Abstract
Research into the genetic basis of bipolar disorder (BD) has reached a turning point. Genome-wide association studies (GWAS), encompassing several thousands of samples, have produced replicated evidence for some novel susceptibility genes; however, the genetic variants implicated so far account for only a fraction of disease liability, a phenomenon not limited to psychiatric phenotypes but characteristic of all complex genetic traits studied to date. It appears that pure genomic approaches such as GWAS alone will not suffice to unravel the genetic basis of a complex illness like BD. Genomic approaches will need to be complemented by a variety of strategies including phenomics, epigenomics, pharmacogenomics, and neurobiology, as well as the study of environmental factors. This review will highlight the most promising findings from recent GWAS and candidate gene studies in BD. It will furthermore sketch out a potential research framework integrating various lines of research into the molecular biological basis of BD.
Keywords: manic-depressive illness, schizophrenia, depression, classification, linkage, association
Introductory remarks
This review of the basic principles and recent advances in genetic research into bipolar disorder (BD) can take advantage of a large, already extant body of work reviewing the evolution of findings from early family, twin, and adoption studies to linkage and association studies in BD 1–16. Rather than reviewing these findings again in a chronological and detailed manner, this review will sketch where we have come from, where we stand right now, how we can use the knowledge gleaned over the course of nearly a century, and how we can incorporate novel molecular and analytical techniques into our quest to unravel the genetics of BD.
Formal genetics, linkage, and association studies
To summarize some well-known facts, BD is a highly heritable disorder. Early 20th century family and twin studies had already observed that BD and other mental disorders aggregate in families, and that they have a heritable basis17–19. Whereas the lifetime prevalence of BD in the general population is around 1–2%, multiple studies have reported that the lifetime morbidity risk for BD in a first-degree relative of an individual with BD lies between 10 to 20% 13. Furthermore, twin studies have repeatedly cemented the heritable component of BD, with heritability estimates ranging between 80 and 90%, and adoption studies similarly support the notion that genetic factors contribute substantially more to the etiology of BD than environmental factors (for a comprehensive review of family, twin, and adoption studies, see 13).
This large body of evidence, established over the course of several decades, across changing diagnostic concepts of BD, and in populations of varying ethnic backgrounds, laid the foundation for gene mapping via linkage and association studies. The early days of this molecular era of genetic research into BD could be frustrating 20, as most linkage findings were not supported by subsequent studies 11. Problems associated with these early linkage studies were manifold: limited sample sizes, sparse genetic maps, and the use of the standard parametric logarithm of the odds (LOD) score method; in this method, originally designed for Mendelian disorders with monogenic inheritance, parameters such as mode of inheritance, penetrance, or the clinically unaffected status of probands’ relatives need to be specified, which is not possible for complex phenotypes.
With the advent of larger, multi-center studies, the availability of denser maps, and the use of non-parametric linkage algorithms (e.g., affected sib pair design), many of these early problems could be alleviated. Half a decade ago, we argued that large-scale linkage studies would in the end succeed in gene-identification, or at least serve as the starting point for systematic molecular genetic research in BD 11. Since then, however, even the largest studies or meta-analyses have left us with a void. Some consistencies of linkage findings hold up in large meta-analyses (e.g. for chromosomes 6q and 8q 21), but most of the reported linkages on virtually every chromosome remain isolated findings. The impact of this lack of replicability on our thinking is significant. For instance, does it imply that the linkage findings are false? If so, linkage analysis as a tool to pinpoint susceptibility genes might be considered a thing of the past. The genetic (or locus) heterogeneity of BD may be so high that we are not able to dissect it by means of linkage analysis (alone), even with very large sample sizes. Nevertheless, incorporating the information gained from linkage studies into future analyses, as well as continuing to collect exact phenotype data of families might still be very valuable and should not be dismissed 22.
As with linkage analysis, positional cloning efforts by means of candidate-gene association mapping have not lived up to their promise. Although several studies, particularly systematic linkage disequilibrium (LD) mapping in linkage regions, have identified potential susceptibility genes for BD 3, 7–9, these findings have been difficult to replicate. While replication at the gene level has been reached for some of these genes, replications at the allelic level—i.e. association with the identical allele of a particular single nucleotide polymorphism (SNP) across studies—are rare 23 (Table 1).
Table 1.
Potential susceptibility genes for bipolar disorder
| Gene | Symbol | Individual study or meta-analysis (MA) | Evidence |
|---|---|---|---|
| Candidate gene association studies | |||
| Serotonin Transporter | SLC6A3 | Anguelova et al. 2003 (MA) | +++ |
| D-amino acid oxidase activator (G72) | DAOA | Detera-Wadleigh & McMahon 2006 (MA) | +++ |
| Brain-derived neurotrophic factor | BDNF | Kanazawa et al. 2007 (MA); Fan & Sklar 2008 (MA) | +++ |
| Disrupted-in-schizophrenia-1 | DISC1 | Hodgkinson et al. 2004; Thomson et al. 2005 ; WTCCC 2007 ; Perlis et al. 2008 | ++ |
| Tryptophan hydroxilase 2 | TPH2 | Harvey et al. 2004 ; Van den Bogaert et al. 2006 ; Lopez et al. 2007 ; Harvey et al. 2007; Cichon et al. 2007 | ++ |
| Aryl hydrocarbon receptor nuclear translocator-like | ARNTL/CLOCK | Mansour et al. 2006 ; Nievergelt et al. 2006 | + |
| Cadherin gene (homolog of the Drosophila tumor suppressor gene fat) | FAT | Blair et al. 2006 ; Abou Jamra et al. 2008 | + |
| Genome-wide association studies | |||
| Diacylglycerol kinase eta | DGKH | Baum et al. 2008a | ++++ |
| alpha-1 subunit of a voltage-dependent calcium channel | CACNA1C | Sklar et al. 2008; Ferreira et al. 2008 | ++++ |
| Ankyrin 3 | ANK3 | Baum et al. 2008a; Ferreira et al. 2008; Smith et al. 2009; Scott et al. 2009 | ++++ |
supported by 2 studies
supported by several studies
supported by meta-analysis of 3 or more samples
genome-wide level of significance (in at least one of the studies)
Genome-wide association studies of bipolar disorder
Despite these rather disheartening experiences, researchers in the field of complex genetics entered the 21st century with great aspirations based on an ever-advancing technology that made genome-wide association studies (GWAS) a practical reality. The first two chapters of this special issue will present this method in detail. GWAS have been performed in the study of several complex disease and physiological trait phenotypes, including type I and type II diabetes 24–28, lung cancer 29–31, body-mass-index and obesity 32–35, coronary heart disease 24, 36, 37, hypertension 24, rheumatoid arthritis 24, age-related macular degeneration 38, 39, Crohn’s disease 24, 40, 41, prostate cancer 42, 43, height 44–47, and pigmentation and hair color48–50. Thus, the study of GWAS in complex disorders has successfully identified and replicated several susceptibility genes 51.
With regards to GWAS of BD, several studies have been published or are in the pipeline 24, 52–56. These have highlighted several novel susceptibility genes. Among these, the genes DGKH, CACNA1C, and ANK3 have been found at robust levels of genome-wide significance and have notably been replicated across samples (52–58; Table 1). DGKH is located within the BD linkage region on chromosome 13q14, and encodes diacylglycerol kinase eta, a key protein in the lithium-sensitive phosphatidyl inositol pathway. The CACNA1C gene encodes an alpha-1 subunit of a voltage-dependent calcium channel. The ANK3 gene encodes ankyrin-G, a large protein whose neural-specific isoforms, localized at the axonal initial segment and nodes of Ranvier, may help maintain ion channels and cell adhesion molecules.
GWAS in BD have taught us several important lessons, and these lessons can be easily generalized to genetic research into other complex psychiatric or somatic disorders:
BD is a polygenic disorder. That means that the contribution of each locus to risk of disease is modest, that cases carry significantly more risk alleles than controls, and that disease risk increases substantially with the total burden of risk alleles carried.
The best findings from GWAS do not necessarily fall within those genes that have previously been widely studied. These “usual suspects” typically included candidate genes studied on the basis of either hypothetical reasoning concerning neurotransmission or linkage findings.
Pursuing a “top-hits-only” strategy may prevent us from understanding the genetic complexity of BD and polygenic disorders in general. Stringent levels of statistical significance such as genome-wide significance are indispensible for confirming any risk gene or polymorphism identified through a GWAS. However, meta-analyses may reveal several points of agreement between independent studies and highlight genes that do not make it to the p-value-defined top of an individual study. A detailed consideration of the wider distribution of association signals across studies may thus prove to be a valuable strategy in complex genetics 59.
Allelic heterogeneity may be an important factor in complex disorders such as BD. Allelic heterogeneity means that a phenotype can be caused by different alleles within a gene; this phenomenon has been extensively observed in monogenic disorders such as cystic fibrosis 60, as well as in BRCA1/2-associated breast cancer 61. In the case of ANK3 and BD, various alleles and haplotypes appear to be independent risk factors 56, 58.
Finally, as with other complex phenotypes, GWAS in BD have brought to light the fact that the identified variants only account for a small fraction of genetic variability. This phenomenon has become known as the “case of the missing heritability” 62.
Consequences for future approaches in the genetics of BD
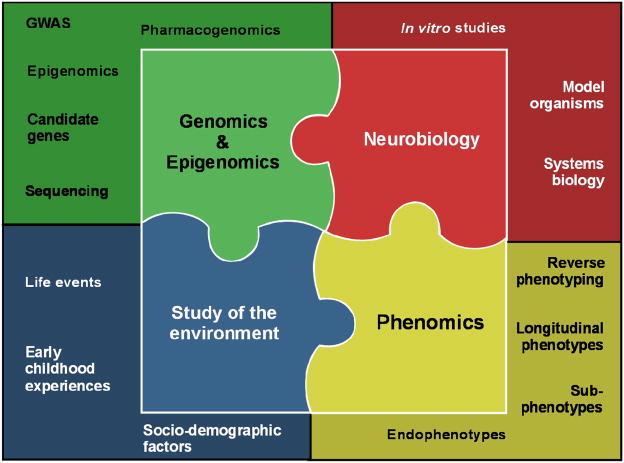
The information detailed above might lead one to believe that GWAS have failed, and that the genetics of a complex disorder such as BD may be too complex to ever be understood. However, in the scientific community there is broad consensus that GWAS results are only a starting point rather than an end point, that many more steps need to be taken in order to put the pieces together, and that GWAS need to be embedded in a framework of complementary approaches 63–65. These include studies focusing on genomic, epigenomic, phenomic, neurobiological, and environmental aspects (Figure 1).
Figure 1.
Conceptual framework for future genetic research into bipolar disorder
Genomics and epigenomics
Most of the world’s largest data collections of individuals with BD and controls have already been analyzed by GWAS, and the results published. Compared to many GWAS of non-psychiatric phenotypes, sample sizes in psychiatric GWAS—usually totaling around two to four thousand cases and controls—are still not large enough to detect risk variants with small effect sizes. Thus, the psychiatric genetic research community has embraced the idea of bringing together all available samples for joint mega- and meta-analyses through a collaborative effort known as the Psychiatric GWAS Consortium (PGC) 66. While this will most certainly help to identify further common vulnerability variants, validate existing findings, and allow the study of allelic heterogeneity and gene-gene interaction, increasing sample size is only one of many necessary steps.
Such collaborative GWAS focusing on common SNP variations (i.e., GWAS to date have typically studied HapMap-SNPs (http://www.hapmap.org/) with a minor allele frequency above 5%) will need to be complemented by studies investigating uncommon (i.e. in the 1–5% range) and rare (i.e. <1%) genetic variations. This includes the genome-wide study of copy number variants (CNVs) presenting with deletions or duplication of variably-sized segments of DNA. The study of CNVs has proven pivotal for identifying structural chromosomal variation as rare sources of genetic susceptibility to psychiatric disorders 67 such as schizophrenia 68, 69 and autism 70–72. With regards to BD, it was recently shown that singleton deletions—deletions that appear only once in the dataset of more than 100 kb in length—were present in 16.2% of BD cases in contrast to 12.3% of controls, and that this effect was more pronounced in patients with an age at onset of mania at or below 18 years of age. This suggests that BD can result from the effects of multiple rare structural variants 73.
The notion that current GWAS designs fail to assess the impact of uncommon or rare variations has led to the development of several resequencing strategies. In contrast to GWAS approaches that use SNP markers derived from HapMap and thus miss rare or idiosyncratic variations, resequencing determines an individual’s DNA sequence. Large-scale international resequencing projects such as the 1,000 Genomes Project (www.1000genomes.org) or the ClinSeq project 74 are well under way. While the latter study focuses on disease traits, it is hoped that both will contribute to the hunt for the lost heritability.
The field of pharmacogenomics may also emerge as an important part of the process of discovery in bipolar disorder, and especially the discovery of genetic variation that is relevant to the management of patients. While the high heritability of psychiatric disorders is well-known, evidence for the heritability of response or side-effect susceptibility to psychiatric medications is less well established; systematic formal genetic studies such as twin studies have not been performed 75. However, in addition to clinical observations supporting the notion that response to treatment is familial, ample justification exists for pursuing pharmacogenetic studies in psychiatry, as reviewed by Peter Zandi in this special issue. Over the last decades, progress has been made in understanding the genetics of pharmacokinetics—broadly defined as mechanisms regarding the blood and tissue concentrations of a drug. As a result, quantitative dose adjustments based on genotype can be calculated for many medications, including antidepressants and antipsychotics. In marked contrast, translation of genetic findings on pharmacodynamic phenotypes (i.e. drug effects) into psychiatric clinical practice is still not feasible 76, 77. However, recent findings on the genetics of response or susceptibility to adverse events to pharmacotherapy in depression have yielded relatively large effect sizes, illustrating that the availability of large and adequately characterized samples will be key to success in this line of research 75, 78–85.
Overall, little research has been done on the genetics of response or susceptibility to adverse events in the treatment of BD. Despite lithium’s proven efficacy, and evidence suggesting that there is a genetic basis of response to lithium treatment 86, pharmacogenetic studies in this area have so far been confined to small sample sizes and varying phenotype definitions 87. Using data from the STEP-BD study sample and collaborators from the UK, Perlis and colleagues 88 performed a GWAS on lithium response that included more than 800 lithium-treated patients. They identified multiple regions of interest but none met the threshold for genome-wide significance 88. Recently, researchers from the International Study Group of Lithium Treated Patients (IGSLI; www.igsli.org) and the National Institute of Mental Health (NIMH) spearheaded the creation of the Consortium on Lithium Genetics (ConLiGen; www.ConLiGen.org). ConLiGen is a worldwide effort to harmonize phenotype definition of response to lithium treatment and to perform GWAS of response and adverse events to lithium in adequately sized samples 89. Efforts like this will be needed to move towards a personalized medicine approach in the treatment of BD. The potential pharmacogenetic target phenotypes are plentiful: acute depressive and manic episodes, prophylaxis, characteristic acute and long-term side effects of mood stabilizers, etc.
The field of epigenomics has recently drawn increased attention, given ample evidence for the involvement of epigenetic mechanisms and of their interplay with environmental factors in the etiology of somatic disorders such as cancer 90. With regards to mental illness, postmortem studies suggest that epigenetic factors play a role in the etiology of schizophrenia and BD 91, 92. Stress-induced imbalances of histone acetylation may also be involved in the transition from acute adaptive response to chronic psychiatric illness 93.
Phenomics
To date, psychiatric genetic studies, including GWAS, have mainly focused on categorical diagnoses such as BD for phenotype definitions. While the use of diagnostic systems such as the DSM-IV has increased diagnostic reliability, categorical diagnoses are by definition artificial constructs. The psychiatric genetic community has thus embraced the idea of thorough genotype-phenotype studies. Here, investigators hope that focusing on phenotypic dissection will help decrease phenotypic—and hence genotypic—heterogeneity; this would allow the delineation of characteristic genotype-phenotype signatures 94–98. Thorough genotype-phenotype studies may also deliver clues in dissecting the phenotypic and genotypic overlap between schizophrenia and BD.
Subphenotype approaches have proven successful in elucidating the genetics of breast cancer 99 and non-syndromal deafness 100. Thus, they may prove valuable in psychiatric genetics. To avoid an insurmountable multiple testing problem, investigators should carefully choose the subphenotypes to be studied out of the plethora of phenotypic variables potentially amenable to phenotypic dissection approaches. This could be done by focusing on well-formulated hypotheses, or by concentrating on variables that show some evidence of heritability or at least familiality. As regards BD, considerable research has established the familiality of clinical variables for subphenotypic analyses. For instance, the following familial phenotypic characteristics have been identified: episode frequency, comorbid panic disorder, comorbid obsessive compulsive disorder (OCD), comorbid anorexia nervosa, comorbid substance abuse, comorbid alcoholism, psychosis, a history of suicide attempts, a history of missed work, a history of psychiatric hospitalizations, (early) age of onset, polarity of onset, puerperal trigger of onset, temperament, rapid cycling, and level of social functioning 97, 101–108. For some of these, heritability has also been established. Most of these variables are currently being used in subphenotype analyses of large GWAS datasets.
One major motivation behind these analyses is the notion that subphenotyping may help define more homogenous subgroups of the disorder, and that this increased homogeneity will improve chances of identifying susceptibility genes. Furthermore, both case-control and cases- only strategies can be pursued in these subphenotype analyses. In the case-control design, cases with (or without) a specific subphenotype are compared to control individuals. In the cases-only design, cases with a specific subphenotype are compared to cases without this subphenotype. These different strategies are believed capable of better disentangling the underlying genetic architecture, as they may help to differentiate between factors such as genetic heterogeneity (e.g., gene A may increase the risk of developing BD with psychosis while gene B may increase the risk of developing BD without psychosis) as well as modifier genes (e.g., gene A may increase risk of developing BD only in the presence of gene B, which by itself confers no risk for BD).
Reverse phenotyping, originally proposed by Schulze & McMahon (2004), is a particular form of subphenotyping 19. Here, genetic marker data are used to drive, or form, the basis of new phenotype definitions. In the case of association studies, reverse phenotyping aims to define phenotypic groupings that are distinguished by more deviant allele frequencies than are seen in traditional diagnostic categories. For instance, we demonstrated that the association between a haplotype in the gene G72 and BD in a large German and Polish sample was driven by an association with the subphenotype “history of persecutory delusions” 98. Furthermore, in the Polish sample, the association was only revealed through this approach, as the overall sample of BD patients showed no association. Thus, the subphenotype “history of persecutory delusions” helped to homogenize the samples. Reverse phenotyping has now also been successfully applied to research in non-mental illnesses 110.
As outlined below, applying phenotypic dissection approaches such as reverse phenotyping to the study of the longitudinal course of psychiatric illness might be another fruitful—though so far largely untraveled—road in psychiatric genetics. To fully understand the phenomic expression of genetic liability to disease, phenomic studies should include the study of endophenotypes, comprising neurophysiological, biochemical, endocrine, neuroanatomical, cognitive, or neuropsychological measurements. The joint consideration of endophenotypes and genetic factors may hold promise for understanding the actual mechanisms leading from genetic variation to phenotypic expression 112. The prerequisite for phenomic studies is the availability of large and adequately characterized samples, as well as sophisticated data management strategies 107, 113.
For decades, biological psychiatric research, in particular psychiatric genetics, has been based on cross-sectional datasets but has not paid much attention to a phenotype that is of utmost relevance to the clinician, the patient, and the patient’s family: the longitudinal course. In the case of schizophrenia, epidemiological studies have shown that there are characteristic subtypes of longitudinal courses in schizophrenia 114, and that these are defined by both relapse pattern and level of impairment. In BD, there is broad consensus that BD is a severe, usually lifelong, chronic condition, as suggested by longitudinal studies like the McLean-Harvard First Episode Project 115–117. According to these data, full recovery from initial episodes is uncommon. When full symptomatic recovery does happen, it occurs much more slowly than early syndromal recovery. Furthermore, initial depression or mixed states predict more depressive episodes and overall morbidity during later stages of the disorder, while initial mania or psychotic features predict more manic episodes and a better prognosis. Characteristic polymorphic patterns with mood-dominated, schizo-affective-dominated and schizo-dominated course have also been described for BD 118. Again, switching between mania and depression within one episode has been suggested to predict poor prognosis 119, and rapid cycling is associated with substantial depressive morbidity and high risk for serious suicide attempts 120. Nevertheless, data are still lacking on the course of BD across the life cycle, encompassing aspects of phenomenology, severity, and impairment 121.
In their recent review on the course and outcome of BD, Treuer & Tohen (2009) urged more research to establish better and more reliable course predictors for individual patients 122. This need is reflected by current efforts for a paradigmatic change in the upcoming DSM-V and the ICD-11; notably, measures of longitudinal course along with dimensional aspects will be core elements of the new classification systems 123. Surprisingly, integrating longitudinal aspects into future classification systems has not yet been paralleled by a similar move in the field of biological, mainly genetic research in psychiatry; rather, current efforts focus on increasing sample sizes of cross-sectional diagnoses, not establishing longitudinal cohorts. In the future, genetic studies of BD and other psychiatric disorders should target the longitudinal course of the illness as an important phenotype of interest. This, of course, will require patience as well as a trained clinician’s eye. Such efforts will have to be paralleled by the establishment of phenotypic databases such as the Bipolar Phenome Project 107, as well as by the development of mathematical algorithms that allow for the robust delineation of phenomic profiles and their genomic signatures. Finally, it is important to note that these genomic, epigenomic, and phenomic approaches outlined above need to be embedded in a framework of “neurobiological vetting”, and that environmental influences must also be modeled.
Neurobiology
Once genetic susceptibility to psychiatric disorders has been identified and, more crucially, robustly replicated, the variants in question become prime targets for focused neurobiological studies to further elucidate their relevance to the mode of action in the pathway leading up to disease presentation 124–127. Such studies should include gene-expression studies and the development and testing of animal models. Ideally, phenotype and endophenotype studies in humans (e.g. brain imaging) can be paralleled with identical approaches in animals (e.g. brain imaging in animals).
Study of environment
As noted previously, classic formal genetic studies demonstrated that heritability estimates in BD range up to 80–90%. Given this high heritability, it is easy to forget that non-genetic factors are also part of the equation describing phenotypic variance. Psychiatric geneticists have long wanted to study the complex interplay between genes and environment. The crucial question, however, has always been how best to do it. A recent, large-scale, meta-analysis of the widely-reported interaction between variation in the serotonin transporter gene and stressful life events 128 found little substance to this finding. Inconsistency of study designs, measures, and analyses proved to be main explanatory factors in this sobering but much-needed finding. Therefore, the question remains whether we will ever be able to account for environmental factors in a robust way. Plugging in retrospectively collected environmental data as covariates into an analysis may prove to be a futile endeavor. Instead, prospective studies may be key to success in this area. Furthermore, we may see a renaissance of studies targeting multiply-affected families, this time not to conduct linkage studies but rather for in-depth sequencing within the framework of longitudinal observation. Families from populations with similarly shared patterns of idiosyncratic environmental exposures (e.g. Amish or Hutterite communities in the Americas) might prove ideal for gene-environment studies.
Concluding remarks
Over the last decade, the psychiatric genetics research community has made considerable progress, and has learned much. Indeed, our most important lesson has been that the complexity of a complex disorder like BD is always a little more complex than we think. As our technological advances become progressively more sophisticated, new layers of complexity will undoubtedly arise; this is, in essence, the nature of science. That said, we should not shy away from these new challenges because we have made some headway. Our research efforts have demonstrated that BD is a truly polygenic disorder and have revealed potential mechanisms beyond those traditionally-hypothesized pathophysiological pathways, as illustrated by replicated GWAS findings of susceptibility genes involved in the architecture of calcium and sodium channels. Furthermore, we now know that we will not find the gene or the genes for BD; in fact we may have to settle for a scenario where we can only sufficiently characterize the joint effect of several hundreds or even thousands of genes on disease presentation. However, the fact that recent replicated findings only explain a fraction of phenotypic variability should serve as a stimulus to think ahead rather than give up. After all, GWAS in somatic phenotypes may have produced smaller p-values, but they do not fare better in terms of explaining. Thus, many areas of research still need to be explored. If explored systematically and in a harmonized way, they will shed more light on the genetics of disorders such as BD.
Acknowledgments
The author is greatly indebted to Francis J. McMahon for highly inspirational discussions and gratefully acknowledges the support of the Intramural Research Program of the National Institute of Mental Health. Ioline Henter provided outstanding editorial assistance. The author gratefully acknowledges Daniela Reich-Erkelenz for assistance in designing graphical presentations.
Footnotes
Disclosure: This work was supported by the Intramural Research Program of the National Institute of Mental Health. Dr. Schulze reports no conflicts of interest or other sources of funding.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baron M. Manic-depression genes and the new millennium: poised for discovery. Mol Psychiatry. 2002;7(4):342–358. doi: 10.1038/sj.mp.4000998. [DOI] [PubMed] [Google Scholar]
- 2.Berrettini WH. Molecular linkage studies of bipolar disorders. Bipolar Disord. 2001;3(6):276–283. doi: 10.1034/j.1399-5618.2001.30603.x. [DOI] [PubMed] [Google Scholar]
- 3.Craddock N, Forty L. Genetics of affective (mood) disorders. Eur J Hum Genet. 2006;14(6):660–668. doi: 10.1038/sj.ejhg.5201549. [DOI] [PubMed] [Google Scholar]
- 4.Craddock N, Jones I. Molecular genetics of bipolar disorder. Br J Psychiatry Suppl. 2001;41:s128–133. [PubMed] [Google Scholar]
- 5.Craddock N, Sklar P. Genetics of bipolar disorder: successful start to a long journey. Trends Genet. 2009;25(2):99–105. doi: 10.1016/j.tig.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Detera-Wadleigh SD, McMahon FJ. Genetic association studies in mood disorders: issues and promise. Int Rev Psychiatry. 2004;16(4):301–310. doi: 10.1080/09540260400014377. [DOI] [PubMed] [Google Scholar]
- 7.Farmer A, Elkin A, McGuffin P. The genetics of bipolar affective disorder. Curr Opin Psychiatry. 2007;20(1):8–12. doi: 10.1097/YCO.0b013e3280117722. [DOI] [PubMed] [Google Scholar]
- 8.Hayden EP, Nurnberger JI., Jr Molecular genetics of bipolar disorder. Genes Brain Behav. 2006;5(1):85–95. doi: 10.1111/j.1601-183X.2005.00138.x. [DOI] [PubMed] [Google Scholar]
- 9.Kato T. Molecular genetics of bipolar disorder and depression. Psychiatry Clin Neurosci. 2007;61(1):3–19. doi: 10.1111/j.1440-1819.2007.01604.x. [DOI] [PubMed] [Google Scholar]
- 10.Prathikanti S, McMahon FJ. Genome scans for susceptibility genes in bipolar affective disorder. Ann Med. 2001;33(4):257–262. doi: 10.3109/07853890108998754. [DOI] [PubMed] [Google Scholar]
- 11.Schulze TG, McMahon FJ. Genetic linkage and association studies in bipolar affective disorder: a time for optimism. Am J Med Genet C Semin Med Genet. 2003;123C(1):36–47. doi: 10.1002/ajmg.c.20012. [DOI] [PubMed] [Google Scholar]
- 12.Schumacher J, Cichon S, Rietschel M, Nothen MM, Propping P. Genetics of bipolar affective disorders. Current status of research for identification of susceptibility genes. Nervenarzt. 2002;73(7):581–592. doi: 10.1007/s00115-002-1304-5. quiz 593–584. [DOI] [PubMed] [Google Scholar]
- 13.Shih RA, Belmonte PL, Zandi PP. A review of the evidence from family, twin and adoption studies for a genetic contribution to adult psychiatric disorders. Int Rev Psychiatry. 2004;16(4):260–283. doi: 10.1080/09540260400014401. [DOI] [PubMed] [Google Scholar]
- 14.Sklar P. Linkage analysis in psychiatric disorders: the emerging picture. Annu Rev Genomics Hum Genet. 2002;3:371–413. doi: 10.1146/annurev.genom.3.022502.103141. [DOI] [PubMed] [Google Scholar]
- 15.Taylor L, Faraone SV, Tsuang MT. Family, twin, and adoption studies of bipolar disease. Curr Psychiatry Rep. 2002;4(2):130–133. doi: 10.1007/s11920-002-0046-1. [DOI] [PubMed] [Google Scholar]
- 16.Tsuang MT, Faraone SV. The Genetics of Mood Disorders. Baltimore: Johns Hopkins University Press; 1990. [Google Scholar]
- 17.Luxenburger H. Psychiatrisch-neurologische Zwillingspathologie. Zbl Ges Neurol Psychiat. 1930;56:145–180. [Google Scholar]
- 18.Rosanoff AJ, Handy LM, Plessett IR. The etiology of manic-depressive syndromes with special reference to their occurrence in twins. Am J Psychiatry. 1934;91:247–286. [Google Scholar]
- 19.Schulze TG, Fangerau H, Propping P. From degeneration to genetic susceptibility, from eugenics to genethics, from Bezugsziffer to LOD score: the history of psychiatric genetics. Int Rev Psychiatry. 2004;16(4):246–259. doi: 10.1080/09540260400014419. [DOI] [PubMed] [Google Scholar]
- 20.Risch N, Botstein D. A manic depressive history. Nat Genet. 1996;12(4):351–353. doi: 10.1038/ng0496-351. [DOI] [PubMed] [Google Scholar]
- 21.McQueen MB, Devlin B, Faraone SV, et al. Combined analysis from eleven linkage studies of bipolar disorder provides strong evidence of susceptibility loci on chromosomes 6q and 8q. Am J Hum Genet. 2005;77(4):582–595. doi: 10.1086/491603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clerget-Darpoux F, Elston RC. Are linkage analysis and the collection of family data dead? Prospects for family studies in the age of genome-wide association. Hum Hered. 2007;64(2):91–96. doi: 10.1159/000101960. [DOI] [PubMed] [Google Scholar]
- 23.Detera-Wadleigh SD, McMahon FJ. G72/G30 in schizophrenia and bipolar disorder: review and meta-analysis. Biol Psychiatry. 2006;60(2):106–114. doi: 10.1016/j.biopsych.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saxena R, Voight BF, Lyssenko V, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316(5829):1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 26.Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316(5829):1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sladek R, Rocheleau G, Rung J, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445(7130):881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 28.Zeggini E, Scott LJ, Saxena R, et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet. 2008;40(5):638–645. doi: 10.1038/ng.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amos CI, Wu X, Broderick P, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40(5):616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hung RJ, McKay JD, Gaborieau V, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452(7187):633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- 31.Thorgeirsson TE, Geller F, Sulem P, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452(7187):638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loos RJ, Lindgren CM, Li S, et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet. 2008;40(6):768–775. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thorleifsson G, Walters GB, Gudbjartsson DF, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009;41(1):18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- 35.Willer CJ, Speliotes EK, Loos RJ, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41(1):25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helgadottir A, Thorleifsson G, Manolescu A, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316(5830):1491–1493. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- 37.McPherson R, Pertsemlidis A, Kavaslar N, et al. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316(5830):1488–1491. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maller J, George S, Purcell S, et al. Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nat Genet. 2006;38(9):1055–1059. doi: 10.1038/ng1873. [DOI] [PubMed] [Google Scholar]
- 40.Hampe J, Franke A, Rosenstiel P, et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39(2):207–211. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- 41.Rioux JD, Xavier RJ, Taylor KD, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39(5):596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gudmundsson J, Sulem P, Manolescu A, et al. Genome-wide association study identifies a second prostate cancer susceptibility variant at 8q24. Nat Genet. 2007;39(5):631–637. doi: 10.1038/ng1999. [DOI] [PubMed] [Google Scholar]
- 43.Yeager M, Orr N, Hayes RB, et al. Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat Genet. 2007;39(5):645–649. doi: 10.1038/ng2022. [DOI] [PubMed] [Google Scholar]
- 44.Lettre G, Jackson AU, Gieger C, et al. Identification of ten loci associated with height highlights new biological pathways in human growth. Nat Genet. 2008;40(5):584–591. doi: 10.1038/ng.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanna S, Jackson AU, Nagaraja R, et al. Common variants in the GDF5-UQCC region are associated with variation in human height. Nat Genet. 2008;40(2):198–203. doi: 10.1038/ng.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weedon MN, Lango H, Lindgren CM, et al. Genome-wide association analysis identifies 20 loci that influence adult height. Nat Genet. 2008;40(5):575–583. doi: 10.1038/ng.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weedon MN, Lettre G, Freathy RM, et al. A common variant of HMGA2 is associated with adult and childhood height in the general population. Nat Genet. 2007;39(10):1245–1250. doi: 10.1038/ng2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han J, Kraft P, Nan H, et al. A genome-wide association study identifies novel alleles associated with hair color and skin pigmentation. PLoS Genet. 2008;4(5):e1000074. doi: 10.1371/journal.pgen.1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sulem P, Gudbjartsson DF, Stacey SN, et al. Two newly identified genetic determinants of pigmentation in Europeans. Nat Genet. 2008;40(7):835–837. doi: 10.1038/ng.160. [DOI] [PubMed] [Google Scholar]
- 50.Sulem P, Gudbjartsson DF, Stacey SN, et al. Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat Genet. 2007;39(12):1443–1452. doi: 10.1038/ng.2007.13. [DOI] [PubMed] [Google Scholar]
- 51.Lango H, Weedon MN. What will whole genome searches for susceptibility genes for common complex disease offer to clinical practice? J Intern Med. 2008;263(1):16–27. doi: 10.1111/j.1365-2796.2007.01895.x. [DOI] [PubMed] [Google Scholar]
- 52.Baum AE, Akula N, Cabanero M, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry. 2008;13(2):197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferreira MA, O’Donovan MC, Meng YA, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40(9):1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scott LJ, Muglia P, Kong XQ, et al. Genome-wide association and meta-analysis of bipolar disorder in individuals of European ancestry. Proc Natl Acad Sci U S A. 2009;106(18):7501–7506. doi: 10.1073/pnas.0813386106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sklar P, Smoller JW, Fan J, et al. Whole-genome association study of bipolar disorder. Mol Psychiatry. 2008;13(6):558–569. doi: 10.1038/sj.mp.4002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith EN, Bloss CS, Badner JA, et al. Genome-wide association study of bipolar disorder in European American and African American individuals. Mol Psychiatry. 2009;14(8):755–763. doi: 10.1038/mp.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ollila HM, Soronen P, Silander K, et al. Findings from bipolar disorder genome-wide association studies replicate in a Finnish bipolar family-cohort. Mol Psychiatry. 2009;14(4):351–353. doi: 10.1038/mp.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schulze TG, Detera-Wadleigh SD, Akula N, et al. Two variants in Ankyrin 3 (ANK3) are independent genetic risk factors for bipolar disorder. Mol Psychiatry. 2009;14(5):487–491. doi: 10.1038/mp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baum AE, Hamshere M, Green E, et al. Meta-analysis of two genome-wide association studies of bipolar disorder reveals important points of agreement. Mol Psychiatry. 2008;13(5):466–467. doi: 10.1038/mp.2008.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009;373(9678):1891–1904. doi: 10.1016/S0140-6736(09)60327-5. [DOI] [PubMed] [Google Scholar]
- 61.Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4(9):665–676. doi: 10.1038/nrc1431. [DOI] [PubMed] [Google Scholar]
- 62.Maher B. Personal genomes: The case of the missing heritability. Nature. 2008;456(7218):18–21. doi: 10.1038/456018a. [DOI] [PubMed] [Google Scholar]
- 63.Cordell HJ. Genome-wide association studies: Detecting gene-gene interactions that underlie human diseases. Nat Rev Genet. 2009 doi: 10.1038/nrg2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ioannidis JP, Thomas G, Daly MJ. Validating, augmenting and refining genome-wide association signals. Nat Rev Genet. 2009;10(5):318–329. doi: 10.1038/nrg2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCarthy MI, Abecasis GR, Cardon LR, et al. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat Rev Genet. 2008;9(5):356–369. doi: 10.1038/nrg2344. [DOI] [PubMed] [Google Scholar]
- 66.Psychiatric GWAS Consortium Steering Committee. A framework for interpreting genome-wide association studies of psychiatric disorders. Mol Psychiatry. 2009;14(1):10–17. doi: 10.1038/mp.2008.126. [DOI] [PubMed] [Google Scholar]
- 67.Cook EH, Jr, Scherer SW. Copy-number variations associated with neuropsychiatric conditions. Nature. 2008;455(7215):919–923. doi: 10.1038/nature07458. [DOI] [PubMed] [Google Scholar]
- 68.Stefansson H, Rujescu D, Cichon S, et al. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455(7210):232–236. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walsh T, McClellan JM, McCarthy SE, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320(5875):539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- 70.Mefford HC, Sharp AJ, Baker C, et al. Recurrent rearrangements of chromosome 1q21.1 and variable pediatric phenotypes. N Engl J Med. 2008;359(16):1685–1699. doi: 10.1056/NEJMoa0805384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sebat J, Lakshmi B, Malhotra D, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316(5823):445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weiss LA, Shen Y, Korn JM, et al. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358(7):667–675. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- 73.Zhang D, Cheng L, Qian Y, et al. Singleton deletions throughout the genome increase risk of bipolar disorder. Mol Psychiatry. 2009;14(4):376–380. doi: 10.1038/mp.2008.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Biesecker L, Mullikin JC, Facio F, et al. The ClinSeq Project: Piloting large-scale genome sequencing for research in genomic medicine. Genome Res. 2009;19(9):1665–74. doi: 10.1101/gr.092841.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perlis RH. Pharmacogenetic studies of antidepressant response: how far from the clinic? Psychiatr Clin North Am. 2007;30(1):125–138. doi: 10.1016/j.psc.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 76.Kirchheiner J, Fuhr U, Brockmoller J. Pharmacogenetics-based therapeutic recommendations--ready for clinical practice? Nat Rev Drug Discov. 2005;4(8):639–647. doi: 10.1038/nrd1801. [DOI] [PubMed] [Google Scholar]
- 77.Kirchheiner J, Nickchen K, Bauer M, et al. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry. 2004;9(5):442–473. doi: 10.1038/sj.mp.4001494. [DOI] [PubMed] [Google Scholar]
- 78.Binder EB, Salyakina D, Lichtner P, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36(12):1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- 79.Hu XZ, Rush AJ, Charney D, et al. Association between a functional serotonin transporter promoter polymorphism and citalopram treatment in adult outpatients with major depression. Arch Gen Psychiatry. 2007;64(7):783–792. doi: 10.1001/archpsyc.64.7.783. [DOI] [PubMed] [Google Scholar]
- 80.Laje G, Paddock S, Manji H, et al. Genetic markers of suicidal ideation emerging during citalopram treatment of major depression. Am J Psychiatry. 2007;164(10):1530–1538. doi: 10.1176/appi.ajp.2007.06122018. [DOI] [PubMed] [Google Scholar]
- 81.Lekman M, Laje G, Charney D, et al. The FKBP5-gene in depression and treatment response--an association study in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Cohort. Biol Psychiatry. 2008;63(12):1103–1110. doi: 10.1016/j.biopsych.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McMahon FJ, Buervenich S, Charney D, et al. Variation in the gene encoding the serotonin 2A receptor is associated with outcome of antidepressant treatment. Am J Hum Genet. 2006;78(5):804–814. doi: 10.1086/503820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paddock S, Laje G, Charney D, et al. Association of GRIK4 with outcome of antidepressant treatment in the STAR*D cohort. Am J Psychiatry. 2007;164(8):1181–1188. doi: 10.1176/appi.ajp.2007.06111790. [DOI] [PubMed] [Google Scholar]
- 84.Perlis RH, Moorjani P, Fagerness J, et al. Pharmacogenetic analysis of genes implicated in rodent models of antidepressant response: association of TREK1 and treatment resistance in the STAR(*)D study. Neuropsychopharmacology. 2008;33(12):2810–2819. doi: 10.1038/npp.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Uher R, Huezo-Diaz P, Perroud N, et al. Genetic predictors of response to antidepressants in the GENDEP project. Pharmacogenomics J. 2009;9(4):225–233. doi: 10.1038/tpj.2009.12. [DOI] [PubMed] [Google Scholar]
- 86.Grof P, Duffy A, Cavazzoni P, et al. Is response to prophylactic lithium a familial trait? J Clin Psychiatry. 2002;63(10):942–947. doi: 10.4088/jcp.v63n1013. [DOI] [PubMed] [Google Scholar]
- 87.Mamdani F, Groisman IJ, Alda M, Turecki G. Pharmacogenetics and bipolar disorder. Pharmacogenomics J. 2004;4(3):161–170. doi: 10.1038/sj.tpj.6500245. [DOI] [PubMed] [Google Scholar]
- 88.Perlis RH, Smoller JW, Ferreira MA, et al. A genomewide association study of response to lithium for prevention of recurrence in bipolar disorder. Am J Psychiatry. 2009;166(6):718–725. doi: 10.1176/appi.ajp.2009.08111633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schulze TG, Alda M, Adli M, et al. The international Consortium on Lithium Genetics (ConLiGen)--an initiative by the NIMH and IGSLI to study the genetic basis of response to lithium treatment. Neuropsychobiology. doi: 10.1159/000314708. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358(11):1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 91.Connor CM, Akbarian S. DNA methylation changes in schizophrenia and bipolar disorder. Epigenetics. 2008;3(2):55–58. doi: 10.4161/epi.3.2.5938. [DOI] [PubMed] [Google Scholar]
- 92.Sharma RP, Grayson DR, Gavin DP. Histone deactylase 1 expression is increased in the prefrontal cortex of schizophrenia subjects: analysis of the National Brain Databank microarray collection. Schizophr Res. 2008;98(1–3):111–117. doi: 10.1016/j.schres.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8(5):355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 94.Craddock N, Owen MJ. Rethinking psychosis: the disadvantages of a dichotomous classification now outweigh the advantages. World Psychiatry. 2007;6(2):84–91. [PMC free article] [PubMed] [Google Scholar]
- 95.Potash JB. Carving chaos: genetics and the classification of mood and psychotic syndromes. Harv Rev Psychiatry. 2006;14(2):47–63. doi: 10.1080/10673220600655780. [DOI] [PubMed] [Google Scholar]
- 96.Rietschel M, Beckmann L, Strohmaier J, et al. G72 and its association with major depression and neuroticism in large population-based groups from Germany. Am J Psychiatry. 2008;165(6):753–762. doi: 10.1176/appi.ajp.2008.07060883. [DOI] [PubMed] [Google Scholar]
- 97.Schulze TG, Hedeker D, Zandi P, Rietschel M, McMahon FJ. What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Arch Gen Psychiatry. 2006;63(12):1368–1376. doi: 10.1001/archpsyc.63.12.1368. [DOI] [PubMed] [Google Scholar]
- 98.Schulze TG, Ohlraun S, Czerski PM, et al. Genotype-phenotype studies in bipolar disorder showing association between the DAOA/G30 locus and persecutory delusions: a first step toward a molecular genetic classification of psychiatric phenotypes. Am J Psychiatry. 2005;162(11):2101–2108. doi: 10.1176/appi.ajp.162.11.2101. [DOI] [PubMed] [Google Scholar]
- 99.Wooster R, Neuhausen SL, Mangion J, et al. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12–13. Science. 1994;265(5181):2088–2090. doi: 10.1126/science.8091231. [DOI] [PubMed] [Google Scholar]
- 100.Tekin M, Arnos KS, Pandya A. Advances in hereditary deafness. Lancet. 2001;358(9287):1082–1090. doi: 10.1016/S0140-6736(01)06186-4. [DOI] [PubMed] [Google Scholar]
- 101.Evans L, Akiskal HS, Keck PE, Jr, et al. Familiality of temperament in bipolar disorder: support for a genetic spectrum. J Affect Disord. 2005;85(1–2):153–168. doi: 10.1016/j.jad.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 102.Fisfalen ME, Schulze TG, DePaulo JR, Jr, DeGroot LJ, Badner JA, McMahon FJ. Familial variation in episode frequency in bipolar affective disorder. Am J Psychiatry. 2005;162(7):1266–1272. doi: 10.1176/appi.ajp.162.7.1266. [DOI] [PubMed] [Google Scholar]
- 103.Jones I, Craddock N. Familiality of the puerperal trigger in bipolar disorder: results of a family study. Am J Psychiatry. 2001;158(6):913–917. doi: 10.1176/appi.ajp.158.6.913. [DOI] [PubMed] [Google Scholar]
- 104.Kassem L, Lopez V, Hedeker D, Steele J, Zandi P, McMahon FJ. Familiality of polarity at illness onset in bipolar affective disorder. Am J Psychiatry. 2006;163(10):1754–1759. doi: 10.1176/ajp.2006.163.10.1754. [DOI] [PubMed] [Google Scholar]
- 105.MacKinnon DF, Zandi PP, Cooper J, et al. Comorbid bipolar disorder and panic disorder in families with a high prevalence of bipolar disorder. Am J Psychiatry. 2002;159(1):30–35. doi: 10.1176/appi.ajp.159.1.30. [DOI] [PubMed] [Google Scholar]
- 106.O’Mahony E, Corvin A, O’Connell R, et al. Sibling pairs with affective disorders: resemblance of demographic and clinical features. Psychol Med. 2002;32(1):55–61. doi: 10.1017/s0033291701004986. [DOI] [PubMed] [Google Scholar]
- 107.Potash JB, Toolan J, Steele J, et al. The bipolar disorder phenome database: a resource for genetic studies. Am J Psychiatry. 2007;164(8):1229–1237. doi: 10.1176/appi.ajp.2007.06122045. [DOI] [PubMed] [Google Scholar]
- 108.Saunders EH, Scott LJ, McInnis MG, Burmeister M. Familiality and diagnostic patterns of subphenotypes in the National Institutes of Mental Health bipolar sample. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(1):18–26. doi: 10.1002/ajmg.b.30558. [DOI] [PubMed] [Google Scholar]
- 109.Schulze TG, McMahon FJ. Defining the phenotype in human genetic studies: forward genetics and reverse phenotyping. Hum Hered. 2004;58(34):131–8. doi: 10.1159/000083539. [DOI] [PubMed] [Google Scholar]
- 110.Iannuzzi MC, Baughman RP. Reverse phenotyping in sarcoidosis. Am J Respir Crit Care Med. 2007;175(1):4–5. doi: 10.1164/rccm.200610-1459ED. [DOI] [PubMed] [Google Scholar]
- 111.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 112.Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7(10):818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- 113.Fangerau H, Ohlraun S, Granath RO, Nothen MM, Rietschel M, Schulze TG. Computer-assisted phenotype characterization for genetic research in psychiatry. Hum Hered. 2004;58(3–4):122–130. doi: 10.1159/000083538. [DOI] [PubMed] [Google Scholar]
- 114.Watt DC, Katz K, Shepherd M. The natural history of schizophrenia: a 5-year prospective follow-up of a representative sample of schizophrenics by means of a standardized clinical and social assessment. Psychol Med. 1983;13(3):663–670. doi: 10.1017/s0033291700048091. [DOI] [PubMed] [Google Scholar]
- 115.Baca-Garcia E, Perez-Rodriguez MM, Basurte-Villamor I, et al. Diagnostic stability and evolution of bipolar disorder in clinical practice: a prospective cohort study. Acta Psychiatr Scand. 2007;115(6):473–480. doi: 10.1111/j.1600-0447.2006.00984.x. [DOI] [PubMed] [Google Scholar]
- 116.Salvatore P, Baldessarini RJ, Tohen M, et al. McLean-Harvard International First-Episode Project: two-year stability of DSM-IV diagnoses in 500 first-episode psychotic disorder patients. J Clin Psychiatry. 2009;70(4):458–466. doi: 10.4088/jcp.08m04227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Salvatore P, Khalsa HM, Hennen J, et al. Psychopathology factors in first-episode affective and non-affective psychotic disorders. J Psychiatr Res. 2007;41(9):724–736. doi: 10.1016/j.jpsychires.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 118.Marneros A, Roettig S, Roettig D, Tscharntke A, Brieger P. The longitudinal polymorphism of bipolar I disorders and its theoretical implications. J Affect Disord. 2008;107(1–3):117–126. doi: 10.1016/j.jad.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 119.Turvey CL, Coryell WH, Solomon DA, et al. Long-term prognosis of bipolar I disorder. Acta Psychiatr Scand. 1999;99(2):110–119. doi: 10.1111/j.1600-0447.1999.tb07208.x. [DOI] [PubMed] [Google Scholar]
- 120.Coryell W, Solomon D, Turvey C, et al. The long-term course of rapid-cycling bipolar disorder. Arch Gen Psychiatry. 2003;60(9):914–920. doi: 10.1001/archpsyc.60.9.914. [DOI] [PubMed] [Google Scholar]
- 121.Goodwin GM, Anderson I, Arango C, et al. ECNP consensus meeting. Bipolar depression. Nice, March 2007. Eur Neuropsychopharmacol. 2008;18(7):535–549. doi: 10.1016/j.euroneuro.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 122.Treuer T, Tohen M. Course and outcome of bipolar disorder -- focusing on depressive aspects. In: Zarate CA, Manji H, editors. Bipolar Depression: Molecular Neurobiology, Clinical Diagnosis and Pharmacotherapy. Basel, Switzerland: Birkhauser; 2009. pp. 29–46. [Google Scholar]
- 123.Regier DA, Narrow WE, Kuhl EA, Kupfer DJ. The conceptual development of DSM-V. Am J Psychiatry. 2009;166:645–650. doi: 10.1176/appi.ajp.2009.09020279. [DOI] [PubMed] [Google Scholar]
- 124.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9(6):437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Newberg AR, Catapano LA, Zarate CA, Manji HK. Neurobiology of bipolar disorder. Expert Rev Neurother. 2008;8(1):93–110. doi: 10.1586/14737175.8.1.93. [DOI] [PubMed] [Google Scholar]
- 126.Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52(1):139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 127.Sawamura N, Sawa A. Disrupted-in-schizophrenia-1 (DISC1): a key susceptibility factor for major mental illnesses. Ann N Y Acad Sci. 2006;1086:126–133. doi: 10.1196/annals.1377.018. [DOI] [PubMed] [Google Scholar]
- 128.Risch N, Herrell R, Lehner T, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301(23):2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]