Abstract
Objective:
The administration of the growth hormone (GH) secretagogue GH-releasing peptide (GHRP)-2, like ghrelin, increases food intake (FI) in lean healthy men. The aim of this study was to investigate whether this effect occurs in obese subjects and whether it is dose-dependent.
Research Methods and Procedures:
Nineteen subjects (10 lean and nine obese), all healthy and weight stable, received a double-blind randomized subcutaneous infusion of GHRP-2 at high dose (HD; 1 μg/kg per hour), low dose (0.1 μg/kg per hour), or placebo for 270 minutes over three study visits. Blood for hormone assays was collected through an intravenous forearm catheter. Hunger and fullness were rated on visual analog scales before and after a fixed breakfast (320 kcal at 120 minutes) and a buffet lunch at 240 minutes. Before lunch, subjects received taped instructions to eat as much as they wanted.
Results:
GHRP-2 infusion significantly increased ad libitum FI in a dose-dependent manner by 10.2 ± 3.9% at low dose (p = 0.011) and by 33.5 ± 5.8% at HD (p = 0.000) compared with placebo. Obesity status did not influence the effect of GHRP-2 on FI. All subjects had greater ratings of appetite before but similar levels of fullness after the meal with the HD GHRP-2. Serum GH levels increased dose dependently in all subjects.
Discussion:
The dual stimulatory effect of GHRP-2 on FI and human GH is dose dependent. Obese individuals retain their ability to respond to GHRP-2 both in terms of FI and human GH.
Keywords: ghrelin, growth hormone-releasing peptide-2, food intake, growth hormone, cortisol
Introduction
Ghrelin, the endogenous ligand for the growth hormone (GH)1 secretagogue (GHS) receptor, which was cloned in 1996 (1), is secreted by the stomach and circulates in the blood at measurable concentrations (2). Ghrelin regulates GH release (3) and plays a role in the regulation of food intake (FI) and energy balance. Ghrelin also stimulates adrenocorticotropin and cortisol (3). Central or peripheral administration of ghrelin increases FI in rodents (4,5). With repeated daily administration for a week, ghrelin maintains its stimulatory effect on FI; as a result, rodents increase their body fat (4,5).
Peripheral ghrelin administration has also been shown to stimulate FI in humans, not only in healthy lean men and women (6), but also in patients with cancer (7) or on peritoneal dialysis (8). Recently, ghrelin was shown to retain its stimulatory effect on FI in obese individuals (9). We have shown recently that the administration of GH-releasing peptide (GHRP)-2 stimulates FI by 36% in healthy lean men (10).
GHRP-2 (DAlaDβNalAlaTrpDPheLysNH2), a synthetic GHS receptor agonist, belongs to a family of GHSs discovered in the 1980s and extensively studied for their effect on GH release (11). Despite the very different chemistry of natural ghrelin and synthetic GHSs, evidence strongly and increasingly supports that they have the same biological actions (12). Like ghrelin, GHSs increase FI (13) and body weight (14,15) in rodents. Appetite ratings increased in children with idiopathic GH deficiency treated chronically with oral GHRP-2 (12). The magnitude of the effect of GHRP-2 on FI in humans (10) is similar to that of ghrelin (6).
In parallel with the data using exogenous administration of ghrelin (6) or GHRP-2 (10), there are data suggesting that circulating ghrelin could be implicated in meal-to-meal regulation. Ghrelin levels increase in anticipation of a meal (16) and are suppressed by food ingestion (17).
The mechanisms of these acute meal-related changes on serum ghrelin levels are multiple. The presence of nutrients in the gut (18,19), the calorie (20) or the carbohydrate content of the meal (21-23), vagal stimulation (24-26), the insulin response to the meal (27,28), or other post-gastric (pre- or post-absorptive) stimulation (28) all have been shown to play a role in meal-to-meal ghrelin regulation. However, the role of the increase of insulin after a meal remains controversial (17,29,30).
Serum ghrelin levels vary as a function of energy balance. Elevated ghrelin levels in patients with anorexia (31-35) decrease with weight restoration (31). The lower levels of circulating ghrelin observed in obese individuals (36-39) increase with diet-induced weight loss (40-42). Obese individuals have less suppression of circulating ghrelin levels postprandially (43) than lean individuals (44). Whether the dysregulation of the ghrelin system plays a part in the pathogenesis of the energy imbalance of obesity is in question.
Thus, ghrelin could be an important player not only in meal-to-meal FI behavior but also in chronic over- and undernutrition (45,46). Because of its dual effects on human GH (hGH) and on energy balance, ghrelin may be a critical hormonal signal of nutritional status to the somatotropic axis, playing a role in integrating energy balance with the growth process (45).
To better understand the role of ghrelin on FI in humans, in particular whether obese individuals, who have low ghrelin levels, could respond to the exogenous administration of ghrelin, we investigated the effect of GHRP-2 at two different doses on ad libitum FI in lean and obese individuals.
Research Methods and Procedures
Study Subjects
A total of 19 subjects were studied, 10 lean (seven men and three women) and nine obese (all women). Subject characteristics are shown in Table 1. All subjects were weight stable, healthy, not on medications, non-smoking, non-dieting, and without any eating disorder. A screening session included a full physical exam, routine laboratory tests, a taste test, and a test meal. Subjects were asked to rate their liking of the food with a score of 5 or more (on a scale of 0 to 7) and were asked to eat at least 2090 kJ (500 kcal) during the screening test meal. No side effects were reported during the GHRP-2 infusions. Fat mass was measured by anthropometrics (47).
Table 1.
Subject characteristics with serum fasting levels
| Lean | Obese | p | |
|---|---|---|---|
| Age (years) | 25.1 ± 1.2 | 26.6 ± 11.5 | 0.433 |
| Weight (kg) | 74.6 ± 2.2 | 88.5 ± 2.3 | 0.000 |
| BMI (kg/m2) | 23.5 ± 0.7 | 31.4 ± 0.7 | 0.000 |
| Fat mass (%) | 23.9 ± 3.3 | 40.2 ± 0.8 | 0.001 |
| Glucose (mg/dL) | 89.7 ± 1.1 | 92.5 ± 2.7 | 0.338 |
| Insulin (μU/mL) | 5.45 ± 0.97 | 14.74 ± 1.14 | 0.000 |
| Leptin (ng/dL) | 5.13 ± 1.95 | 22.18 ± 3.44 | 0.000 |
| Human growth hormone (μg/L) | 0.65 ± 0.41 | 1.24 ± 0.64 | 0.155 |
Study Design
Subjects were studied after an overnight fast on 3 non-consecutive days separated by at least a week. The same study design as previously described (10), was used in this study. GHRP-2 (0.1 μg/kg per hour), GHRP-2 (1 μg/kg per hour), or placebo (PLAC) was administered in a randomized, double-blind fashion for the entire duration of the experiment (including meal time) using a subcutaneous catheter attached to a pump (MiniMed 508; Medtronic Diabetes, Northridge, CA). Blood was collected for subsequent hormone measurement through an intravenous catheter placed in the forearm, sampled every 30 minutes until the beginning of the meal. The buffet meal included several choices of food served in excess to accommodate a large range of appetite. While eating in the General Clinical Research Center laboratory, the subjects were observed with a camera to ensure their safety and that they did not dispose of the food in any way other than eating. A fixed, standard test breakfast was given 120 minutes after the beginning of the infusion, and the ad libitum lunch was offered at 240 minutes. The breakfast consisted of 1.5 English muffins, 5 grams of butter, and 125 mL of apple juice (1254 kJ, 65% carbohydrates, 4% protein, 31% fat). Before the buffet lunch, subjects listened to taped instructions to “eat as much as they wanted.” The items served during the buffet lunch were pasta, bread rolls, peas and carrots, chocolate chip cookies, apple sauce, breaded chicken fillets, and water. Food was weighed before and after the meal, and the caloric intake was calculated. After the meal, the infusion was stopped. Visual analog scales (VASs; from 0, not at all to 150 mm, extremely) to assess hunger, desire to eat, and fullness were administered before starting the infusion, before and after breakfast, and before and after completion of the lunch meal. The positions of the marks on the 150-mm scale were measured in millimeters by a blinded investigator.
Assays
Serum GH concentrations were measured in duplicate by a radioisotopic kit for quantitative determination of hGH from Nichols Institute Diagnostic (San Juan, CA). The assay sensitivity was 0.2 μg/L, and the median intra- and inter-assay coefficients of variation were 2.9% and 7.5%, respectively. Serum cortisol levels were measured by radio-immunoassay (kit from DSL, Webster, TX). The assay sensitivity was 0.11 ng/dL. The median intra- and inter-assay variations were 3.8% and 5.9%, respectively. Hormonal results are incomplete; therefore, data from 14 subjects are reported for GH and from 17 subjects for cortisol.
Statistical Methods
An appetite score was calculated as hunger + (150 mm − fullness) + prospective consumption (48). General linear model for repeated measure was used to test the effect of treatment [GHRP-2 low dose (LD), GHRP-2 high dose (HD), and PLAC] on FI, with gender and BMI as between-subjects factors. Comparison between groups (lean/obese, men/women) was done by independent Student's t tests. Hormonal response was assessed as area under the curve as measured by the trapezoidal method. A p value of 0.05 was used for level of significance. Data are mean ± standard error unless otherwise stated. SPSS/PC statistical program (version 11.5 for Windows; SPSS, Inc., Chicago, IL) was used for statistical analysis.
Results
FI
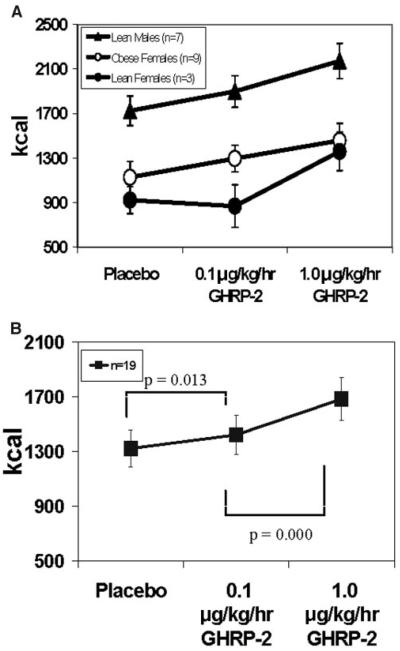
GHRP-2 infusion significantly increased FI (n = 19) in a dose-dependent manner by 10.2 ± 3.9% at LD (1422 ± 142 kcal, p = 0.011) and by 33.5 ± 5.8% at HD (1684 ± 158 kcal, p = 0.000) compared with PLAC (1297 ± 133 kcal) (Figure 1B). Overall, there was no effect of obesity status on FI stimulation by GHRP-2.
Figure 1.
Ad libitum FI in (A) lean men (triangle), lean women (closed circle), and obese women (open circle) and (B) all 19 subjects with placebo and GHRP-2 at 0.1 and 1 μg/kg per hour.
When the data from the lean subjects were analyzed separately, GHRP-2 did not increase FI at LD (p = 0.194) but did at HD by 34.2 ± 8.3% (p = 0.001) vs. PLAC. There was a gender effect in the response to GHRP-2 in the lean group, with a treatment-by-gender interaction at LD (p = 0.016) but not HD. The three lean women were non-responders at LD (Figure 1A). When the data from the lean men were analyzed separately, GHRP-2 increased FI both at LD by 10.6 ± 2.9% (p = 0.022) and at HD by 26.1 ± 6.6% (p = 0.009) vs. PLAC. In obese subjects, GHRP-2 increased FI in a dose-dependent manner by 18.3 ± 5.3% (p = 0.039) at LD and by 32.8 ± 8.7% (p = 0.005) at HD compared with PLAC.
Macronutrient Intake
Obese subjects ate more fat at each meal than the lean (46.8% vs. 41.3%, p = 0.024 in the PLAC condition). In the lean group, there was no gender difference in the macronutrient composition of meals. Macronutrient intake was not different between conditions (GHRP-2 at 2 doses and placebo). The absence of effect of GHRP-2 on the macronutrient composition of the meals was not affected by gender or BMI.
Appetite and Hunger Rating by VASs
Lean and obese subjects did not differ in terms of hunger feelings, fullness, desire to eat, or appetite score at baseline before the infusion started. With HD, subjects reported more hunger after the breakfast preload (p = 0.034) and had greater appetite score (p = 0.002) before lunch compared with PLAC. Similarly, GHRP-2 HD increased the desire to eat a meal after breakfast (p = 0.000) and before lunch (p = 0.012) and the desire to eat a favorite food after breakfast (p = 0.031) and before lunch (p = 0.018), compared with PLAC. Subjects were less full before lunch (p = 0.023) with GHRP-2 HD vs. PLAC. However, all subjects experienced the same level of fullness after the buffet lunch. There was no gender or obesity effect for any of the results obtained with VAS rating. GHRP-2 at LD had no effect on any VAS compared with PLAC.
Hormonal Data
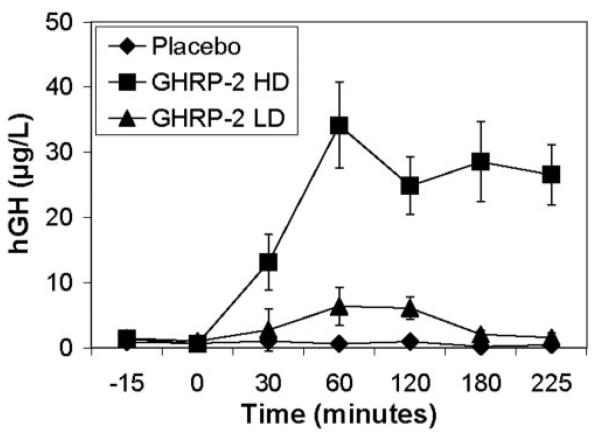
Levels of hGH increased significantly and dose dependently during the GHRP-2 infusions, at LD (680 ± 160 μg/L per time, p = 0.013) and HD (4955 ± 664 μg/L per time, p = 0.000), compared with PLAC (236 ± 76 μg/L per time). The effect of GHRP-2 on hGH was different between LD and HD (p = 0.000), and between LD and PLAC (p = 0.011) (Figure 2). The maximum serum levels of hGH after GHRP-2 were 37.9 ± 5.9 μg/L for lean men and 28.4 ± 6.2 μg/L for obese women. This was not statistically different. Serum cortisol levels (cortisol area under the curve) increased significantly with GHRP-2 at HD compared with PLAC (2569 ± 153 vs. 1591 ± 123 ng/dL per time, p = 0.000) but not at LD (p = 0.193). There was no gender and/or obesity effect on hGH or cortisol changes with GHRP-2. There was a negative correlation between fasting leptin levels and GH response to HD GHRP-2 (r2 = 0.673, p = 0.006) but no correlation between fasting insulin and/or leptin and FI response to GHRP-2.
Figure 2.
Serum GH levels over time (n = 14).
Discussion
The administration of ghrelin stimulates FI (5) and adiposity (4,5) in rodents and FI in human lean (6) and obese (9) subjects. The ghrelin analog GHRP-2, similarly to ghrelin, stimulates FI in lean men (10). In this study, GHRP-2 increased FI and GH release in obese individuals. Obese individuals have lower basal and postprandial circulating total ghrelin levels (43,44). Lower total serum ghrelin levels in obese patients increase after diet-induced weight loss (49) but not after gastric bypass surgery (40). This latter fact (50,51) remains controversial (52-54). The dysregulation of the ghrelin system could play a part in the pathogenesis of the energy imbalance of obesity; thus, we speculated that the FI response of obese individuals to GHRP-2 could be different than their lean counterparts. Surprisingly, and contrary to our hypothesis, our data show that GHRP-2 administration increases FI in obese women. This is in agreement with recently published data from Druce et al. (9), in which the administration of HD ghrelin stimulated FI in both obese and lean individuals. The magnitude of the ad libitum FI response to GHRP-2 was similar in obese and lean individuals. These results differ from the data obtained with ghrelin in which obese subjects had a greater response than lean subjects at the HD (9). These study discrepancies could be attributed to a difference in the compound, effective dose, and/or protocol design, the last because GHRP-2 was infused for 240 minutes whereas ghrelin was infused for 75 minutes (9). In addition, it is possible that partial desensitization of the GHRP-2 effect on FI occurred. The redundancies of the physiological regulators of FI are multiple, but the singular role of ghrelin in this regulation merits emphasis because so far it is the only known peripheral hormone that increases FI in humans.
A second aim of our study was to test whether the effect of GHRP-2 on FI was dose dependent. The effect of GHRP-2 on FI occurs at 1 μg/kg per minute, a dose that pharmacologically stimulates hGH and cortisol, whereas at the lower dose of 0.1 μg/kg per minute, these hormonal changes are minimal and are within the physiological range (hGH) or without any hormonal changes (cortisol). GHRP-2 has been shown to increase GH secretion dose dependently (55). Our data now demonstrate that the GHRP-2 effect on FI is also dose dependent. A smaller but statistically significant stimulation of FI at LD in lean men and obese women (0.1 μg/kg per minute) is a key finding. With the administration of LD GHRP-2, there are minimal or no detectable hormonal changes of GH and cortisol. This supports a dissociation between the FI and GH secretory effect of GHRP-2. Previous animal data have shown that the effect of ghrelin/GHRP-2 on FI can be dissociated from its hormonal effects (4), and the effect is, in part (56-58), mediated by the neuropeptide Y/Agouti gene-related protein neurons (59) and the melanocortin system in the hypothalamic arcuate nucleus (60,61).
Our data reveal that lean women do not have increased FI at the LD infusion of GHRP-2. The small number of lean women in this study does not allow us to comment on these results. Moreover, the absence of men in the obese group prevents any comments on gender/dose interaction. Future studies will address the possible role of gender in the GHRP-2/ghrelin effect on FI.
Data suggest that circulating total ghrelin could be implicated in meal-to-meal regulation. Total ghrelin levels increase in anticipation of a meal (16) and are suppressed by food ingestion (16,17). Postprandial suppression of total ghrelin levels is impaired both in obese (43) and anorectic (31) human subjects. The potential mechanisms by which serum ghrelin levels are suppressed after a meal are multiple. The role of macronutrients is apparent in well-controlled rat studies (18). Although debated (17), the role of meal-related insulin excursion is possible (27,62). Recently, fat digestion (63) was shown to be required, and the role of other gut hormones such as GLP-1 and somatostatin was postulated (28).
Although the mechanism is not entirely elucidated, the intake of macronutrients seems to play a role in the meal-to-meal regulation of ghrelin secretion. Whether ghrelin/GHRP-2 influences macronutrient choice and intake has been considered. If ghrelin administration stimulates macronutrient intake differentially, a possible regulatory loop system may exist. However, GHRP-2 had no effect on macronutrient dietary choices in our first study in lean individuals (10) or in this study in obese individuals. Similarly, studies by Druce et al. (9) showed that ghrelin administration to humans did not show any effect on macronutrient dietary composition. In our study, obese women ingested more fat than lean subjects at baseline, and this difference was not altered with GHRP-2. Therefore, GHRP-2, like ghrelin, stimulates greater intake of calories but does not preferentially influence types of dietary macronutrient.
There were no significant changes of any of the VAS scores at LD GHRP-2, and yet the FI was significantly greater with GHRP-2 than PLAC. It is possible that the VAS tools we used were insufficiently sensitive to detect changes in perception of fullness and hunger at LD GHRP-2. With GHRP-2 HD, all subjects reported similar levels of hunger and fullness. Gender and BMI had no particular effect on feeling of appetite or fullness. Particularly, the level of fullness after the ad libitum meal was identical between lean and obese, although obese individuals have been shown to have a decreased postprandial sensation of fullness (64).
The sensitivity effect of GHRP-2 on hGH release was dose dependent, both in lean and in obese subjects, as previously shown (55). This is contrary to other studies that have shown an impairment of the hGH response to HD ghrelin administration in obese individuals (9,63). GH changes with GHRP-2 were not significantly different between lean and obese subjects. Also, in other studies (9,63), the older age of the obese subjects could explain a smaller hGH response to ghrelin. In our study, subjects from the lean and obese groups were of a similar young age. Other relevant experimental differences compared with previous studies include different agents, dosages, and, in particular, mode of administration, i.e., subcutaneous infusion in present study vs. intravenous bolus administration in other studies (9).
GHRP-2, like ghrelin (3), stimulates the adrenocorticotropin/cortisol axis, as demonstrated by an increase of serum cortisol levels with HD GHRP-2. Although glucocorticoids have been implicated in FI regulation (65), it is unlikely that the GHRP-2 effect on FI is mediated by the rise in cortisol because LD GHRP-2 stimulates FI without significant changes of serum cortisol levels.
The GH response to GHRP-2 is inversely correlated to leptin levels. It is probable that the greater the obesity, the higher the circulating leptin and the lower the GHRP-2 GH response. However, this correlation probably does not imply that leptin inhibits the GHRP-2 GH response. Indeed, our data on FI indicates that obese patients are highly sensitive to GHRP-2. Elevated leptin levels do not inhibit the GHRP-2/ghrelin FI response. Moreover, the effect of GHRP-2 on FI is unlikely to be mediated by leptin because the infusion of GHRP-2 at LD and HD does not modify leptin levels (data not shown).
In summary, these data confirm that peripheral subcutaneous infusion of GHRP-2 potently stimulates short-term FI in healthy human lean and obese subjects. This effect is dose-dependent and of similar magnitude in obese women and lean men; effects in obese men are yet to be determined. These results further support a role for peripheral circulating ghrelin in the control of FI in humans. So far, these limited data do not support a dysregulation of the ghrelin system in obese individuals. Additional studies are needed to investigate lean-obese differences as a function of age, gender, duration, severity, and stage of obesity, as well as many other factors known to be involved in the regulation of FI. Whether the acute effect of ghrelin/GHRP-2 on FI persists with chronic administration will determine possible clinical uses in malnutrition.
Acknowledgments
This work was supported by NIH Grant DK 02572-01, General Clinical Research Center Grants RR 00645 and 05096, and by Obesity Research Center Grant 26687. We thank Medtronic Diabetes (Northridge, CA) for lending us the infusion pump and the pump supplies.
Footnotes
Nonstandard abbreviations: GH, growth hormone; FI, food intake; GHRP, GH-releasing peptide; GHS, GH secretagogue; PLAC, placebo; VAS, visual analog scale; LD, low dose; HD, high dose; hGH, human GH.
References
- 1.Howard AD, Feighner SD, Cully DF, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–7. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- 2.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 3.Takaya K, Ariyasu H, Kanamoto N, et al. Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab. 2000;85:4908–11. doi: 10.1210/jcem.85.12.7167. [DOI] [PubMed] [Google Scholar]
- 4.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–13. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 5.Wren AM, Small CJ, Ward HL, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–8. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- 6.Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 7.Neary NM, Small CJ, Wren AM, et al. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:2832–6. doi: 10.1210/jc.2003-031768. [DOI] [PubMed] [Google Scholar]
- 8.Wynne K, Giannitsopoulou K, Small CJ, et al. Subcutaneous ghrelin enhances acute food intake in malnourished patients who receive maintenance peritoneal dialysis: a randomized, placebo-controlled trial. J Am Soc Nephrol. 2005;16:2111–8. doi: 10.1681/ASN.2005010039. [DOI] [PubMed] [Google Scholar]
- 9.Druce MR, Wren AM, Park AJ, et al. Ghrelin increases food intake in obese as well as lean subjects. Int J Obes Relat Metab Disord. 2005;29:1130–6. doi: 10.1038/sj.ijo.0803001. [DOI] [PubMed] [Google Scholar]
- 10.Laferrère B, Abraham C, Russell CD, Bowers CY. Growth hormone releasing peptide-2 (GHRP-2), like ghrelin, increases food intake in healthy men. J Clin Endocrinol Metab. 2005;90:611–4. doi: 10.1210/jc.2004-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowers CY, Momany FA, Reynolds GA, Hong A. On the in vitro and in vivo activity of a new synthetic hexapeptide that acts on the pituitary to specifically release growth hormone. Endocrinology. 1984;114:1537–45. doi: 10.1210/endo-114-5-1537. [DOI] [PubMed] [Google Scholar]
- 12.Mericq V, Cassorla F, Bowers CY, Avila A, Gonen B, Merriam GR. Changes in appetite and body weight in response to long-term oral administration of the ghrelin agonist GHRP-2 in growth hormone deficient children. J Pediatr Endocrinol Metab. 2003;16:981–5. doi: 10.1515/jpem.2003.16.7.981. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, York DA, Bray GA. Regulation of ghrelin gene expression in stomach and feeding response to a ghrelin analogue in two strains of rats. Peptide. 2004;25:2171–7. doi: 10.1016/j.peptides.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Kuriyama H, Hotta M, Wakabayashi I, Shibasaki T. A 6-day intracerebroventricular infusion of the growth hormone-releasing peptide KP-102 stimulates food intake in both non-stressed and intermittently-stressed rats. Neurosci Lett. 2000;282:109–12. doi: 10.1016/s0304-3940(00)00882-x. [DOI] [PubMed] [Google Scholar]
- 15.Alba M, Fintini D, Bowers CY, Parlow AF, Salvatori R. Effects of long term treatment with growth hormone releasing peptide-2 in the GHRH knock out mouse. Am J Physiol Endocrinol Metab. 2005;289:E762–7. doi: 10.1152/ajpendo.00203.2005. [DOI] [PubMed] [Google Scholar]
- 16.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–9. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 17.Caixas A, Bashore C, Nash W, Pi-Sunyer F, Laferrère B. Insulin, unlike food intake, does not suppress ghrelin in human subjects. J Clin Endocrinol Metab. 2002;87:1902. doi: 10.1210/jcem.87.4.8538. [DOI] [PubMed] [Google Scholar]
- 18.Overduin J, Frayo RS, Grill HJ, Kaplan JM, Cummings DE. Role of the duodenum and macronutrient type in ghrelin regulation. Endocrinology. 2005;146:845–50. doi: 10.1210/en.2004-0609. [DOI] [PubMed] [Google Scholar]
- 19.Parker BA, Doran S, Wishart J, Horowitz M, Chapman IM. Effects of small intestinal and gastric glucose administration on the suppression of plasma ghrelin concentrations in healthy older men and women. Clin Endocrinol (Oxf) 2005;62:539–46. doi: 10.1111/j.1365-2265.2005.02254.x. [DOI] [PubMed] [Google Scholar]
- 20.Callahan HS, Cummings DE, Pepe MS, Breen PA, Matthys CC, Weigle DS. Postprandial suppression of plasma ghrelin level is proportional to ingested caloric load but does not predict intermeal interval in humans. J Clin Endocrinol Metab. 2004;89:1319–24. doi: 10.1210/jc.2003-031267. [DOI] [PubMed] [Google Scholar]
- 21.Monteleone P, Bencivenga R, Longobardi N, Serritella C, Maj M. Differential responses of circulating ghrelin to high-fat or high-carbohydrate meal in healthy women. J Clin Endocrinol Metab. 2003;88:5510–4. doi: 10.1210/jc.2003-030797. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez J, Oliver P, Pico C, Palou A. Diurnal rhythms of leptin and ghrelin in the systemic circulation and in the gastric mucosa are related to food intake in rats. Pflugers Arch. 2004;448:500–6. doi: 10.1007/s00424-004-1283-4. [DOI] [PubMed] [Google Scholar]
- 23.Teff KL, Elliott SS, Tschop M, et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89:2963–72. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- 24.Arosio M, Ronchi CL, Beck-Peccoz P, et al. Effects of modified sham feeding on ghrelin levels in healthy human subjects. J Clin Endocrinol Metab. 2004;89:5101–4. doi: 10.1210/jc.2003-032222. [DOI] [PubMed] [Google Scholar]
- 25.Simonian HP, Kresge KM, Boden GH, Parkman HP. Differential effects of sham feeding and meal ingestion on ghrelin and pancreatic polypeptide levels: evidence for vagal efferent stimulation mediating ghrelin release. Neurogastroenterol Motil. 2005;17:348–54. doi: 10.1111/j.1365-2982.2004.00634.x. [DOI] [PubMed] [Google Scholar]
- 26.Williams J, Mobarhan S. A critical interaction: leptin and ghrelin. Nutr Rev. 2003;61:391–3. doi: 10.1301/nr.2003.nov.391-393. [DOI] [PubMed] [Google Scholar]
- 27.Murdolo G, Lucidi P, Di Loreto C, et al. Insulin is required for prandial ghrelin suppression in humans. Diabetes. 2003;52:2923–7. doi: 10.2337/diabetes.52.12.2923. [DOI] [PubMed] [Google Scholar]
- 28.Lippl F, Kircher F, Erdmann J, Allescher HD, Schusdziarra V. Effect of GIP, GLP-1, insulin and gastrin on ghrelin release in the isolated rat stomach. Regul Pept. 2004;119:93–8. doi: 10.1016/j.regpep.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Spranger J, Ristow M, Otto B, et al. Post-prandial decrease of human plasma ghrelin in the absence of insulin. J Endocrinol Invest. 2003;26:RC19–22. doi: 10.1007/BF03347349. [DOI] [PubMed] [Google Scholar]
- 30.Broglio F, Benso A, Castiglioni C, et al. The endocrine response to ghrelin as a function of gender in humans in young and elderly subjects. J Clin Endocrinol Metab. 2003;88:1537–42. doi: 10.1210/jc.2002-021504. [DOI] [PubMed] [Google Scholar]
- 31.Otto B, Tschop M, Fruhauf E, et al. Postprandial ghrelin release in anorectic patients before and after weight gain. Psychoneuroendocrinology. 2005;30:577–81. doi: 10.1016/j.psyneuen.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Misra M, Miller KK, Stewart V, et al. Ghrelin and bone metabolism in adolescent girls with anorexia nervosa and healthy adolescents. J Clin Endocrinol Metab. 2005;90:5082–7. doi: 10.1210/jc.2005-0512. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka M, Nakahara T, Kojima S, et al. Effect of nutritional rehabilitation on circulating ghrelin and growth hormone levels in patients with anorexia nervosa. Regul Pept. 2004;122:163–8. doi: 10.1016/j.regpep.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Inui A. Acyl and desacyl ghrelin in anorexia nervosa. Psychoneuroendocrinology. 2005;30:115. doi: 10.1016/j.psyneuen.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Nakai Y, Hosoda H, Nin K, et al. Short-term secretory regulation of the active form of ghrelin and total ghrelin during an oral glucose tolerance test in patients with anorexia nervosa. Eur J Endocrinol. 2004;150:913–4. doi: 10.1530/eje.0.1500913. [DOI] [PubMed] [Google Scholar]
- 36.Tschop M, Wawarta R, Riepl RL, et al. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest. 2001;24:RC19–21. doi: 10.1007/BF03351037. [DOI] [PubMed] [Google Scholar]
- 37.Stock S, Leichner P, Wong AC, et al. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J Clin Endocrinol Metab. 2005;90:2161–8. doi: 10.1210/jc.2004-1251. [DOI] [PubMed] [Google Scholar]
- 38.Inui A, Asakawa A, Bowers CY, et al. Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J. 2004;18:439–56. doi: 10.1096/fj.03-0641rev. [DOI] [PubMed] [Google Scholar]
- 39.Monteleone P, Martiadis V, Rigamonti AE, et al. Investigation of peptide YY and ghrelin responses to a test meal in bulimia nervosa. Biol Psychiatry. 2005;57:926–31. doi: 10.1016/j.biopsych.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–30. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 41.Soriano-Guillen L, Barrios V, Lechuga-Sancho A, Chowen JA, Argente J. Response of circulating ghrelin levels to insulin therapy in children with newly diagnosed type 1 diabetes mellitus. Pediatr Res. 2004;55:830–5. doi: 10.1203/01.PDR.0000120679.92416.70. [DOI] [PubMed] [Google Scholar]
- 42.Weigle DS, Cummings DE, Newby PD, et al. Roles of leptin and ghrelin in the loss of body weight caused by a low fat, high carbohydrate diet. J Clin Endocrinol Metab. 2003;88:1577–86. doi: 10.1210/jc.2002-021262. [DOI] [PubMed] [Google Scholar]
- 43.le Roux CW, Patterson M, Vincent RP, Hunt C, Ghatei MA, Bloom SR. Postprandial plasma ghrelin is suppressed proportional to meal calorie content in normal-weight but not obese subjects. J Clin Endocrinol Metab. 2005;90:1068–71. doi: 10.1210/jc.2004-1216. [DOI] [PubMed] [Google Scholar]
- 44.English PJ, Ghatei MA, Malik IA, Bloom SR, Wilding JP. Food fails to suppress ghrelin levels in obese humans. J Clin Endocrinol Metab. 2002;87:2984. doi: 10.1210/jcem.87.6.8738. [DOI] [PubMed] [Google Scholar]
- 45.Horvath TL, Diano S, Sotonyi P, Heiman M, Tschop M. Minireview: ghrelin and the regulation of energy balance: a hypothalamic perspective. Endocrinology. 2001;142:4163–9. doi: 10.1210/endo.142.10.8490. [DOI] [PubMed] [Google Scholar]
- 46.Muccioli G, Tschop M, Papotti M, Deghenghi R, Heiman M, Ghigo E. Neuroendocrine and peripheral activities of ghrelin: implications in metabolism and obesity. Eur J Pharmacol. 2002;440:235–54. doi: 10.1016/s0014-2999(02)01432-2. [DOI] [PubMed] [Google Scholar]
- 47.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- 48.Anderson GH, Catherine NL, Woodend DM, Wolever TM. Inverse association between the effect of carbohydrates on blood glucose and subsequent short-term food intake in young men. Am J Clin Nutr. 2002;76:1023–30. doi: 10.1093/ajcn/76.5.1023. [DOI] [PubMed] [Google Scholar]
- 49.Hansen TK, Dall R, Hosoda H, et al. Weight loss increases circulating levels of ghrelin in human obesity. Clin Endocrinol (Oxf) 2002;56:203–6. doi: 10.1046/j.0300-0664.2001.01456.x. [DOI] [PubMed] [Google Scholar]
- 50.Fruhbeck G, Rotellar F, Hernandez-Lizoain JL, et al. Fasting plasma ghrelin concentrations 6 months after gastric bypass are not determined by weight loss or changes in insulinemia. Obes Surg. 2004;14:1208–15. doi: 10.1381/0960892042386904. [DOI] [PubMed] [Google Scholar]
- 51.Leonetti F, Silecchia G, Iacobellis G, et al. Different plasma ghrelin levels after laparoscopic gastric bypass and adjustable gastric banding in morbid obese subjects. J Clin Endocrinol Metab. 2003;88:4227–31. doi: 10.1210/jc.2003-030133. [DOI] [PubMed] [Google Scholar]
- 52.Holdstock C, Engstrom BE, Ohrvall M, Lind L, Sundbom M, Karlsson FA. Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. J Clin Endocrinol Metab. 2003;88:3177–83. doi: 10.1210/jc.2002-021734. [DOI] [PubMed] [Google Scholar]
- 53.Faraj M, Havel PJ, Phelis S, Blank D, Sniderman AD, Cianflone K. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88:1594–602. doi: 10.1210/jc.2002-021309. [DOI] [PubMed] [Google Scholar]
- 54.Korner J, Bessler M, Cirilo LJ, et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90:359–65. doi: 10.1210/jc.2004-1076. [DOI] [PubMed] [Google Scholar]
- 55.Bowers CY, Granda-Ayala R. Growth hormone/insulin-like growth factor-1 response to acute and chronic growth hormone-releasing peptide-2, growth hormone-releasing hormone 1–44NH2 and in combination in older men and women with decreased growth hormone secretion. Endocrine. 2001;14:79–86. doi: 10.1385/ENDO:14:1:079. [DOI] [PubMed] [Google Scholar]
- 56.Chen C. Growth hormone secretagogue actions on the pituitary gland: multiple receptors for multiple ligands? Clin Exp Pharmacol Physiol. 2000;27:323–9. doi: 10.1046/j.1440-1681.2000.03258.x. [DOI] [PubMed] [Google Scholar]
- 57.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes. 2001;50:2438–43. doi: 10.2337/diabetes.50.11.2438. [DOI] [PubMed] [Google Scholar]
- 58.Tamura H, Kamegai J, Shimizu T, Ishii S, Sugihara H, Oikawa S. Ghrelin stimulates GH but not food intake in arcuate nucleus ablated rats. Endocrinology. 2002;143:3268–75. doi: 10.1210/en.2002-220268. [DOI] [PubMed] [Google Scholar]
- 59.Tschop M, Flora DB, Mayer JP, Heiman ML. Hypophysectomy prevents ghrelin-induced adiposity and increases gastric ghrelin secretion in rats. Obes Res. 2002;10:991–9. doi: 10.1038/oby.2002.135. [DOI] [PubMed] [Google Scholar]
- 60.Shaw AM, Irani BG, Moore MC, Haskell-Luevano C, Millard WJ. Ghrelin-induced food intake and growth hormone secretion are altered in melanocortin 3 and 4 receptor knockout mice. Peptides. 2005;26:1720–7. doi: 10.1016/j.peptides.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 61.Bjursell M, Egecioglu E, Gerdin AK, et al. Importance of melanin-concentrating hormone receptor for the acute effects of ghrelin. Biochem Biophys Res Commun. 2005;326:759–65. doi: 10.1016/j.bbrc.2004.11.116. [DOI] [PubMed] [Google Scholar]
- 62.Blom WA, Stafleu A, de Graaf C, Kok FJ, Schaafsma G, Hendriks HF. Ghrelin response to carbohydrate-enriched breakfast is related to insulin. Am J Clin Nutr. 2005;81:367–75. doi: 10.1093/ajcn.81.2.367. [DOI] [PubMed] [Google Scholar]
- 63.Feinle-Bisset C, Patterson M, Ghatei MA, Bloom SR, Horowitz M. Fat digestion is required for the suppression of ghrelin and stimulation of peptide YY and pancreatic polypeptide secretion by intraduodenal lipid. Am J Physiol Endocrinol Metab. 2005;289:E948–53. doi: 10.1152/ajpendo.00220.2005. [DOI] [PubMed] [Google Scholar]
- 64.Guss JL, Kissileff HR, Walsh BT, Devlin MJ. Binge eating behavior in patients with eating disorders. Obes Res. 1994;2:355–363. doi: 10.1002/j.1550-8528.1994.tb00075.x. [DOI] [PubMed] [Google Scholar]
- 65.Dallman MF, La Fleur SE, Pecoraro NC, Gomez F, Houshyar H, Akana SF. Minireview: glucocorticoids: food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology. 2004;145:2633–8. doi: 10.1210/en.2004-0037. [DOI] [PubMed] [Google Scholar]