Abstract
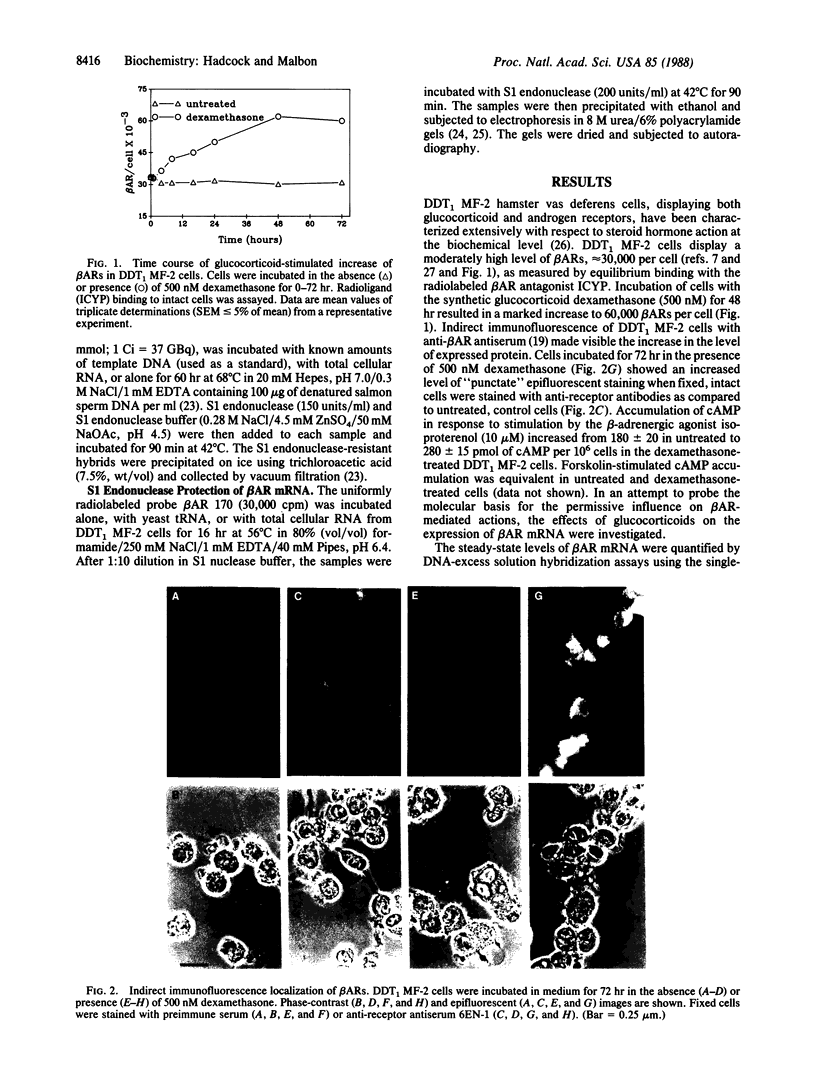
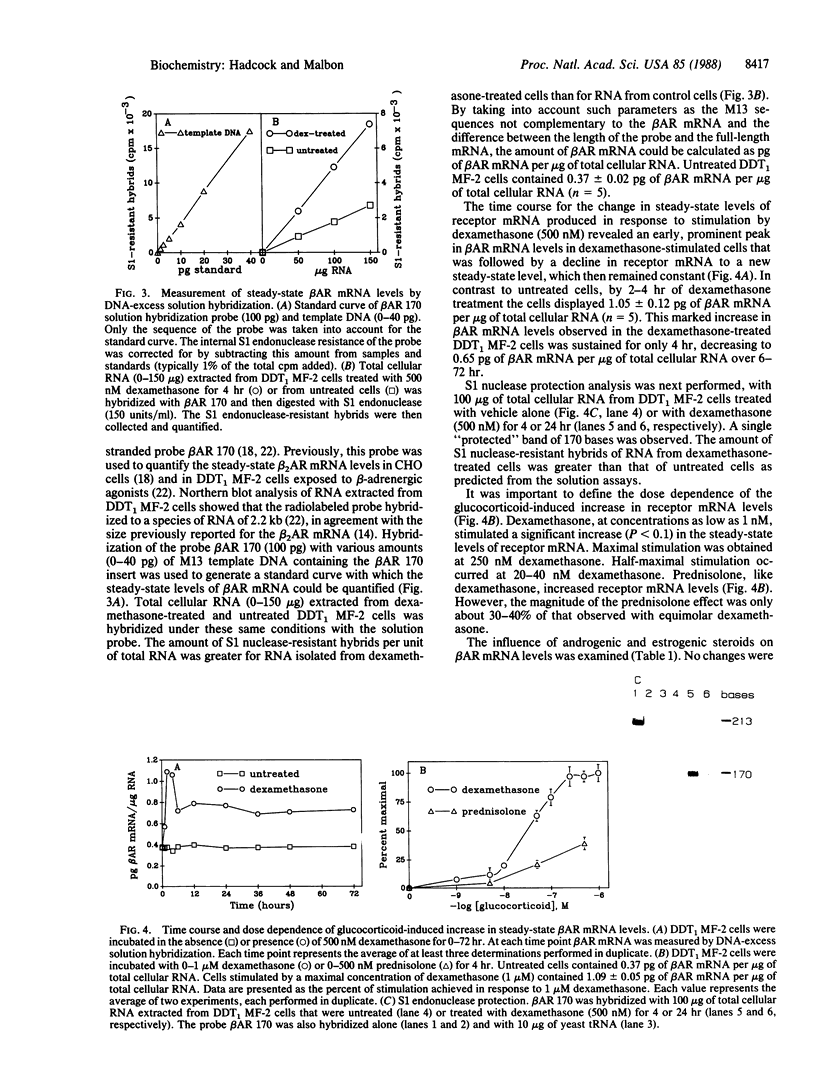
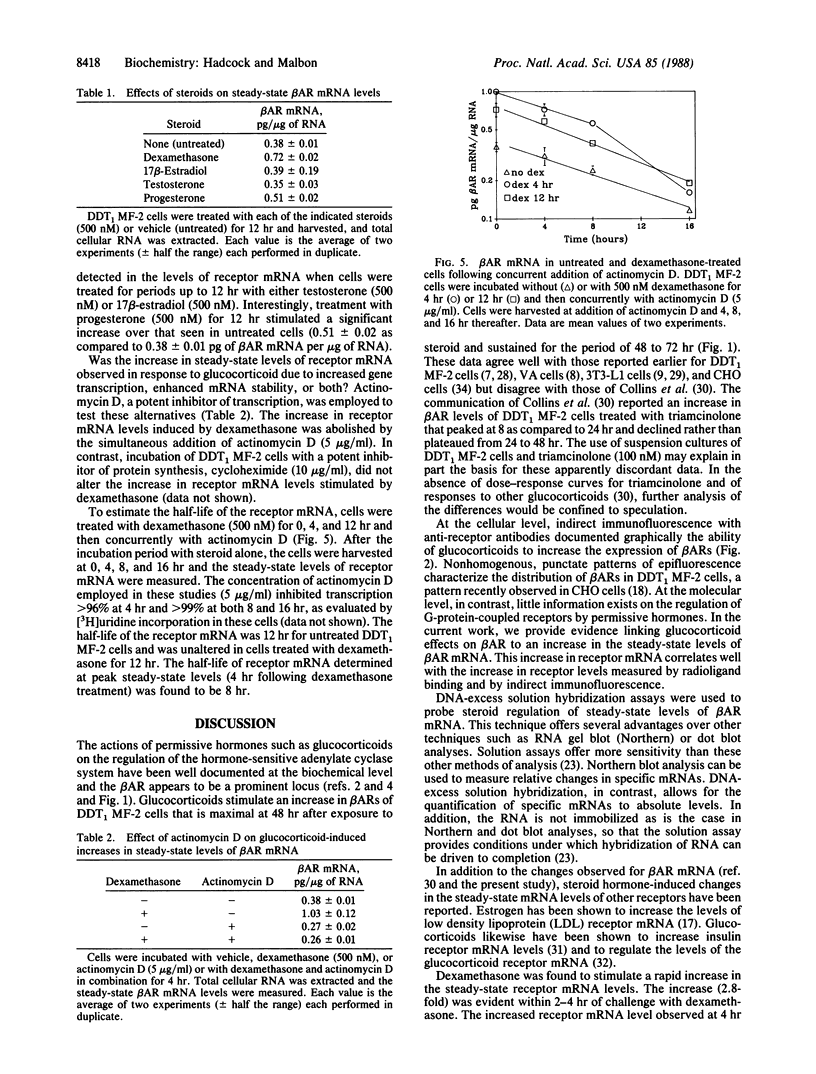
Incubation of DDT1 MF-2 hamster vas deferens cells with glucocorticoids results in a marked increase in beta-adrenergic receptor (beta AR) number. The increase in receptor number was visualized by indirect immunofluorescence with antiserum specific for the beta AR and was verified by radioligand binding. The steady-state levels of beta AR mRNA were quantified in untreated (control) and glucocorticoid-treated cells by DNA-excess solution hybridization using a single-stranded probe corresponding to nucleotides +12 to 182 of the hamster beta 2AR cDNA coding region. The steady-state level increased from 0.37 pg of beta AR mRNA per microgram of total cellular RNA in untreated cells to 1.05 pg of beta AR mRNA per microgram of RNA in cells treated with dexamethasone (500 nM) for 2-4 hr. After this sharp transient peak, the steady-state level of receptor mRNA declined by 6 hr to a level approximately twice that of the untreated cells. Half-maximal effects were achieved at 20-40 nM dexamethasone. Testosterone (500 nM) and 17 beta-estradiol (500 nM), in contrast, did not alter the steady-state levels of beta AR mRNA. Actinomycin D, a potent inhibitor of transcription, abolished the dexamethasone-induced increase in beta AR mRNA, suggesting that the permissive hormone effect was exerted on gene transcription. The half-life of the receptor mRNA measured in the presence of actinomycin D was found to be 12 hr in both the untreated and the dexamethasone-treated cells. These studies provide a molecular explanation for the well-known regulation of GTP-binding protein (G-protein)-linked cell-surface receptors by permissive hormones.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chung F. Z., Lentes K. U., Gocayne J., Fitzgerald M., Robinson D., Kerlavage A. R., Fraser C. M., Venter J. C. Cloning and sequence analysis of the human brain beta-adrenergic receptor. Evolutionary relationship to rodent and avian beta-receptors and porcine muscarinic receptors. FEBS Lett. 1987 Jan 26;211(2):200–206. doi: 10.1016/0014-5793(87)81436-9. [DOI] [PubMed] [Google Scholar]
- Collins S., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptors in hamster smooth muscle cells are transcriptionally regulated by glucocorticoids. J Biol Chem. 1988 Jul 5;263(19):9067–9070. [PubMed] [Google Scholar]
- Davies A. O., De Lean A., Lefkowitz R. J. Myocardial beta-adrenergic receptors from adrenalectomized rats: impaired formation of high-affinity agonist-receptor complexes. Endocrinology. 1981 Feb;108(2):720–722. doi: 10.1210/endo-108-2-720. [DOI] [PubMed] [Google Scholar]
- Davies A. O., Lefkowitz R. J. Corticosteroid-induced differential regulation of beta-adrenergic receptors in circulating human polymorphonuclear leukocytes and mononuclear leukocytes. J Clin Endocrinol Metab. 1980 Sep;51(3):599–605. doi: 10.1210/jcem-51-3-599. [DOI] [PubMed] [Google Scholar]
- Davies A. O., Lefkowitz R. J. Regulation of beta-adrenergic receptors by steroid hormones. Annu Rev Physiol. 1984;46:119–130. doi: 10.1146/annurev.ph.46.030184.001003. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Kobilka B. K., Strader D. J., Benovic J. L., Dohlman H. G., Frielle T., Bolanowski M. A., Bennett C. D., Rands E., Diehl R. E. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986 May 1;321(6065):75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Emorine L. J., Marullo S., Delavier-Klutchko C., Kaveri S. V., Durieu-Trautmann O., Strosberg A. D. Structure of the gene for human beta 2-adrenergic receptor: expression and promoter characterization. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6995–6999. doi: 10.1073/pnas.84.20.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster S. J., Harden T. K. Dexamethasone increases beta-adrenoceptor density in human astrocytoma cells. Biochem Pharmacol. 1980 Aug 1;29(15):2151–2153. doi: 10.1016/0006-2952(80)90189-6. [DOI] [PubMed] [Google Scholar]
- Fraser C. M., Venter J. C. The synthesis of beta-adrenergic receptors in cultured human lung cells: induction by glucocorticoids. Biochem Biophys Res Commun. 1980 May 14;94(1):390–397. doi: 10.1016/s0006-291x(80)80233-6. [DOI] [PubMed] [Google Scholar]
- George S. T., Berrios M., Hadcock J. R., Wang H. Y., Malbon C. C. Receptor density and cAMP accumulation: analysis in CHO cells exhibiting stable expression of a cDNA that encodes the beta 2-adrenergic receptor. Biochem Biophys Res Commun. 1988 Jan 29;150(2):665–672. doi: 10.1016/0006-291x(88)90443-3. [DOI] [PubMed] [Google Scholar]
- Hadcock J. R., Malbon C. C. Down-regulation of beta-adrenergic receptors: agonist-induced reduction in receptor mRNA levels. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5021–5025. doi: 10.1073/pnas.85.14.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilka B. K., Frielle T., Dohlman H. G., Bolanowski M. A., Dixon R. A., Keller P., Caron M. G., Lefkowitz R. J. Delineation of the intronless nature of the genes for the human and hamster beta 2-adrenergic receptor and their putative promoter regions. J Biol Chem. 1987 May 25;262(15):7321–7327. [PubMed] [Google Scholar]
- Lai E., Rosen O. M., Rubin C. S. Differentiation-dependent expression of catecholamine-stimulated adenylate cyclase. Roles of the beta-receptor and G/F protein in differentiating 3T3-L1 adipocytes. J Biol Chem. 1981 Dec 25;256(24):12866–12874. [PubMed] [Google Scholar]
- Lee T. P., Reed C. E. Effects of steroids on the regulation of the levels of cyclic AMP in human lymphocytes. Biochem Biophys Res Commun. 1977 Oct 10;78(3):998–1004. doi: 10.1016/0006-291x(77)90520-4. [DOI] [PubMed] [Google Scholar]
- Ma P. T., Yamamoto T., Goldstein J. L., Brown M. S. Increased mRNA for low density lipoprotein receptor in livers of rabbits treated with 17 alpha-ethinyl estradiol. Proc Natl Acad Sci U S A. 1986 Feb;83(3):792–796. doi: 10.1073/pnas.83.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malbon C. C., Hadcock J. R. Evidence that glucocorticoid response elements in the 5'-noncoding region of the hamster beta 2-adrenergic receptor gene are obligate for glucocorticoid regulation of receptor mRNA levels. Biochem Biophys Res Commun. 1988 Jul 29;154(2):676–681. doi: 10.1016/0006-291x(88)90192-1. [DOI] [PubMed] [Google Scholar]
- Malbon C. C., Rapiejko P. J., Watkins D. C. Permissive hormone regulation of hormone-sensitive effector systems. Trends Pharmacol Sci. 1988 Jan;9(1):33–36. doi: 10.1016/0165-6147(88)90240-4. [DOI] [PubMed] [Google Scholar]
- Mano K., Akbarzadeh A., Townley R. G. Effect of hydrocortisone on beta-adrenergic receptors in lung membranes. Life Sci. 1979 Nov 26;25(22):1925–1930. doi: 10.1016/0024-3205(79)90614-3. [DOI] [PubMed] [Google Scholar]
- Marone G., Lichtenstein L. M., Plaut M. Hydrocortisone and human lymphocytes: increases in cyclic adenosine 3':5'-monophosphate and potentiation of adenylate cyclase-activating agents. J Pharmacol Exp Ther. 1980 Nov;215(2):469–478. [PubMed] [Google Scholar]
- Moxham C. P., George S. T., Graziano M. P., Brandwein H. J., Malbon C. C. Mammalian beta 1- and beta 2-adrenergic receptors. Immunological and structural comparisons. J Biol Chem. 1986 Nov 5;261(31):14562–14570. [PubMed] [Google Scholar]
- Nakada M. T., Stadel J. M., Poksay K. S., Crooke S. T. Glucocorticoid regulation of beta-adrenergic receptors in 3T3-L1 preadipocytes. Mol Pharmacol. 1987 Apr;31(4):377–384. [PubMed] [Google Scholar]
- Norris J. S., Brown P., Cohen J., Cornett L. E., Kohler P. O., MacLeod S. L., Popovich K., Robey R. B., Sifford M., Syms A. J. Glucocorticoid induction of beta-adrenergic receptors in the DDT1 MF-2 smooth muscle cell line involves synthesis of new receptor. Mol Cell Biochem. 1987 Mar;74(1):21–27. doi: 10.1007/BF00221909. [DOI] [PubMed] [Google Scholar]
- Norris J. S., Kohler P. O. The coexistence of androgen and glucocorticoid receptors in the DDT1 cloned cell line. Endocrinology. 1977 Mar;100(3):613–618. doi: 10.1210/endo-100-3-613. [DOI] [PubMed] [Google Scholar]
- Rosewicz S., McDonald A. R., Maddux B. A., Goldfine I. D., Miesfeld R. L., Logsdon C. D. Mechanism of glucocorticoid receptor down-regulation by glucocorticoids. J Biol Chem. 1988 Feb 25;263(6):2581–2584. [PubMed] [Google Scholar]
- Scarpace P. J., Baresi L. A., Sanford D. A., Abrass I. B. Desensitization and resensitization of beta-adrenergic receptors in a smooth muscle cell line. Mol Pharmacol. 1985 Dec;28(6):495–501. [PubMed] [Google Scholar]
- Shelness G. S., Williams D. L. Apolipoprotein II messenger RNA. Transcriptional and splicing heterogeneity yields six 5'-untranslated leader sequences. J Biol Chem. 1984 Aug 10;259(15):9929–9935. [PubMed] [Google Scholar]
- Shibasaki Y., Sakura H., Odawara M., Shibuya M., Kanazawa Y., Akanuma Y., Takaku F., Kasuga M. Glucocorticoids increase insulin binding and the amount of insulin-receptor mRNA in human cultured lymphocytes. Biochem J. 1988 Feb 1;249(3):715–719. doi: 10.1042/bj2490715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles G. L., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptors: biochemical mechanisms of physiological regulation. Physiol Rev. 1984 Apr;64(2):661–743. doi: 10.1152/physrev.1984.64.2.661. [DOI] [PubMed] [Google Scholar]
- Strasser R. H., Benovic J. L., Caron M. G., Lefkowitz R. J. Beta-agonist- and prostaglandin E1-induced translocation of the beta-adrenergic receptor kinase: evidence that the kinase may act on multiple adenylate cyclase-coupled receptors. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6362–6366. doi: 10.1073/pnas.83.17.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. L., Newman T. C., Shelness G. S., Gordon D. A. Measurement of apolipoprotein mRNA by DNA-excess solution hybridization with single-stranded probes. Methods Enzymol. 1986;128:671–689. doi: 10.1016/0076-6879(86)28099-4. [DOI] [PubMed] [Google Scholar]