Abstract
Strong statistical associations between soil transmitted helminths and schistosomes are frequently observed in co-endemic human populations, although the underlying explanations remain poorly understood. This study investigates the contribution of host genetics and domestic environment to hookworm and Schistosoma mansoni infection intensity and evaluates the role of genetic and non-genetic factors in covariation of infection intensity. Detailed genealogical information allowed assignment of 1,303 individuals living in the Brazilian community of Americaninhas, Minas Gerais state, to 25 pedigrees (containing between two and 1,159 members) residing in 303 households. The prevalence of co-infection with both hookworms and schistosomes was high (38.5%), with significant correlation between Necator americanus and S. mansoni faecal egg counts (r = 0.242; P < 0.0001). Bivariate variance component analysis demonstrated a modest but significant species-specific heritability for intensity of N. americanus (h2 = 0.196) and S. mansoni infection (h2 = 0.230). However, after accounting for demographic, socio-economic and household risk factors, no evidence for common genetic control of intensity of hookworm and schistosome infection was observed (genetic correlation ρG = 0.15; P = 0.52). There was some evidence for residual clustering within households (household correlation ρC = 0.45; P = 0.06), but the majority (63%) of the covariance between N. americanus and S. mansoni infection intensity remained specific to the individual and could not be explained by shared genes, shared environment or other shared demographic, socio-economic or environmental risk factors. Our results emphasize the importance of exposure to hookworm and schistosome infection in driving the association between levels of infection with these species in hosts resident in areas of high transmission and suggest that much of this common exposure occurs outside the home.
Keywords: Variance component analysis, Heritability, Schistosoma mansoni, Necator americanus, Co-infection
1. Introduction
Numerous epidemiological studies indicate that individuals infected with multiple helminths, particularly soil-transmitted helminths (STH; especially hookworms and Ascaris lumbricoides) and schistosomes, harbour heavier worm burdens for each species than those infected with a single species (Booth et al., 1998; Needham et al., 1998; Brooker et al., 2000; Tchuem Tchuente et al., 2003; Fleming et al., 2006). Presence (and intensity) of co-infection may result from environmental and/or behavioural factors of the human host; for example, factors specific to the peri-domiciliary environment (Holland et al., 1988; Bethony et al., 2001; Raso et al., 2005; Hotez, 2007) or to family-specific behaviours (Bethony et al., 2001; Clennon et al., 2004). Higher levels of infection in co-infected individuals may alternatively be explained by host immunological or physiological factors (Fulford et al., 1998), phenotypes which in turn may be influenced by host genetics (Bethony et al., 1999), nutrition (Bundy and Golden, 1987) or the parasites themselves (Pearce and MacDonald, 2002; Loukas et al., 2005). Although evidence from quantitative genetic studies and genome scans of humans living in helminth endemic areas suggests a genetic basis for intensity of helminth infection (reviewed by Quinnell, 2003; Bethony and Quinnell, 2008), few studies have investigated whether phenotypic correlation in intensity of helminth infections can be attributed to common host genetic influences in humans. A recent quantitative genetic analysis of individuals infected with multiple helminths in rural Jiangxi Province, China, suggested that, whilst there was some evidence that multiple helminth infections aggregated within families, the risk of multiple infection for an individual was largely environmentally influenced (Ellis et al., 2007).
In a previous study, we explored the role of exposure-related factors in defining the epidemiological and spatial patterns of co-infection with the hookworm Necator americanus and Schistosoma mansoni in a rural Brazilian community (Pullan et al., 2008). While this study revealed that a limited number of household and environmental factors explained much of the spatial variability in the presence of co-infection, the majority (66%) of between-household variation in the occurrence of co-infection could not be explained by these factors, pointing to the need to evaluate the involvement of additional behavioural and genetic elements. On this basis we applied a multiple outcome approach to separate the effects of host genetic factors, common domestic environmental effects and residual individual variation to evaluate the evidence for shared genetic and non-genetic control of covariation in N. americanus and S. mansoni infection intensity. The extension of univariate quantitative genetic analysis to investigate multivariate outcomes has been described in detail (Hopper and Matthews, 1982; Lange and Boehnke, 1983; Carey, 1988), but this is the first time, to our knowledge, that it has been applied in the context of parasitic diseases in humans.
2. Materials and methods
2.1. Study area, population and recruitment
The study was conducted in Americaninhas, a region in the municipality of Nova Oriente, in northeastern Minas Gerais state, Brazil in 2004. Details of the study area, recruitment and enrolment procedures, as well as cross-sectional parasitological and survey data have been provided elsewhere (Brooker et al., 2006; Fleming et al., 2006; Brooker et al., 2007b), with only a summary provided here. Briefly, the study was designed as a total population survey, with the research team visiting all households in a 10 km2 area to obtain informed consent using a written and verbal consent form approved by the ethical committee of the Centro de Pesquisas René Rachou-FIOCRUZ, the Brazilian National Committee for Ethics in Research (CONEP), George Washington University Medical Center (USA), and the London School of Hygiene and Tropical Medicine (UK). Each house was assigned a unique household identification number (HHID), and each resident a unique personal identifier (PID).
2.2. Mapping, household questionnaire and parasitological survey
All households in the study area were geo-referenced and information on household socio-economic and physical characteristics was collected using a pre-tested, standardized household questionnaire. Remotely sensed environmental data were extracted for May 2001 from the Advanced Spaceborne Thermal Emission and Reflection Radiometer (ASTER) satellite sensor at 30 m spatial resolution. ASTER provides information on Normalized Difference Vegetation Index (NDVI), a proxy of vegetation density and soil moisture, and digital elevation (Tatem et al., 2004). During the parasitological survey, stool samples were collected over the course of 2 days. Those confirmed as egg-positive for any helminth species using formalin-ether sedimentation were subsequently examined by Kato–Katz faecal thick smear to quantify the intensity of the infection as eggs per gram of faeces (epg). Two slides were taken from each day’s faecal sample for a total of up to four slides from each individual. Individuals positive by formalin-ether sedimentation but negative by Kato-Katz were assigned an egg count of 3 epg, half the Kato-Katz detection limit. Hookworm was confirmed as exclusively N. americanus by both morphological examination and PCR (Pullan et al., 2008).
2.3. Pedigree collection and identification
To establish kinship relationships within and between households, questionnaires were administered to either the head male or female of the household to elicit genealogical and demographic information on each household member, including their age, sex and names of parents. Relationships between all household members were recorded, and records were also made of any other first- or second-degree relatives living in other households in the study area to establish genetic ties between households. Individuals were then defined as belonging to the same extended pedigree if they were related to anyone else in the pedigree or were married to anyone in the pedigree. Pedigrees were assembled using PEDSYS (Dyke, 1999), and the number of relative pairs calculated using the ‘kinship’ function. Doubtful pedigree relationships were confirmed by re-interviewing relevant heads of households. The 1,303 sampled individuals who provided pedigree and household information and at least one phenotype could be assembled into one large multi-generational pedigree comprising 1,159 people, and 24 smaller pedigrees of two to 11 phenotyped individuals each; 36 individuals had no phenotyped relatives in the study area. There were 303 households, with one to 12 (mean 4.3) phenotyped individuals per household.
2.4. Data management and preliminary statistical analysis
The outcomes used in this analysis were intensity of infection with N. americanus and S. mansoni. The methods used assume the phenotype to be normally distributed; therefore, because of the skewed distribution of infection intensities (faecal egg counts), individual infection intensities were assessed after log-transformation as ln(epg+1). Analysis was not undertaken for A. lumbricoides because egg counts were truncated at 500 eggs per slide (12,000 epg).
Preliminary investigation of covariates was performed using Stata 9.1 (STATA Corporation, Houston, TX, USA). All available demographic (age, sex), household (toilet facilities, household crowding, flooring material, relative socio-economic status) and environmental (sector, NDVI, altitude and population and household density) covariates were included in a full fixed effect regression model, and non-significant (P > 0.1) variables removed sequentially to derive a minimally adequate model. Standard errors were adjusted for dependence between individuals within households using a clustered sandwich estimator. Visual examination of scatter-plots was used to investigate non-linear relationships. Covariates were analysed separately for each infection. Subsequently, the retained covariates were built into bivariate mixed linear regression models outlined below (genetic variance component analysis (VCA)).
2.5. Genetic VCA
Quantitative genetic analyses were conducted by means of a maximum likelihood–based variance decomposition approach implemented in the computer package SOLAR 4.2.0 (Almasy and Blangero, 1998). In brief, this approach incorporates information on genetic relationships within and between households in order to partition the total variation in infection intensity into its genetic, household and other causes. The a priori expectation for the covariance between any two individuals is determined by the degree of relatedness between them (equal to twice the kinship coefficient (φ), which represents a genome-averaged probability that two individuals will share an allele identical by descent (Hopper and Matthews, 1982)). Incorporation of a “kinship coefficient matrix” into a random effect model therefore allows the covariance between relatives to depend upon the degree of relatedness between them, providing an estimate of the total cumulative effect of individual genes (the “additive genetic” effect) on phenotypic variation. The relative contribution of fixed covariates to total variation in infection intensity (total phenotypic variance) was estimated by comparing the trait S.D. in models with and without fixed covariates.
For each infection, four initial models were investigated. The ‘sporadic’ model attributes variation in faecal egg excretion entirely to a random, individual-specific effect (e2). The ‘household’ model introduces an additional household-level random effect (c2), allowing estimation of the effect of unmeasured factors associated with the common domestic environment, whilst the ‘polygenic’ model introduces a random effect describing additive genetic variation (h2 heritability) by incorporating a “relationship matrix”. Finally, in the ‘saturated’ model, all of these effects are modelled and estimated together. The variance parameters were standardised by dividing by the total phenotypic variation (i.e. the proportion of total variance attributable to each parameter was estimated). Chi-squared testing based on likelihood ratios was then used to test the significance of variance parameters.
Bivariate variance components were fitted to estimate the role of additive genetics and shared household in the covariance between two traits (e.g. ln(epg+1) of hookworm infection and ln(epg+1) of schistosome infection). By this approach, the variance-covariance matrix is partitioned into additive genetic, shared-household and random, individual-specific effects, providing information on the pair-wise covariance between traits. The variance-covariance matrices for these bivariate outcome models provided estimates of three additional parameters: the additive genetic correlation (ρG), the shared-household correlation (ρC) and unexplained correlation (ρE) between infection intensity. Respectively, these correlations are estimates of the common additive effects of genes on the phenotypic variance of each trait, and the effects of domestic environmental factors and individual non-genetic factors common to both traits (Carey, 1988). The significance of each of the additional estimated parameters was evaluated by likelihood-ratio tests comparing the general model, in which the covariance parameter is estimated, with a restricted model in which the covariance parameter is constrained to zero (i.e. no correlation between infections for that effect). Shared genetic control is indicated by an additive genetic correlation (ρG) that is significantly different from zero.
An unbiased estimate of the total phenotypic correlation (ρP) between the two species can be calculated based on these pair-wise correlations using the following equation:
where the sub-script 1 and 2 represent variance component estimates for each trait, respectively.
3. Results
3.1. Parasitological and demographic data
In total, 1,303 individuals provided pedigree and household information and at least one parasitological phenotype (87% of the total population): intensity of hookworm infection was available for 1,294 individuals, whilst intensity of S. mansoni infection was available for 1,302. Overall, the prevalence of N. americanus was 70.9% (mean epg = 1,441), and S. mansoni was 47.2% (mean epg = 131); 38.5% of individuals were co-infected with both species. A significant positive phenotypic correlation was observed between log transformed N. americanus and S. mansoni infection intensities (r = 0.242, n = 1,293, P < 0.0001), as has been described previously (Fleming et al., 2006). Intensity of hookworm infection rose during childhood, reaching a peak between 5 and 10 years of age where it remained constant throughout adulthood; mean intensity was greater in males than females. In contrast S. mansoni infection intensity peaked between 10 and 25 years of age and declined throughout adulthood.
Findings from unadjusted linear regression models reflect our previous multinomial analysis of N. americanus – S. mansoni co-infection: N. americanus infection intensity was positively associated with characteristics associated with lower socio-economic status (low socio-economic index, household crowding) and with higher NDVI, whilst both infections showed significant associations with lack of toilet facilities. Mean hookworm infection intensity was lower in the village area (sector 1) compared with the rural sectors (sectors 3-7); whilst for S. mansoni mean infection intensity was highest in the village area.
3.2. Variance components analysis of individual helminth infection intensity
To define the contribution of hereditary and environmental influences on infection intensity a quantitative genetic variance component approach was applied. Table 1 describes the number of relative pairs included in these analyses. In total, when considering up to seven degrees of relatedness, 40,476 relative pairs were available. Four models were initially developed: sporadic, household, polygenic and saturated. Maximum-likelihood estimates of the variance components, and the corresponding model log-likelihoods, are provided in Table 2. Results for N. americanus and S. mansoni are similar. Examination of log-likelihood values reveals that infection intensity was best described by a saturated model; in the absence of covariates, heritability (h2) was 0.30 for N. americanus and 0.29 for S. mansoni, with a further 0.15 and 0.17 of variation in egg counts attributable to the domestic environment.
Table 1.
Distribution of relative pairs by degree of relationship (n = 1303)
| Relationship | Degree of relatedness |
Co-efficient of relatedness |
Number of pairs |
|---|---|---|---|
| Husband - Wife | 0 | 0 | 554 |
| Parent - offspring | 1 | 0.5 | 1,373 |
| Sibling | 1 | 0.5 | 1,281 |
| Other first degree (inc. complex relationships) | 1 | 0.5-0.563 | 211 |
| Half sibling | 2 | 0.25 | 243 |
| Grandparent | 2 | 0.25 | 885 |
| Avuncular | 2 | 0.25 | 2,292 |
| Other second degree (inc. complex relationships) | 2 | 0.25-0.375 | 638 |
| Cousin | 3 | 0.125 | 3,117 |
| Half avuncular | 3 | 0.125 | 472 |
| Grand avuncular | 3 | 0.125 | 1,354 |
| Great grand parent | 3 | 0.125 | 90 |
| Other third degree (inc. complex relationships) | 3 | 0.125-0.219 | 2,158 |
| Higher-degree relative | 4 - 7 | 0.0039-0.117 | 26,358 |
|
| |||
| Total | - | 40,476 | |
Table 2.
Variance component analysis for variation in infection intensity with Necator americanus and Schistosoma mansoni showing standardised variance parameter estimates (h2 additive genetic variation; c2 household-level variation; e2 individual-level unexplained variation) and estimated log likelihood values.
| Hypothesis | N. americanus |
S. mansoni |
||||||
|---|---|---|---|---|---|---|---|---|
| h2 | c2 | e2 | lnL | h2 | c2 | e2 | lnL | |
| No Covariates: | ||||||||
| Sporadic | 1.00 | −2174.3 | 1.00 | −1838.0 | ||||
| Shared household | 0.34 ± 0.03 | 0.66 ± 0.03 | −2076.5 | 0.35 ± 0.03 | 0.65 ± 0.03 | −1711.3 | ||
| Additive genetic | 0.48 ± 0.05 | 0.52 ± 0.05 | −2067.7 | 0.47 ± 0.04 | 0.53 ± 0.04 | −1704.1 | ||
| Saturated | 0.30 ± 0.07 | 0.15 ± 0.05 | 0.55 ± 0.04 | −2060.8 | 0.29 ± 0.06 | 0.17 ± 0.04 | 0.54 ± 0.04 | −1693.5 |
| With Covariatesa | ||||||||
| Sporadic | 1.00 | −1955.3 | 1.00 | −1655.3 | ||||
| Shared household | 0.22 ± 0.03 | 0.78 ± 0.03 | −1909.0 | 0.22 ± 0.03 | 0.78 ± 0.03 | −1610.5 | ||
| Additive genetic | 0.38 ± 0.05 | 0.62 ± 0.05 | −1906.2 | 0.37 ± 0.05 | 0.63 ± 0.05 | −1605.5 | ||
| Saturated | 0.23 ± 0.06 | 0.12 ± 0.04 | 0.65 ± 0.05 | −1900.2 | 0.24 ± 0.06 | 0.11 ± 0.04 | 0.65 ± 0.05 | −1600.8 |
Necator americanus infection adjusted for age, sex, toilet facilities, overcrowding, sector and Normalised Difference Vegetation Index (NDVI); S. mansoni infection adjusted for age (age and age2), toilet facilities and sector.
At an individual level, N. americanus intensity was modelled by a sex-dependent linear increase with age from 0-5 years, with no change in older hosts; intensity was higher in adult males than females. Other covariates included in the N. americanus model were the absence of toilet facilities, household overcrowding (> 1 people/room), relative socio-economic index (linear), NDVI and sector; the S. mansoni model included age (modelled as age and age2), absence of toilet facilities, and sector. Introduction of these demographic, socio-economic and environmental covariates led to a slight reduction in the proportion of remaining variation explained by the additive genetic (to 0.23-0.24) and shared household variance components (to 0.12 for N. americanus and 0.11 for S. mansoni). It did not however lead to different conclusions for the hypothesis tests; for both infections both additive genetic effects and household effects were highly significant (P ≤ 0.001 for χ2 tests comparing the saturated model with simpler (household or polygenic) models).
3.3. Bivariate variance components analysis of N. americanus and S. mansoni intensity
To estimate the contribution of additive genetic effects and domestic environment to the phenotypic correlation between hookworm and S. mansoni intensities we used a bivariate variance component analysis. Estimates of the covariance components are presented in Table 3. Total unbiased correlation between infection intensities (ρP) was 0.27, which reduced to 0.24 after adjusting for covariates. The relatively small influence of covariates (which explained only 11% of the correlation between these two traits) is unsurprising given that the only common explanatory factors identified were age, toilet facilities and sector. Correlation between individuals’ hookworm and S. mansoni worm burdens was unrelated to the degree of relationship between them, as indicated by the lack of evidence for covariance between the additive genetic effects for each trait (ρG = 0.147; P = 0.52). Evidence for positive correlation due to shared domestic environment was weak (ρC = 0.451; P = 0.06). However, there was strong evidence for residual (non-genetic, non-household) correlation between intensity of infection at the individual level (ρE = 0.233; P << 0.001); after adjusting for covariates an estimated 5% of residual phenotypic variance could be attributed to additional unmeasured factors common to both species (ρE2 = 0.054).
Table 3.
Correlation components for covariance between Necator americanus and Schistosoma mansoni faecal egg excretion.
| Total phenotypic correlation (ρP) | 0.24 |
|---|---|
| Additive genetic: | |
| Additive genetic correlation(ρG)(SE) | 0.147 (0.216) |
| % of total correlation (ρP) | 12.8% |
| P b | 0.52 |
| Shared-household: | |
| Household correlation(ρC)(SE) | 0.451 (0.201) |
| % of total correlation (ρP) | 24.5% |
| P b | 0.06 |
| Non-genetic individual: | |
| Unexplained correlation(ρE)(SE) | 0.233 (0.048) |
| % of total correlation (ρP) | 62.6% |
| P b | <<0.001 |
Necator americanus infection adjusted for age, sex, toilet facilities, overcrowding, sector and Normalised Difference Vegetation Index (NDVI); S. mansoni infection adjusted for age (age and age2), toilet facilities and sector.
P value for likelihood ratio test comparing general model with restricted model in which correlation for this effect is zero.
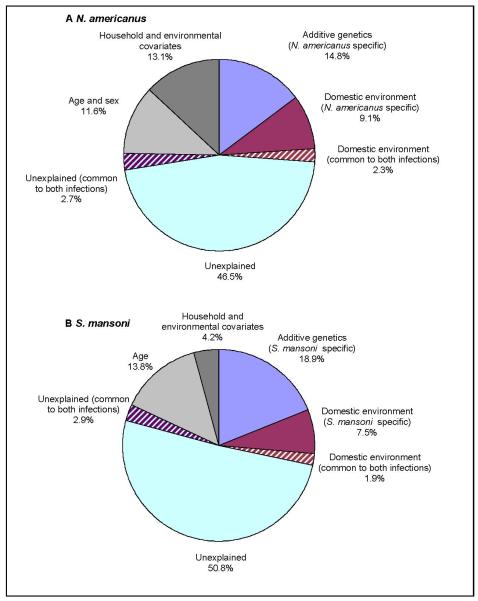
The estimates of all fixed effects and variance parameters are shown in Table 4, and resulting estimates of shared and species-specific sources of variation are shown in Fig. 1. Heritability was comparable to that estimated from univariate models; for N. americanus h2 = 0.196, for S. mansoni h2 = 0.230. A strong age dependency was observed for intensity of infection for both helminths; for the intensity of hookworm infection, this effect was greater in males than females. Together, age and sex explained 11.6% of total variation of hookworm burden and 13.8% of S. mansoni burden. Socio-economic and geographical environmental covariates associated with the household explained substantially more of the total variation for hookworm infection intensity than for S. mansoni infection intensity (a further 13.1% and 4.2%, respectively), with a further 9.9-11.4% attributable to other factors associated with the domestic environment. When expressed as a proportion of total, rather than residual, variation the contribution of host genetics to the variation in faecal egg excretion was 14.8% for hookworm and 18.9% for schistosomiasis. A remaining 46.5-50.8% of the heterogeneity in faecal egg counts was not explained by any of the factors included in this model.
Table 4.
Covariate and variance estimates from bivariate quantitative genetic analysis of Necator americanus and Schistosoma mansoni infection intensity expressed as ln(epg+1).
|
N. americanus ln[epg+1] |
S. mansoni ln[epg+1] | |||
|---|---|---|---|---|
| Covariate coefficients (SE): | ||||
| Demography | ||||
| Age a | 1.04 | (0.09) | 0.075 | (0.009) |
| Age2 | −0.001 | (0.0001) | ||
| Sex (female vs male) | −0.49 | (0.14) | ||
| Sex × age interaction | −0.27 | (0.13) | ||
| Household characteristics | ||||
| Toilet (present vs absent) | −0.66 | (0.32) | −0.44 | (0.21) |
| Crowding b (yes vs no) | 0.50 | (0.22) | ||
| SES index c | −0.23 | (0.09) | ||
| Location characteristics | ||||
| Remotely sensed data: | ||||
| NDVI c | 2.41 | (0.75) | ||
| Sector (vs. sector 1): | ||||
| Sector 3 | 1.50 | (0.37) | 0.71 | (0.26) |
| Sector 4 | 1.26 | (0.46) | −1.82 | (0.34) |
| Sector 5 | −0.90 | (0.56) | −2.41 | (0.42) |
| Sector 6 | 1.49 | (0.63) | −1.82 | (0.48) |
| Sector 7 | 0.23 | (0.41) | −1.63 | (0.30) |
|
Variance components as proportions of residual variation (SE) after controlling
for covariates: | ||||
| Additive genetic | 0.196 | (0.065) | 0.230 | (0.065) |
| Shared domestic environment | 0.151 | (0.043) | 0.116 | (0.042) |
| Individual-specific | 0.653 | (0.047) | 0.654 | (0.047) |
For N. americanus, age was modelled as a linear increase up to 5 years old; for S. mansoni age was modelled as a quadratic.
A crowded household is defined as having < 1 room/person
Socio-economic (SES) index and Normalised Difference Vegetation Index (NDVI) are modelled a continuous variables.
epg, eggs per gram of faeces; SE, standard error.
Fig. 1.
Results of bivariate quantitative genetic analysis of infection intensity for Necator americanus and Schistosoma mansoni, adjusting for covariates. Total phenotypic variance in (A) N. americanus and (B) S. mansoni infection intensity is partitioned into that due to covariates, species-specific additive genetic effects, domestic environmental effects (species-specific and those common to both species) and unexplained, individual-specific effects (species-specific and those common to both species).
4. Discussion
In this analysis, we build upon recent literature exploring infection intensity for human helminth infection. By modelling the intensity of N. americanus and S. mansoni infection as a bivariate outcome, we were able to assess host genetic and non-genetic influences unique to each infection and common to both. As frequently observed in co-endemic communities, there was a strong positive phenotypic correlation between the intensity of infection for these two species (ρp = 0.24 when adjusting for covariates). However, when modelled in the presence of shared domestic environment, no evidence was found for common genetic control of infection intensity, although there was weak evidence of a household contribution to the correlation between egg counts for these species within individual hosts (ρC = 0.45; P = 0.06). Importantly, the majority (63%) of the covariance between N. americanus and S. mansoni infection intensity remained specific to the individual and could not be explained by host genetics, the domestic environment or known demographic, socio-economic and environmental risk factors.
Our conclusions are based on the results of a bivariate genetic variance components analysis. This method has been used extensively to model relationships between biological phenotypes (Edwards et al., 1999; Hokanson et al., 2003; Njajou et al., 2006; Lichtenstein et al., 2009), although we believe this is the first time it has been applied in the context of human parasitic helminth infection. We demonstrate a modest but significant heritability for intensity of N. americanus (h2 = 0.196) and S. mansoni infection (h2 = 0.230) in hosts from an endemic area. Heritability estimates are population-specific, depending on the magnitude of both genetic and environmental variability; here, heritability is derived from a single large pedigree, which may result in lower genetic and/or environmental variance. However, our estimates are consistent with previous studies establishing a role for host genetics in determining infection burden for the major human STH (Williams-Blangero et al., 1997, 1999, 2002a; Breitling et al., 2008) and schistosome infections (Bethony et al., 2002; King et al., 2004; Seto et al., 2005; Ellis et al., 2006). Importantly, our results suggest that there is no, or very little, shared genetic control of N. americanus and S. mansoni infection intensity in this population, with a low and non-significant genetic correlation of 0.147.
Strong positive genetic correlations have been previously demonstrated between Trichuris suis and Ascaris suum burdens in experimentally infected pigs, and between Haemonchus contortus and Trichostrongylus colubriformis in sheep, suggesting that resistance to intestinal nematodes of different species may involve regulation by overlapping sets of genes (Gruner et al., 2004; Nejsum et al., 2009). The lack of a positive genetic correlation in our study may reflect greater biological differences between hookworms and schistosomes (including substantially different transmission cycles, locations within the host and interactions with the host immune system). However, it should be noted that assessing evidence for biological pleiotropy from statistical correlations can be difficult (Carey, 1988). For example, dominance and epistatic effects that differ across traits, linkage disequilibrium, gene-environment interactions and simple pedigree errors can all serve to decrease (or increase) the estimate of additive genetic correlation (Carey, 1988). Identification of the genes involved in determining intensity of infection for each species will be needed to confirm whether there are is any common genetic control. Whilst some loci controlling S. mansoni (Marquet et al., 1996) A. lumbricoides (Williams-Blangero et al., 2002b) and Trichuris trichiura (Williams-Blangero et al., 2008) infection have been mapped, to our knowledge there have been no such studies carried out for either of the hookworm species, an area warranting further investigation.
After accounting for socio-economic, environmental and household covariates, further unmeasured factors associated with the domestic environment accounted for up to 25% of the correlation in intensity of infection between species in this community. This finding is in keeping with a previous observation that occurrences of co-infections with Schistosoma japonicum – T. trichiura or with A. lumbricoides – T. trichiura were aggregated within households, but not within families, in a rural Chinese population (Ellis et al., 2007), and suggests that, despite differences in the mode of transmission between schistosomes and STHs, common exposures shared by members of the same household may play an important role in transmission of both species. These observations emphasise that improvements in sanitation and health awareness within the home, as well as targeted treatment of those living in poor conditions, will impact significantly on the transmission of multiple helminth species.
Importantly however, most of the phenotypic correlation between these two species could be explained by neither the domestic environment nor host genetics, suggesting that common exposures outside the home may be driving the relationship. Although (as mentioned above) the ecology and transmission of S. mansoni and hookworm differ considerably, occupation, hygiene behaviours, health knowledge and water-contact patterns may all potentially influence exposure to both infections (Watts et al., 1998; Bethony et al., 2004; Matthys et al., 2007). For example, a recent analysis of risk factors for S. mansoni and hookworm in urban farming communities in western Côte d’Ivoire revealed that both infections were related to certain agricultural practices, including contact with irrigation wells that may provide hiding places for open defecation (Matthys et al., 2007). Similarly, water contact was a significant risk factor for both S. japonicum, T. trichiura and A. lumbricoides in the Poyang Lake region in China (Ellis et al., 2007). Interestingly, there was very little role for individual-level factors in determining predisposition to hookworm infection in this Brazilian community, suggesting that environmental determinants of co-infection may be variable through time (Quinnell et al., in press). Alternatively, the unexplained correlation between N. americanus and S. mansoni infection intensity may reflect a direct biological or immunological interaction between species. Schistosoma mansoni infection influences immune responses to other helminth infections in mice (Curry et al., 1995; Yoshida et al., 1999), but whether helminth-induced immuno-suppression can influence the intensity of concomitant infection in humans requires further study (for review see Geiger, 2008).
As for single outcome genetic VCA, shared household is only a proxy measure for the influence of domestic environmental factors on phenotype. Given that environmental conditions may be common to multiple households within a community, it is important that potential sources of heterogeneity in environmental exposure to infection be measured and included as covariates. To our knowledge this is the first study of the genetic epidemiology of human helminth infection to include detailed information on socio-economic indicators, physical environment and spatial location (including remotely sensed environmental data) allowing greater quantification of sources of heterogeneities in exposure in this community. Despite these included covariates, nearly half of the total variation in infection intensity remained unexplained. Much of this may be due to measurement error, which is known to be large for faecal egg counts, even when (as in the current study) several slides were counted on separate days (Yu et al., 1998). Moreover, faecal egg counts represent an indirect measure of underlying worm burden; it is likely that density-dependent fecundity and possibly other host factors (including host genetics) may influence this association (Anderson and Schad, 1985; Agnew et al., 1996). Given the location in which the adult worms lay their eggs (the gut lumen versus mesenteric blood vessels) comparability between these two proxies of infection intensity may also be limited. As a consequence, studies such as ours that rely on faecal egg count for the quantitative assessment of the intensity of infection risk underestimating the genetic and non-genetic components (and correlations) for these infections. Unexplained variation in helminth infection intensity may also result from genetic heterogeneity in the parasite population. Molecular studies have revealed allelic and nucleotide diversity in the genomes of human helminth parasite populations (Hawdon et al., 2001; Curtis et al., 2002; Stohler et al., 2005), and further study of the relative importance of parasite genetic diversity in driving patterns of heterogeneity within human populations is warranted.
We believe this study represents the first analysis of the intensity of co-infection with S. mansoni and N. americanus to quantify the relative importance of host genetic and household factors in the commonly observed correlation in infection levels for these two helminth species. Our results emphasise the importance of environmental factors in driving this association and suggest that whilst around a quarter of correlation can be explained by factors associated with the domestic environment, much of this common exposure occurs outside the home. Conflicting opinions on the nature of interactions between concomitant infections, particularly in relation to interactions between helminths and malaria, have highlighted the need for robust approaches to studying co-infection (Nacher, 2004; Booth, 2006; Mwangi et al., 2006; Brooker et al 2007a). Numerous studies support the view that, for the majority of infectious diseases, host susceptibility is likely to be polygenic, with the immunomodulatory effects of certain genetic polymorphisms influencing several diseases (Frodsham and Hill, 2004). However, it still remains unclear whether the associations frequently observed between parasitic infections are dictated by an individual’s genotype, by direct synergistic or antagonistic interactions between species, or whether they simply reflect common patterns of exposure. By providing insight into the patterns of genetic and non-genetic relationships between traits, bivariate genetic VCA provides us with a valuable tool to help unravel the complex interplay between these infectious diseases.
Acknowledgements
We are very grateful to inhabitants of Americaninhas who kindly participated in the study, to the many members of the Centro de Pesquisas René Rachou staff for their technical assistance, and to Lutz Breitling (University of Leeds) for assembling the pedigrees. We are also most appreciative of those who were responsible for carrying out the fieldwork, which made this analysis possible. Fieldwork was financially supported by the Human Hookworm Vaccine Initiative (HHVI) of the Sabin Institute, which receives support from the Bill and Melinda Gates Foundation. RP is supported by a Medical Research Council DTA-funded studentship and SB is supported by a Career Development Fellowship (081673) from the Wellcome Trust. JB is supported by grants from the Sabin Institute, the Bill and Melinda Gates Foundation and NIAID/NIH.
References
- Agnew A, Fulford A, Mwanje MT, Gachuhi K, Gutsmann V, Kriger FW, Sturrock RF, Vennervald BJ, Ouma JH, Butterworth AE, Deelder AM. Age-dependent reduction of schistosome fecundity in Schistosoma haematobium but not Schistosoma mansoni infections in humans. Am. J. Trop. Med. Hyg. 1996;55:338–343. doi: 10.4269/ajtmh.1996.55.338. [DOI] [PubMed] [Google Scholar]
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am. J. Hum. Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Schad GA. Hookworm burdens and faecal egg counts: an analysis of the biological basis of variation. Trans. R. Soc. Trop. Med. Hyg. 1985;79:812–825. doi: 10.1016/0035-9203(85)90128-2. [DOI] [PubMed] [Google Scholar]
- Bethony J, Silveira AM, Alves-Oliveira LF, Thakur A, Gazzinelli G, Correa-Oliveira R, LoVerde PT. Familial resemblance in humoral immune response to defined and crude Schistosoma mansoni antigens in an endemic area in Brazil. J. Infect. Dis. 1999;180:1665–1673. doi: 10.1086/315069. [DOI] [PubMed] [Google Scholar]
- Bethony J, Williams JT, Kloos H, Blangero J, Alves-Fraga L, Buck G, Michalek A, Williams-Blangero S, Loverde PT, Correa-Oliveira R, Gazzinelli A. Exposure to Schistosoma mansoni infection in a rural area in Brazil. II: Household risk factors. Trop. Med. Int. Health. 2001;6:136–145. doi: 10.1046/j.1365-3156.2001.00685.x. [DOI] [PubMed] [Google Scholar]
- Bethony J, Williams JT, Blangero J, Kloos H, Gazzinelli A, Soares-Filho B, Coelho L, Alves-Fraga L, Williams-Blangero S, Loverde PT, Correa-Oliveira R. Additive host genetic factors influence fecal egg excretion rates during Schistosoma mansoni infection in a rural area in Brazil. Am. J. Trop. Med. Hyg. 2002;67:336–343. doi: 10.4269/ajtmh.2002.67.336. [DOI] [PubMed] [Google Scholar]
- Bethony J, Williams JT, Brooker S, Gazzinelli A, Gazzinelli MF, LoVerde PT, Correa-Oliveira R, Kloos H. Exposure to Schistosoma mansoni infection in a rural area in Brazil. Part III: household aggregation of water-contact behaviour. Trop. Med. Int. Health. 2004;9:381–389. doi: 10.1111/j.1365-3156.2004.01203.x. [DOI] [PubMed] [Google Scholar]
- Bethony JM, Quinnell RJ. Genetic epidemiology of human schistosomiasis in Brazil. Acta Trop. 2008;108:166–174. doi: 10.1016/j.actatropica.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Booth M, Bundy DA, Albonico M, Chwaya HM, Alawi KS, Savioli L. Associations among multiple geohelminth species infections in schoolchildren from Pemba Island. Parasitology. 1998;116:85–93. doi: 10.1017/s003118209700190x. [DOI] [PubMed] [Google Scholar]
- Booth M. The role of residential location in apparent helminth and malaria associations. Trends Parasitol. 2006;22:359–362. doi: 10.1016/j.pt.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Breitling LP, Wilson AJ, Raiko A, Lagog M, Siba M, Shaw MA, Quinnell RJ. Heritability of human hookworm infection in Papua New Guinea. Parasitology. 2008;135:1407–1415. doi: 10.1017/S0031182008004976. [DOI] [PubMed] [Google Scholar]
- Brooker S, Miguel EA, Moulin S, Luoba AI, Bundy DA, Kremer M. Epidemiology of single and multiple species of helminth infections among school children in Busia District, Kenya. East African Medicine Journal. 2000;77:157–161. doi: 10.4314/eamj.v77i3.46613. [DOI] [PubMed] [Google Scholar]
- Brooker S, Alexander N, Geiger S, Moyeed RA, Stander J, Fleming F, Hotez PJ, Correa-Oliveira R, Bethony J. Contrasting patterns in the small-scale heterogeneity of human helminth infections in urban and rural environments in Brazil. Int. J. Parasitol. 2006;36:1143–1151. doi: 10.1016/j.ijpara.2006.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker S, Akhwale WS, Pullan R, Estambale B, Clarke S, Snow RW, Hotez PJ. Epidemiology of Plasmodium-Helminth coinfection in Africa: potential impact on anaemia and prospects for combining control. Am. J. Trop. Med. Hyg. 2007a;77:88–98. [PMC free article] [PubMed] [Google Scholar]
- Brooker S, Jardim-Botelho A, Quinnell RJ, Geiger SM, Caldas IR, Fleming F, Hotez PJ, Correa-Oliveira R, Rodrigues LC, Bethony JM. Age-related changes in hookworm infection, anaemia and iron deficiency in an area of high Necator americanus hookworm transmission in southeastern Brazil. Trans. R. Soc. Trop. Med. Hyg. 2007b;101:146–154. doi: 10.1016/j.trstmh.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Bundy DAP, Golden MH. The impact of host nutrition on gastrointestinal helminth populations. Parasitology. 1987;95:623–635. doi: 10.1017/s0031182000058042. [DOI] [PubMed] [Google Scholar]
- Carey G. Inference about genetic correlations. Behav. Genet. 1988;18:329–338. doi: 10.1007/BF01260933. [DOI] [PubMed] [Google Scholar]
- Clennon JA, King CH, Muchiri EM, Karuiki HC, Ouma JH, Mungai P, Kitron U. Spatial patterns of urinary schistosomiasis infection in a highly endemic area of coastal Kenya. Am. J. Trop. Med. Hyg. 2004;70:443–448. [PubMed] [Google Scholar]
- Curry AJ, Else KJ, Jones F, Bancroft A, Grencis RK, Dunne DW. Evidence that cytokine-mediated immune interactions induced by Schistosoma mansoni alter disease outcome in mice concurrently infected wtih Trichuris muris. J. Exp. Med. 1995;181:769–774. doi: 10.1084/jem.181.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis J, Sorensen RE, Minchella DJ. Schistosome genetic diversity : the implications of population structure as detected with microsatellite markers. Parasitology. 2002;125:S51–S59. doi: 10.1017/s0031182002002020. [DOI] [PubMed] [Google Scholar]
- Dyke B. PEDSYS: A Pedigree Data Management System. Southwest Foundation for Biomedical Research; San Antonio, TX: 1999. [Google Scholar]
- Edwards KL, Mahaney MC, Motulsky AG, Austin MA. Pleiotropic genetic effects on LDL size, plasma triglyceride and HDL cholesterol in families. Arterioscler. Thromb. Vasc. Biol. 1999;19:2456–2464. doi: 10.1161/01.atv.19.10.2456. [DOI] [PubMed] [Google Scholar]
- Ellis MK, Li Y, Rong Z, Chen H, McManus DP. Familial aggregation of human infection with Schistosoma japonicum in the Poyang Lake region, China. Int. J. Parasitol. 2006;36:71–77. doi: 10.1016/j.ijpara.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis MK, Raso G, Li Y-S, Rong Z, Chen H-G, McManus DP. Familial aggregation of human susceptibility to co- and multiple helminth infections in a population from the Poyang Lake region, China. Int J Parasitol. 2007;37:1153–61. doi: 10.1016/j.ijpara.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming FM, Brooker S, Geiger SM, Caldas IR, Correa-Oliveira R, Hotez PJ, Bethony JM. Synergistic associations between hookworm and other helminth species in a rural community in Brazil. Trop. Med. Int. Health. 2006;11:56–64. doi: 10.1111/j.1365-3156.2005.01541.x. [DOI] [PubMed] [Google Scholar]
- Frodsham AJ, Hill AVS. Genetics of infectious diseases. Hum. Mol. Genet. 2004;13:R187–R194. doi: 10.1093/hmg/ddh225. [DOI] [PubMed] [Google Scholar]
- Fulford AJ, Webster M, Ouma JH, Kimani G, Dunne DW. Puberty and age-related changes in susceptibility to schistosome infection. Parasitol. Today. 1998;14:23–26. doi: 10.1016/s0169-4758(97)01168-x. [DOI] [PubMed] [Google Scholar]
- Geiger S. Immuno-epidemiology of Schistosoma mansoni infections in endemic populations co-infected with soil-transmitted helminths: present knowledge, challenges, and the need for further studies. Acta Trop. 2008;108:118–123. doi: 10.1016/j.actatropica.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Gruner L, Bouix J, Brunel JC. High genetic correlation between resistance to Haemonchus contortus and to Trichostrongylus colubriformis in INRA 401 sheep. Vet. Parasitol. 2004;119:51–58. doi: 10.1016/j.vetpar.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Hawdon JM, Li T, Zhan B, Blouin MS. Genetic structure of populations of the human hookworm, Necator americanus, in China. Mol. Ecol. 2001;10:1433–1437. doi: 10.1046/j.1365-294x.2001.01296.x. [DOI] [PubMed] [Google Scholar]
- Hokanson JE, Langefeld CD, Mitchell BD, Lange LA, Goff DCJ, Haffner SM, Saad MF, Rotter JI. Pleiotropy and heterogeneity in the expression of atherogenic lipoproteins: the IRAS Family Study. Hum. Hered. 2003;55:46–50. doi: 10.1159/000071809. [DOI] [PubMed] [Google Scholar]
- Holland CV, Taren DL, Crompton DW, Nesheim MC, Sanjur D, Barbeau I, Tucker K, Tiffany J, Rivera G. Intestinal helminthiases in relation to the socioeconomic environment of Panamanian children. Soc. Sci. Med. 1988;26:209–213. doi: 10.1016/0277-9536(88)90241-9. [DOI] [PubMed] [Google Scholar]
- Hopper JL, Matthews JD. Extensions to multivariate normal models for pedigree analysis. Ann. Hum. Genet. 1982;46:373–383. doi: 10.1111/j.1469-1809.1982.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Hotez PJ. Hookworm and poverty. Ann. New York Acad. Sci. 2007;1136:38–44. doi: 10.1196/annals.1425.000. [DOI] [PubMed] [Google Scholar]
- King CH, Blanton RE, Muchiri EM, Ouma JH, Kariuki HC, Mungai P, Magak P, Kadzo H, Ireri E, Koech DK. Low heritable component of risk for infection intensity and infection-associated disease in urinary schistosomiasis among Wadigo village populations in Coast Province, Kenya. Am. J. Trop. Med. Hyg. 2004;70:57–62. [PubMed] [Google Scholar]
- Lange K, Boehnke M. Extensions to pedigree analysis, IV: covariance components models for multivariate traits. Am. J. Med. Genet. 1983;14:513–524. doi: 10.1002/ajmg.1320140315. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukas A, Constant SL, Bethony JM. Immunobiology of hookworm infection. FEMS Immunol. Med. Microbiol. 2005;43:115–124. doi: 10.1016/j.femsim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Marquet S, Abel L, Hillaire D, Dessein H, Kalil J, Feingold J, Weissenbach J, Dessein AJ. Genetic localization of a locus controlling the intensity of infection by Schistosoma mansoni on chromosome 5q31-q33. Nat. Genet. 1996;14:181–184. doi: 10.1038/ng1096-181. [DOI] [PubMed] [Google Scholar]
- Matthys B, Tschannen AB, Tian-Bi NT, Comoé H, Diabaté S, Traoré M, Vounatsou P, Raso G, Gosoniu L, Tanner M, Cissé G, N’Goran EK, Utzinger J. Risk factors for Schistosoma mansoni and hookworm in urban farming communities in western Côte d’Ivoire. Trop. Med. Int. Health. 2007;12:709–723. doi: 10.1111/j.1365-3156.2007.01841.x. [DOI] [PubMed] [Google Scholar]
- Mwangi TW, Bethony JM, Brooker S. Malaria and helminth interactions in humans: an epidemiological viewpoint. Ann. Trop. Med. Parasitol. 2006;100:551–570. doi: 10.1179/136485906X118468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacher M. Interactions between worm infections and malaria. Clin. Rev. Allergy Immunol. 2004;26:85–92. doi: 10.1007/s12016-004-0003-3. [DOI] [PubMed] [Google Scholar]
- Needham C, Kim HT, Hoa NV, Cong LD, Michael E, Drake L, Hall A, Bundy DA. Epidemiology of soil-transmitted nematode infections in Ha Nam Province, Vietnam. Trop. Med. Int. Health. 1998;3:904–912. doi: 10.1046/j.1365-3156.1998.00324.x. [DOI] [PubMed] [Google Scholar]
- Nejsum P, Roepstorff A, Jorgensen CB, Fredholm M, Goring HHH, Anderson TJC, Thamsborg SM. High heritability for Ascaris and Trichuris infection levels in pigs. Heredity. 2009;102:357–364. doi: 10.1038/hdy.2008.131. [DOI] [PubMed] [Google Scholar]
- Njajou OT, Alizadeh BZ, Aulchenko Y, Zillikens MC, Pols HA, Oostra BA, Swinkels DW, van Duijn CM. Heritability of serum iron, ferritin and transferrin saturation in a genetically isolated population, the Erasmus Rucphen Family (ERF) Study. Hum. Hered. 2006;61:222–228. doi: 10.1159/000094777. [DOI] [PubMed] [Google Scholar]
- Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- Pullan R, Bethony J, Geiger S, Cundill B, Correa-Oliveira R, Quinnell RJ, Brooker S. Human helminth co-infection: an analysis of spatial heterogeneity and household and environmental risk factors in a Brazilian community. PLoS Negl. Trop. Dis. 2008;2:e352. doi: 10.1371/journal.pntd.0000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinnell RJ. Genetics of susceptibility to human helminth infection. Int. J. Parasitol. 2003;33:1219–1231. doi: 10.1016/s0020-7519(03)00175-9. [DOI] [PubMed] [Google Scholar]
- Quinnell RJ, Pullan RL, Breitling L.Ph., Geiger S, Cundill B, Correa-Oliveira R, Brooker S, Bethony JM. Genetic and household determinants of predisposition to human hookworm infection in a Brazilian community. J. Infect. Dis. doi: 10.1086/655813. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raso G, Utzinger J, Silue KD, Ouattara A, Yapi A, Toty A, Matthys B, Vounatsou P, Tanner M, N’Goran E,K. Disparities in parasitic infections, perceived ill health and access to health care among poorer and less poor schoolchildren of rural Côte d’Ivoire. Trop. Med. Int. Health. 2005;10:42–57. doi: 10.1111/j.1365-3156.2004.01352.x. [DOI] [PubMed] [Google Scholar]
- Seto EY, Zhong B, Kouch J, Hubbard A, Spear RC. Genetic and household risk factors for Schistosoma japonicum infection in the presence of larger scale environmental differences in the mountainous transmission areas of China. Am. J. Trop. Med. Hyg. 2005;73:1145–1150. [PubMed] [Google Scholar]
- Stohler RA, Curtis J, Minchella DJ. A comparison of microsatellite polymorphism and heterozygosity among field and laboratory populations of Schistosoma mansoni. Int. J. Parasitol. 2004;34:595–601. doi: 10.1016/j.ijpara.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Tatem AJ, Goetz SJ, Hay SI. Terra and Aqua: new data for epidemiology and public health. Int. J. App. Earth Obs. and Geo. 2004;6:33–46. doi: 10.1016/j.jag.2004.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchuem Tchuente LA, Behnke JM, Gilbert FS, Southgate VR, Vercruysse J. Polyparasitism with Schistosoma haematobium and soil-transmitted helminth infections among school children in Loum, Cameroon. Trop. Med. Int. Health. 2003;8:975–986. doi: 10.1046/j.1360-2276.2003.01120.x. [DOI] [PubMed] [Google Scholar]
- Watts S, Khallaayoune K, Bensefia R, Laamrani H, Gryseels B. The study of human behavior and schistosomiasis transmission in an irrigated area in Morocco. Soc. Sci. Med. 1998;46:755–765. doi: 10.1016/s0277-9536(97)00171-8. [DOI] [PubMed] [Google Scholar]
- Williams-Blangero S, Blangero J, Bradley M. Quantitative genetic analysis of susceptibility to hookworm infection in a population from rural Zimbabwe. Hum. Biol. 1997;69:201–208. [PubMed] [Google Scholar]
- Williams-Blangero S, Subedi J, Upadhayay RP, Manral DB, Rai DR, Jha B, Robinson ES, Blangero J. Genetic analysis of susceptibility to infection with Ascaris lumbricoides. Am. J. Trop. Med. Hyg. 1999;60:921–926. doi: 10.4269/ajtmh.1999.60.921. [DOI] [PubMed] [Google Scholar]
- Williams-Blangero S, McGarvey ST, Subedi J, Wiest PM, Upadhayay RP, Rai DR, Jha B, Olds GR, Guanling W, Blangero J. Genetic component to susceptibility to Trichuris trichiura: evidence from two Asian populations. Genet. Epidemiol. 2002a;22:254–264. doi: 10.1002/gepi.0187. [DOI] [PubMed] [Google Scholar]
- Williams-Blangero S, VandeBerg JL, Subedi J, Aivaliotis MJ, Rai DR, Upadhayay RP, Jha B, Blangero J. Genes on chromosomes 1 and 13 have significant effects on Ascaris infection. Proc. Natl. Acad. Sci. USA. 2002b;99:5533–5538. doi: 10.1073/pnas.082115999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Blangero S, Vandeberg JL, Subedi J, Jha B, Dyer TD, Blangero J. Two quantitative trait loci influence whipworm (Trichuris trichiura) infection in a Nepalese population. J. Infect. Dis. 2008;197:1198–1203. doi: 10.1086/533493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Maruyama H, Yabu Y, Amano T, Kobayakawa T, Ohta N. Immune response against protozoal and nematodal infection in mice with underlying Schistosoma mansoni infection. Parasitol. Int. 1999;48:73–79. doi: 10.1016/s1383-5769(99)00006-9. [DOI] [PubMed] [Google Scholar]
- Yu JM, De Vlas SJ, Yuan HC, Gryseels B. Variations in fecal Schistosoma japonicum egg counts. Am. J. Trop. Med. Hyg. 1998;59:370–375. doi: 10.4269/ajtmh.1998.59.370. [DOI] [PubMed] [Google Scholar]