All analyses were performed using SAS version 9.1.3 Cary software, North Carolina SAS Institute Inc. 2000 – 2004.
Results
Of the 944 participants who were included in the analysis, 93 men and 103 women were HIV-seropositive with TB disease; 145 men and 104 women were HIV-seronegative with TB disease; 22 men and 61 women were HIV-seropositive without TB disease; while 160 men and 256 women were HIV-seronegative without TB disease (Table 1). In Table 1, among 420 men and 524 women regardless of HIV status there were no significant differences in average age between participants with and participants without TB disease.
Table 1.
Demographics and Laboratory Characteristics of HIV-seropositive and HIV-seronegative Men and Women with or without Pulmonary Tuberculosis
| Men (n=420) | Women (n=524) | |||||||
|---|---|---|---|---|---|---|---|---|
| HIV-seropositive (n=115) | HIV-seronegative (n=305) | HIV-seropositive (n=164) | HIV-seronegative (360) | |||||
| Characteristic1 | TB+ (n=93) | TB− (n=22) | TB+ (n=145) | TB− (n=160) | TB+ (n=103) | TB− (n=61) | TB+ (n=104) | TB− (n=256) |
| Age, y | 33.7 ± 8.1 | 35.7 ± 8.8 | 29.3 ± 9.7 | 29.4 ± 11.6 | 30.6 ± 8.4 | 31.8 ± 8.6 | 27.2 ± 9.2 | 30.2 ± 13.3 |
| Index cases, n | 88 | - | 140 | - | 97 | - | 101 | - |
| CD4+ lymphocytes2, cells/μL2 | 200 ± 189 | 375 ± 205b | - | - | 238 ± 192 | 352 ± 226b | - | - |
| CD4+ lymphocytes < 200 cells/μL2, % | 57 (30/53) | 25 (3/12) b | - | - | 49 (30/61) | 27 (10/37) b | - | - |
| Hemoglobin3, g/L | 115 ± 25 | 140 ± 14a | 126 ± 21 | 158 ± 16a | 104 ± 20 | 129 ± 11a | 115 ± 17 | 134 ± 15a |
| Anemic4, % | 75 (70/93) | 32 (7/22)a | 59 (85/145) | 4 (6/158)a | 78 (80/103) | 18 (11/61)a | 59 (61/104) | 13 (34/252)a |
p-value < 0.001,
p-value < 0.05. TNF-α = tumor necrosis alpha, TB− = no tuberculosis disease, TB+ = Tuberculosis disease.
Values are means ± standard deviation for continuous variables.
CD4+ lymphocytes cell counts were not measured in 50 men and 66 women with HIV infection; 302 men and 358 women without HIV infection.
Hemoglobin was not measured in 50 men with HIV infection; 2 men and 4 women without HIV infection.
Hemoglobin <12.0 g/L for men, <13.0 g/L for women.
HIV-seropositive participants with TB disease had significantly lower CD4 cell count and hemoglobin levels, higher proportions of individuals with anemia and CD4 < 200 cell counts compared to HIV-seropositive participants without TB disease regardless of gender (Table 1). Similarly, HIV-seronegative participants with TB disease had significantly lower hemoglobin, and higher proportions of individuals with anemia compared to HIV-seronegative participants without TB disease regardless of gender. HIV-seropositive participants with TB disease were significantly older, had significantly lower hemoglobin levels and higher proportion of individuals with anemia compared to HIV-seronegative participants with TB disease regardless of gender (Table 1).
HIV-seropositive men and women with TB disease had signs of nutritional deficiency. Compared to individuals without TB disease, patients with TB disease had lower BMI, weight, body cell mass, and fat mass regardless of gender and HIV status. For example, among men body cell mass was 22.83 versus 25.74 (P < 0.001) and 23.01 versus 26.35 (P < 0.001) for HIV-seropositive and negative status, with and without TB disease respectively (Table 2). A greater proportion of TB cases had lower BMI < 18.5 kg/m2, and an increased extra-cellular mass/body cell mass ratio than participants without TB disease. Among men and women with TB disease, measurement of anthropometric and BIA body composition did not differ between HIV-seropositive and HIV-seronegative patients. A similar relationship was observed among men and women without TB disease (Table 2).
Table 2.
Anthropometric and BIA Body Composition among HIV-seropositive and HIV-seronegative Men and Women with or without Pulmonary Tuberculosis
| Men (n=420) | Women (n=524) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic1 | HIV-seropositive (n=115) | HIV-seronegative (n=305) | HIV-seropositive (n=164) | HIV-seronegative (n=360) | ||||
| TB+ (n=93) | TB− (n=22) | TB+ (n=145) | TB− (n=160) | TB+ (n=103) | TB− (n=61) | TB+ (n=104) | TB− (n=256) | |
| Weight, kg | 52.97 ± 7.45 | 59.14 ± 6.87a | 53.26 ± 7.34 | 61.22 ± 10.38a | 49.51 ± 8.35 | 61.17 ± 14.22a | 51.17 ± 9.66 | 60.21 ± 13.40a |
| Height, cm | 168 ± 7 | 168 ± 5 | 169 ± 7 | 168 ± 7 | 158 ± 6 | 159 ± 7 | 158 ± 6 | 157 ± 6 |
| BMI, kg/m2 | 18.7 ± 2.4 | 21.0 ± 1.9a | 18.5 ± 1.9 | 21.6 ± 3.2a | 19.9 ± 3.1 | 24.0 ± 5.1a | 20.4 ± 4.4 | 24.3 ± 4.9a |
| BMI < 18.5, % | 49 (46/93) | 9 (2/22)a | 47 (68/145) | 12 (19/160)a | 31 (32/103) | 8 (5/61)a | 29 (30/104) | 5 (14/256)a |
| Reactance, Ω | 59.5 ± 16.2 | 62.1 ± 9.3 | 59.2 ± 10.9 | 64.6 ± 9.3a | 63.6 ± 13.4 | 64.7 ± 9.4 | 64.4 ± 11.2 | 65.2 ± 11.0 |
| Resistance, Ω | 598.3 ± 118.1 | 540.1 ± 68.6b | 594.5 ± 90.7 | 548.6 ± 79.8a | 678.4 ± 102.5 | 602.1 ± 79.7a | 658.5 ± 79.4 | 614.4 ± 84.9a |
| BCM, kg | 22.83 ± 4.58 | 25.74 ± 3.14a | 23.01 ± 4.14 | 26.35 ± 3.88a | 15.56 ± 2.37 | 17.90 ± 2.23a | 16.15 ± 2.30 | 17.28 ± 2.47a |
| ECM, kg | 24.38 ± 2.65 | 25.08 ± 2.24 | 24.52 ± 2.53 | 25.37 ± 3.35b | 23.45 ± 2.60 | 24.81 ± 2.58a | 23.69 ± 2.21 | 24.14 ± 2.42 |
| ECM/BCM | 1.11 ± 0.23 | 0.98 ± 0.10b | 1.10 ± 0.20 | 0.98 ± 0.14a | 1.53 ± 0.22 | 1.39 ± 0.09a | 1.48 ± 0.17 | 1.41 ± 0.14a |
| Fat mass, kg | 5.97 ± 2.99 | 8.31 ± 2.69a | 5.78 ± 2.63 | 9.50 ± 4.87 a | 11.07 ± 6.42 | 18.32 ± 10.93a | 11.62 ± 6.92 | 18.87 ± 10.58a |
| Percent fat, % | 10 ± 5 | 14 ± 3b | 10 ± 4 | 15 ± 5a | 20 ± 10 | 28 ± 10a | 21 ± 10 | 29 ± 10a |
p-value < 0.001,
p-value < 0.05.
Values are means ± standard deviation for continuous variables. TB− = no tuberculosis disease, TB+ = Tuberculosis disease, BCM = body cell mass, ECM = extracellular mass.
We found that men with TB disease weighed approximately 8 kg less than men without TB disease regardless of HIV serostatus (Figure 1). Similarly, women with TB disease weighed between 9 and 11 kg less than women without TB disease; however, among women, HIV-seropositive TB cases had greater weight difference than did their HIV seronegative counterparts with TB disease (Figure 1). Moreover, we found that the pattern of weight difference differed between men and women. The average weight difference in men comprised of body cell mass and fat mass in equal proportions of about 43% whereas in women, the average weight difference for HIV-seropositive and HIV-seronegative with TB disease comprised predominantly of fat mass of 73% to 80% with minimal body cell mass loss of 13% to 16%, respectively. The pattern of weight, body fat, percent body fat, and body cell mass differences among men and women did not differ according to HIV serostatus (Table 2); however, the pattern differed remarkably according to gender status. Men were heavier and had higher body cell mass than women while women had higher body fat and percent by weight body fat than men regardless of TB and HIV status respectively (Figures 2 and 3).
FIGURE 1.
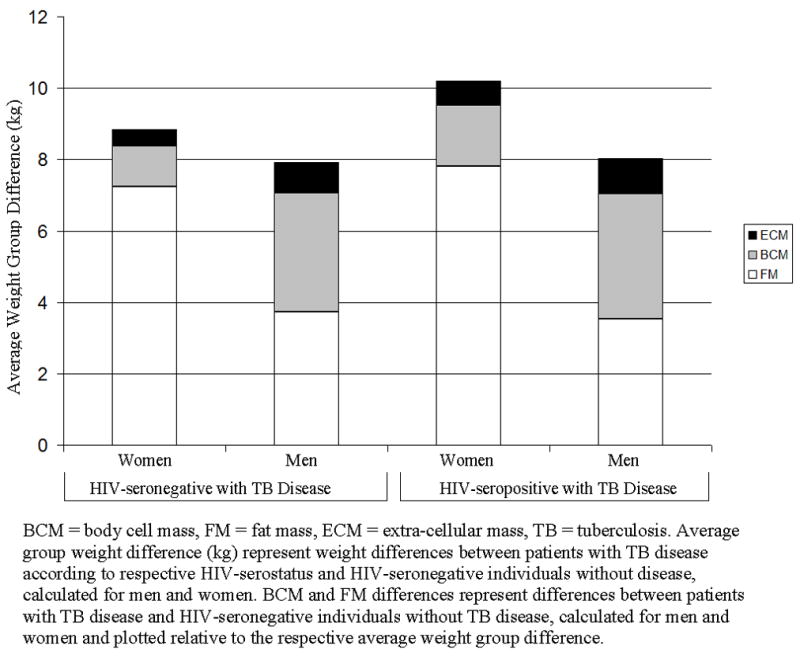
Body Cell Mass and Fat Mass Difference Relative to Average Weight Group Difference for Men and Women among Patients with Pulmonary Tuberculosis Disease.
FIGURE 2.
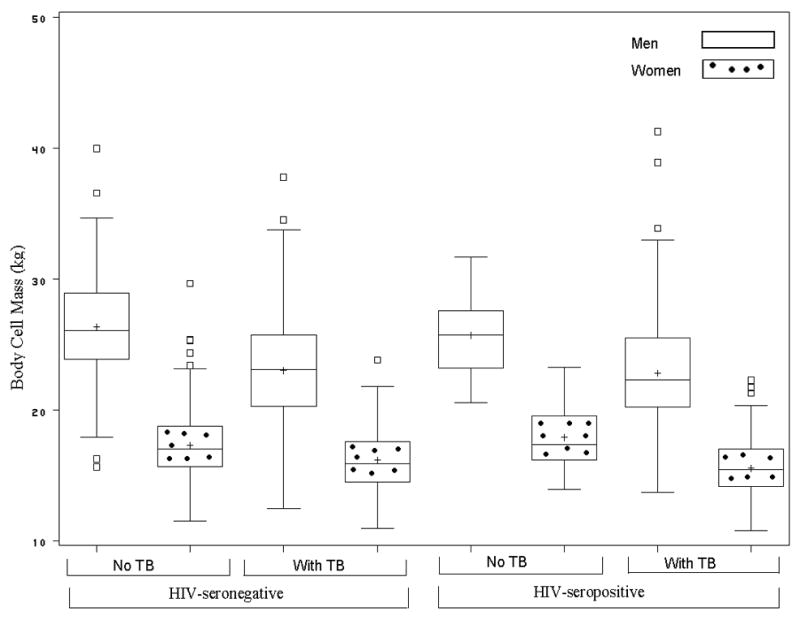
Body Cell Mass Box Plots for Men and Women among HIV-seronegative and HIV-positive Participants with or without Pulmonary Tuberculosis.
FIGURE 3.
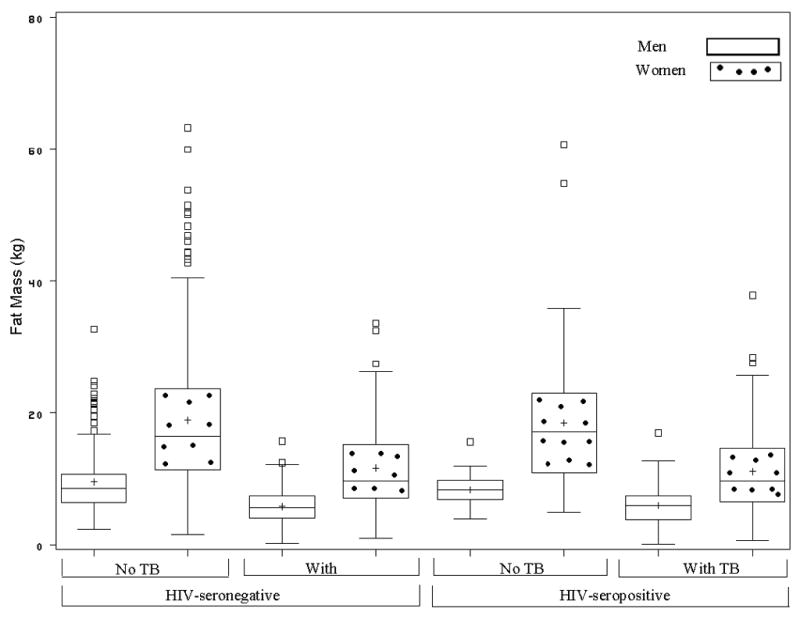
Fat Mass Box Plots for Men and Women among HIV-seronegative and HIV-positive Participants with or without Pulmonary Tuberculosis.
There were no significant differences between anthropometric and BIA body composition parameters when compared among 60 HIV-seropositive adults with pulmonary TB who had CD4 cell counts 200 and 54 who had CD4 cell counts > 200 (Table 3).
Table 3.
Anthropometric and BIA Body Composition among HIV-seropositive Adults with Pulmonary Tuberculosis by CD4+ Lymphocyte count
| Characteristic1 | CD4+ Lymphocyte count ≤ 200 cells/μL (n=60) | > 200 cells/μL (n=54) | p-value |
|---|---|---|---|
| Weight, kg | 51.2 ± 8.2 | 51.6 ± 7.5 | 0.565 |
| Height, cm | 162.6 ± 7.7 | 162.3 ± 7.7 | 0.907 |
| BMI, kg/m2 | 19.3 ± 2.7 | 19.6 ± 3.0 | 0.876 |
| BMI < 18.5, % | 38 (23/60) | 44 (24/54) | 0.508 |
| Reactance, Ω | 55.1 ± 13.7 | 58.4 ± 11.3 | 0.263 |
| Resistance, Ω | 641.5 ± 128.8 | 645.3 ± 112.1 | 0.570 |
| Body cell mass (BCM), kg | 18.8 ± 5.0 | 18.8 ± 5.1 | 0.889 |
| Extracellular mass (ECM), kg | 24.4 ± 3.0 | 23.9 ± 1.9 | 0.845 |
| ECM/BCM | 1.36 ± 0.29 | 1.35 ± 0.28 | 0.740 |
| Fat mass, kg | 8.23 ± 5.15 | 9.29 ± 5.80 | 0.292 |
| Percent fat, % | 15 ± 9 | 17 ± 9 | 0.396 |
Values are means ± standard deviation for continuous variables. TB− = no tuberculosis disease, TB+ = Tuberculosis disease.
Discussion
In this study of 944 Ugandan adults, the poor nutritional status associated with TB disease differed among men and women, yet was not affected by HIV serostatus. The average weight difference in men with TB disease comprised of body cell mass and fat mass in equal proportion whereas women, the average weight difference was predominantly fat mass. This pattern of average weight difference did not differ by HIV-serostatus regardless of gender.
Our findings suggest that TB disease and not HIV infection may be the dominant factor driving the wasting process in co-infected patients with TB disease. Among both men and women, patients with TB had lower body cell mass and fat mass as compared to individuals without TB according to HIV status. However, we did not detect differences in body composition by HIV status in patients without TB. This is in agreement with the postmortem study in Uganda in which results showed TB disease to be the common underlying likely cause of HIV-associated wasting and is also in keeping with studies of weight loss in HIV that linked most wasting episodes with opportunistic infections (26, 27). Furthermore, our study findings are consistent with the study by Paton and Ng (28) who used both a reference method (dual-energy x-ray absorptiometry) and BIA prediction method in measuring body composition in an Asian population. In the Paton and Ng study (28), patients with TB had lower body cell mass (21.0 versus 25.6 kg) and fat mass (6.2 versus 12.6 kg) than the controls without TB. Body composition measurements were not different in patients with and without HIV co-infection. Although the methodology is less robust in our study, because we did not have the referent method available, it does confirm the findings by Paton and Ng in an African population. It also extends their observations to include both men and women with suitable sample sizes. Compared to previous studies on TB and HIV (10, 14, 28, 29), our study included a full panel of control groups of HIV-seropositive and HIV-seronegative individuals without TB disease to demonstrate the absolute contribution of HIV or TB to wasting and the independent effect of TB disease regarding body wasting.
This study found that the changes in body composition for men and women with TB disease differed. Compared to controls without active TB, men with active TB waste both fat and body cell mass whereas women with TB predominantly waste body fat. There are two potential explanations for this finding. First, men may begin to waste body protein sooner than women because fat stores in men are proportionately smaller than in women, as seen in this study. This assumes that fat is wasted at a constant rate that is similar for men and women. Second, it is possible that this finding reflect a preferential loss of fat mass and a relative preservation of body cell mass seen in women (30). The gender differences in body composition may relate to the higher fat mass in women before disease onset and that the rate of tissue lost is related to the starting fat mass and or to the hormonal differences. Regardless of the mechanism, at the time of TB diagnosis, men may have begun to metabolize the body cell mass and have less energy reserve than women for complications of therapy or new opportunistic infections. Although the survival of HIV-infected men and women is similar, one study showed a trend toward better survival as women were 40% less likely to die during the first year after treatment of TB (13).
The striking lower hemoglobin levels among patients with TB disease compared to HIV-seronegative individuals without TB disease may indicate severe malnutrition or inflammation (31). Several studies have shown that decreased body cell mass and hypoalbuminemia as indicators of malnutrition (2, 32, 33) predict shorter survival in AIDS patients; however, the prognostic value of such parameters and hemoglobin are yet to be explored in TB patients.
The findings of our study may reflect uncomplicated, partial starvation (reduction in energy intake) rather than hyper-metabolism. We found the ratios of body cell mass differences relative to average weight difference regardless of gender (Figure 1) to be much less than the ratio of 0.67 seen in classic longitudinal study of chronic starvation (34). The pattern of body composition in TB disease appears to be most consistent with a mixture of calorie deficit, i.e., starvation and cachexia. Pure starvation produces body composition in which weight loss is invariably accompanied by more fat loss than lean tissues or sometimes in equal proportions (35–37). Thus, there is relative sparing of lean tissues. However, individuals with meager fat stores are forced to burn protein when faced with energy deficits (36, 37). In cachexia, there is disproportionate loss of lean tissue (or fat-free mass), which comprises of body cell mass and connective tissues, over losses of body fat mass, relative preservation of the extracellular body water (35, 36, 38, 39). The observed changes in cachexia result from increase in metabolic rate, with catabolism predominating over anabolism and elevations in resting energy expenditure. The metabolic events are affected by cytokines including TNF-α, interleukin-1, interleukin-6, and interferon alpha (40). Furthermore, there may be behavioral factors that restrict both dietary intake and activity. In a cross-sectional study such as this one, it is not possible to infer the underlying mechanisms that led to the observed patterns of body wasting. Regardless of the mechanism, TB patients need adequate nutrition, before and after the diagnosis of the disease.
In this study, we did not use a reference measurement of body composition, such as the dual-energy x-ray absorptiomety. Furthermore, the BIA prediction method used has not yet been validated in the local population. As a result, our findings of body composition may be biased because of variations in hydration across ethnic groups (18). We have, however, used equations that were previously cross-validated in individuals of different race (white, black, and Hispanics) among men and women, who were both health controls and HIV-infected patients (20). Moreover, the equations have been used widely in other studies from Africa with meaningful findings (10, 12, 17). We also took care to make measurements at rest, with proper placement of leads, in participants who had not exercised or taken alcohol, in participants with voided bladder and ambient temperature. However, we made measurements in patients with underlying illness that may cause shifts in body water compartments, thereby affecting measurements of fat mass. Our findings are also limited by the lack of dietary intake assessment to give further insight in the interpretation of gender differences in body composition.
Despite the limitations of our study, we have found that during TB disease there are remarkable gender differences in body composition changes that further evaluation is needed to understand how these changes influence survival and response to antiTB therapy. Among patients with TB and HIV co-infection, TB seems to be the dominant factor driving the wasting process and HIV infection does not appear to alter the body composition changes in TB wasting.
Acknowledgments
The study was supported in part by the AIDS International Training Research Program Fogarty International Center, grant number TW00011 based at Case Western Reserve University Department of Epidemiology and Biostatistics.
We thank all study staff members of the Case Western Reserve University and Makerere University research collaboration at the Tuberculosis Research Unit in the United States and in Uganda for their assistance; the faculty staff at Case Western Reserve University Department of Epidemiology and Biostatistics for the guidance in analyzing the project; and the Fogarty International Center, for the continued support.
List of Abbreviations and Acronyms
- TB
Tuberculosis
- HIV
Human immunodeficiency virus
- BIA
Bioelectrical impedance analysis
- BCM
Body cell mass
- FM
Fat mass
- TNF-α
Tumor necrosis factor alpha
- BMI
Body mass index
- AIDS
Acquired immunodeficiency syndrome
- ELISA
Enzyme-linked immunosorbet assay
Footnotes
Author disclosures: Mupere E, Zalwango S, Chiunda A, Okwera A, Mugerwa R, and Whalen CC, no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rubin SA. Tuberculosis. Captain of all these men of death. Radiol Clin North Am. 1995 Jul;33(4):619–39. [PubMed] [Google Scholar]
- 2.Schwenk A, Macallan DC. Tuberculosis, malnutrition and wasting. Curr Opin Clin Nutr Metab Care. 2000 Jul;3(4):285–91. doi: 10.1097/00075197-200007000-00008. [DOI] [PubMed] [Google Scholar]
- 3.World, Health, Organization. WHO Report 2004. Geneva: 2004. Global Tuberculosis Control: Surveillance, Planning, Financing. Contract No.: Document Number|. [Google Scholar]
- 4.van Lettow M, Fawzi WW, Semba RD. Triple trouble: the role of malnutrition in tuberculosis and human immunodeficiency virus co-infection. Nutr Rev. 2003 Mar;61(3):81–90. doi: 10.1301/nr.2003.marr.81-90. [DOI] [PubMed] [Google Scholar]
- 5.Lucas SB, De Cock KM, Hounnou A, Peacock C, Diomande M, Honde M, et al. Contribution of tuberculosis to slim disease in Africa. Bmj. 1994 Jun 11;308(6943):1531–3. doi: 10.1136/bmj.308.6943.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paton NI, Castello-Branco LR, Jennings G, Ortigao-de-Sampaio MB, Elia M, Costa S, et al. Impact of tuberculosis on the body composition of HIV-infected men in Brazil. J Acquir Immune Defic Syndr Hum Retrovirol. 1999 Mar 1;20(3):265–71. doi: 10.1097/00042560-199903010-00008. [DOI] [PubMed] [Google Scholar]
- 7.Paton NI, Ng YM, Chee CB, Persaud C, Jackson AA. Effects of tuberculosis and HIV infection on whole-body protein metabolism during feeding, measured by the [15N]glycine method. Am J Clin Nutr. 2003 Aug;78(2):319–25. doi: 10.1093/ajcn/78.2.319. [DOI] [PubMed] [Google Scholar]
- 8.Macallan DC, McNurlan MA, Kurpad AV, de Souza G, Shetty PS, Calder AG, et al. Whole body protein metabolism in human pulmonary tuberculosis and undernutrition: evidence for anabolic block in tuberculosis. Clin Sci (Lond) 1998 Mar;94(3):321–31. doi: 10.1042/cs0940321. [DOI] [PubMed] [Google Scholar]
- 9.Zachariah R, Spielmann MP, Harries AD, Salaniponi FM. Moderate to severe malnutrition in patients with tuberculosis is a risk factor associated with early death. Trans R Soc Trop Med Hyg. 2002 May-Jun;96(3):291–4. doi: 10.1016/s0035-9203(02)90103-3. [DOI] [PubMed] [Google Scholar]
- 10.Shah S, Whalen C, Kotler DP, Mayanja H, Namale A, Melikian G, et al. Severity of human immunodeficiency virus infection is associated with decreased phase angle, fat mass and body cell mass in adults with pulmonary tuberculosis infection in Uganda. J Nutr. 2001 Nov;131(11):2843–7. doi: 10.1093/jn/131.11.2843. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy N, Ramsay A, Uiso L, Gutmann J, Ngowi FI, Gillespie SH. Nutritional status and weight gain in patients with pulmonary tuberculosis in Tanzania. Trans R Soc Trop Med Hyg. 1996 Mar-Apr;90(2):162–6. doi: 10.1016/s0035-9203(96)90123-6. [DOI] [PubMed] [Google Scholar]
- 12.Van Lettow M, Kumwenda JJ, Harries AD, Whalen CC, Taha TE, Kumwenda N, et al. Malnutrition and the severity of lung disease in adults with pulmonary tuberculosis in Malawi. Int J Tuberc Lung Dis. 2004 Feb;8(2):211–7. [PubMed] [Google Scholar]
- 13.Whalen CC, Nsubuga P, Okwera A, Johnson JL, Hom DL, Michael NL, et al. Impact of pulmonary tuberculosis on survival of HIV-infected adults: a prospective epidemiologic study in Uganda. Aids. 2000 Jun 16;14(9):1219–28. doi: 10.1097/00002030-200006160-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niyongabo T, Henzel D, Idi M, Nimubona S, Gikoro E, Melchior JC, et al. Tuberculosis, human immunodeficiency virus infection, and malnutrition in Burundi. Nutrition. 1999 Apr;15(4):289–93. doi: 10.1016/s0899-9007(99)00003-9. [DOI] [PubMed] [Google Scholar]
- 15.Niyongabo T, Mlika-Cabanne N, Barihuta T, Henzel D, Aubry P, Larauze B. Malnutrition, tuberculosis and HIV infection in Burundi. Aids. 1994 Jun;8(6):851–2. [PubMed] [Google Scholar]
- 16.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974 Jul;32(1):77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- 17.Villamor E, Saathoff E, Mugusi F, Bosch RJ, Urassa W, Fawzi WW. Wasting and body composition of adults with pulmonary tuberculosis in relation to HIV-1 coinfection, socioeconomic status, and severity of tuberculosis. Eur J Clin Nutr. 2006 Feb;60(2):163–71. doi: 10.1038/sj.ejcn.1602281. [DOI] [PubMed] [Google Scholar]
- 18.Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gomez J, et al. Bioelectrical impedance analysis-part II: Utilization in clinical practice. Clin Nutr. 2004 Dec;23(6):1430–53. doi: 10.1016/j.clnu.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Kyle UG, Piccoli A, Pichard C. Body composition measurements: interpretation finally made easy for clinical use. Curr Opin Clin Nutr Metab Care. 2003;6:387–393. doi: 10.1097/01.mco.0000078988.18774.3d. [DOI] [PubMed] [Google Scholar]
- 20.Kotler DP, Burastero S, Wang J, Pierson RN., Jr Prediction of body cell mass, fat-free mass, and total body water with bioelectrical impedance analysis: effects of race, sex, and disease. Am J Clin Nutr. 1996 Sep;64(3 Suppl):489S–97S. doi: 10.1093/ajcn/64.3.489S. [DOI] [PubMed] [Google Scholar]
- 21.Sun SS, Chumlea WC, Heymsfield SB, Lukaski HC, Schoeller D, Friedl K, et al. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. Am J Clin Nutr. 2003 Feb;77(2):331–40. doi: 10.1093/ajcn/77.2.331. [DOI] [PubMed] [Google Scholar]
- 22.Roubenoff R. Applications of bioelectrical impedance analysis for body composition to epidemiologic studies. Am J Clin Nutr. 1996 Sep;64(3 Suppl):459S–62S. doi: 10.1093/ajcn/64.3.459S. [DOI] [PubMed] [Google Scholar]
- 23.Jackson AS, Pollock ML, Graves JE, Mahar MT. Reliability and validity of bioelectrical impedance in determining body composition. J Appl Physiol. 1988 Feb;64(2):529–34. doi: 10.1152/jappl.1988.64.2.529. [DOI] [PubMed] [Google Scholar]
- 24.Ott M, Fischer H, Polat H, Helm EB, Frenz M, Caspary WF, et al. Bioelectrical impedance analysis as a predictor of survival in patients with human immunodeficiency virus infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995 May 1;9(1):20–5. [PubMed] [Google Scholar]
- 25.Andres R, Elahi D, Tobin JD, Muller DC, Brant L. Impact of age on weight goals. Ann Intern Med. 1985 Dec;103(6 Pt 2):1030–3. doi: 10.7326/0003-4819-103-6-1030. [DOI] [PubMed] [Google Scholar]
- 26.Lucas SB, Diomande M, Hounnou A, Beaumel A, Giordano C, Kadio A, et al. HIV- associated lymphoma in Africa: an autopsy study in Cote d’Ivoire. Int J Cancer. 1994 Oct 1;59(1):20–4. doi: 10.1002/ijc.2910590106. [DOI] [PubMed] [Google Scholar]
- 27.Macallan DC, Noble C, Baldwin C, Foskett M, McManus T, Griffin GE. Prospective analysis of patterns of weight change in stage IV human immunodeficiency virus infection. Am J Clin Nutr. 1993 Sep;58(3):417–24. doi: 10.1093/ajcn/58.3.417. [DOI] [PubMed] [Google Scholar]
- 28.Paton NI, Ng YM. Body composition studies in patients with wasting associated with tuberculosis. Nutrition. 2006 Mar;22(3):245–51. doi: 10.1016/j.nut.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Swaminathan S, Padmapriyadarsini C, Sukumar B, Iliayas S, Kumar SR, Triveni C, et al. Nutritional status of persons with HIV infection, persons with HIV infection and tuberculosis, and HIV-negative individuals from southern India. Clin Infect Dis. 2008 Mar 15;46(6):946–9. doi: 10.1086/528860. [DOI] [PubMed] [Google Scholar]
- 30.Swanson B, Hershow RC, Sha BE, Benson CA, Cohen M, Gunfeld C. Body composition in HIV-infected women. Nutrition. 2000 Nov-Dec;16(11–12):1064–8. doi: 10.1016/s0899-9007(00)00432-9. [DOI] [PubMed] [Google Scholar]
- 31.Heymsfields SB. Modern Nutrition in Health and Disease. 7. Philadelphia: Williams and Wilkins; 1988. Nutritional Assessment by Clinical and Biochemiacl Methods. [Google Scholar]
- 32.Suttmann U, Ockenga J, Selberg O, Hoogestraat L, Deicher H, Muller MJ. Incidence and prognostic value of malnutrition and wasting in human immunodeficiency virus-infected outpatients. J Acquir Immune Defic Syndr Hum Retrovirol. 1995 Mar 1;8(3):239–46. doi: 10.1097/00042560-199503010-00004. [DOI] [PubMed] [Google Scholar]
- 33.Kotler DP, Tierney AR, Wang J, Pierson RN., Jr Magnitude of body-cell-mass depletion and the timing of death from wasting in AIDS. Am J Clin Nutr. 1989 Sep;50(3):444–7. doi: 10.1093/ajcn/50.3.444. [DOI] [PubMed] [Google Scholar]
- 34.Keys A. The Biology of human starvation. Minneapolis: University of Minnesota Press; 1950. [Google Scholar]
- 35.Morley JE, Thomas DR, Wilson MM. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr. 2006 Apr;83(4):735–43. doi: 10.1093/ajcn/83.4.735. [DOI] [PubMed] [Google Scholar]
- 36.Forbes GB. Lean body mass-body fat interrelationships in humans. Nutr Rev. 1987 Aug;45(8):225–31. doi: 10.1111/j.1753-4887.1987.tb02684.x. [DOI] [PubMed] [Google Scholar]
- 37.Forbes GB. Body fat content influences the body composition response to nutrition and exercise. Ann N Y Acad Sci. 2000 May;904:359–65. doi: 10.1111/j.1749-6632.2000.tb06482.x. [DOI] [PubMed] [Google Scholar]
- 38.Argiles JM, Lopez-Soriano J, Busquets S, Lopez-Soriano FJ. Journey from cachexia to obesity by TNF. FASEB J. 1997 Aug;11(10):743–51. doi: 10.1096/fasebj.11.10.9271359. [DOI] [PubMed] [Google Scholar]
- 39.Thomas DR. Distinguishing starvation from cachexia. Clin Geriatr Med. 2002 Nov;18(4):883–91. doi: 10.1016/s0749-0690(02)00032-0. [DOI] [PubMed] [Google Scholar]
- 40.Yeh SS, Blackwood K, Schuster MW. The cytokine basis of cachexia and its treatment: are they ready for prime time? J Am Med Dir Assoc. 2008 May;9(4):219–36. doi: 10.1016/j.jamda.2008.01.003. [DOI] [PubMed] [Google Scholar]


