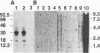
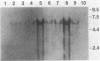
Abstract
All retroviruses encode a nucleic acid-binding or nucleocapsid protein that is believed to be associated with RNA in the virion. Further, all retroviral nucleocapsid proteins contain either one or two copies of the sequence Cys-Xaa2-Cys-Xaa4-His-Xaa4-Cys. The conservation of this sequence suggested that it is important for virus replication, and its resemblance to the "zinc-finger" sequences found in eukaryotic transcription factors raised the possibility that it recognizes specific sequences in viral RNA during retrovirus assembly. We used oligonucleotide-directed mutagenesis to generate a series of mutations in the nucleocapsid protein-coding region of Moloney murine leukemia virus. These mutations changed single amino acids, including each of the cysteines, to serine. The mutant viral genomes direct the synthesis of virus particles; these particles lack detectable viral RNA but do contain significant levels of cellular RNAs. Thus it appears that the mutations have destroyed the ability of the viral proteins to specifically package viral RNA during virus assembly. We propose that the conserved sequence in retroviral nucleocapsid proteins functions in RNA sequence recognition and suggest that it is evolutionarily related to the zinc fingers that recognize specific sequences in double-stranded DNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Weaver C. A. Characterization of murine sarcoma virus (Kirsten) transformation of mouse and human cells. J Gen Virol. 1971 Nov;13(2):245–252. doi: 10.1099/0022-1317-13-2-245. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Rapid cell culture assay technic for murine leukaemia viruses. Nature. 1971 Feb 19;229(5286):564–566. doi: 10.1038/229564b0. [DOI] [PubMed] [Google Scholar]
- Bender M. A., Palmer T. D., Gelinas R. E., Miller A. D. Evidence that the packaging signal of Moloney murine leukemia virus extends into the gag region. J Virol. 1987 May;61(5):1639–1646. doi: 10.1128/jvi.61.5.1639-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J. M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986 Apr 25;232(4749):485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- Colicelli J., Goff S. P. Identification of endogenous retroviral sequences as potential donors for recombinational repair of mutant retroviruses: positions of crossover points. Virology. 1987 Oct;160(2):518–522. doi: 10.1016/0042-6822(87)90030-4. [DOI] [PubMed] [Google Scholar]
- Covey S. N. Amino acid sequence homology in gag region of reverse transcribing elements and the coat protein gene of cauliflower mosaic virus. Nucleic Acids Res. 1986 Jan 24;14(2):623–633. doi: 10.1093/nar/14.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey L., Roberts S. B., Heintz N. RNA polymerase II transcription factors H4TF-1 and H4TF-2 require metal to bind specific DNA sequences. Mol Cell Biol. 1987 Dec;7(12):4582–4584. doi: 10.1128/mcb.7.12.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J., Scherer M., Tsai W. P., Long C. Low-molecular- weight Rauscher leukemia virus protein with preferential binding for single-stranded RNA and DNA. J Virol. 1976 May;18(2):709–718. doi: 10.1128/jvi.18.2.709-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M., Hollenberg S. M. Zinc fingers: gilt by association. Cell. 1988 Jan 15;52(1):1–3. doi: 10.1016/0092-8674(88)90522-3. [DOI] [PubMed] [Google Scholar]
- Fu X. D., Katz R. A., Skalka A. M., Leis J. Site-directed mutagenesis of the avian retrovirus nucleocapsid protein, pp 12. Mutation which affects RNA binding in vitro blocks viral replication. J Biol Chem. 1988 Feb 15;263(5):2140–2145. [PubMed] [Google Scholar]
- Gerwin B. I., Rein A., Levin J. G., Bassin R. H., Benjers B. M., Kashmiri S. V., Hopkins D., O'Neill B. J. Mutant of B-tropic murine leukemia virus synthesizing an altered polymerase molecule. J Virol. 1979 Sep;31(3):741–751. doi: 10.1128/jvi.31.3.741-751.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. P., Lobel L. I. Mutants of murine leukemia viruses and retroviral replication. Biochim Biophys Acta. 1987 Jul 8;907(2):93–123. doi: 10.1016/0304-419x(87)90001-1. [DOI] [PubMed] [Google Scholar]
- Green S., Chambon P. Oestradiol induction of a glucocorticoid-responsive gene by a chimaeric receptor. Nature. 1987 Jan 1;325(6099):75–78. doi: 10.1038/325075a0. [DOI] [PubMed] [Google Scholar]
- Hanas J. S., Hazuda D. J., Bogenhagen D. F., Wu F. Y., Wu C. W. Xenopus transcription factor A requires zinc for binding to the 5 S RNA gene. J Biol Chem. 1983 Dec 10;258(23):14120–14125. [PubMed] [Google Scholar]
- Henderson L. E., Copeland T. D., Sowder R. C., Smythers G. W., Oroszlan S. Primary structure of the low molecular weight nucleic acid-binding proteins of murine leukemia viruses. J Biol Chem. 1981 Aug 25;256(16):8400–8406. [PubMed] [Google Scholar]
- Johnston M. Genetic evidence that zinc is an essential co-factor in the DNA binding domain of GAL4 protein. Nature. 1987 Jul 23;328(6128):353–355. doi: 10.1038/328353a0. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Carner K. R., Masiarz F. R., Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987 Dec 24;51(6):1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- Karpel R. L., Henderson L. E., Oroszlan S. Interactions of retroviral structural proteins with single-stranded nucleic acids. J Biol Chem. 1987 Apr 15;262(11):4961–4967. [PubMed] [Google Scholar]
- Linial M., Medeiros E., Hayward W. S. An avian oncovirus mutant (SE 21Q1b) deficient in genomic RNA: biological and biochemical characterization. Cell. 1978 Dec;15(4):1371–1381. doi: 10.1016/0092-8674(78)90062-4. [DOI] [PubMed] [Google Scholar]
- Long C. W., Henderson L. E., Oroszlan S. Isolation and characterization of low-molecular-weight DNA-binding proteins from retroviruses. Virology. 1980 Jul 30;104(2):491–496. doi: 10.1016/0042-6822(80)90352-9. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- McCoy M. S., Bargmann C. I., Weinberg R. A. Human colon carcinoma Ki-ras2 oncogene and its corresponding proto-oncogene. Mol Cell Biol. 1984 Aug;4(8):1577–1582. doi: 10.1128/mcb.4.8.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méric C., Spahr P. F. Rous sarcoma virus nucleic acid-binding protein p12 is necessary for viral 70S RNA dimer formation and packaging. J Virol. 1986 Nov;60(2):450–459. doi: 10.1128/jvi.60.2.450-459.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszlan S., Copeland T. D. Primary structure and processing of gag and env gene products of human T-cell leukemia viruses HTLV-ICR and HTLV-IATK. Curr Top Microbiol Immunol. 1985;115:221–233. doi: 10.1007/978-3-642-70113-9_14. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Long C. W., Gilden R. V. Isolation of murine type-C virus p30 precursor protein by DNA-cellulose chromatography. Virology. 1976 Jul 15;72(2):523–526. doi: 10.1016/0042-6822(76)90182-3. [DOI] [PubMed] [Google Scholar]
- Rein A., Benjers B. M., Gerwin B. I., Bassin R. H., Slocum D. R. Rescue and transmission of a replication-defective variant of moloney murine leukemia virus. J Virol. 1979 Feb;29(2):494–500. doi: 10.1128/jvi.29.2.494-500.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A. Interference grouping of murine leukemia viruses: a distinct receptor for the MCF-recombinant viruses in mouse cells. Virology. 1982 Jul 15;120(1):251–257. doi: 10.1016/0042-6822(82)90024-1. [DOI] [PubMed] [Google Scholar]
- Rein A., McClure M. R., Rice N. R., Luftig R. B., Schultz A. M. Myristylation site in Pr65gag is essential for virus particle formation by Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7246–7250. doi: 10.1073/pnas.83.19.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulein M., Burnette W. N., August J. T. Stoichiometry and specificity of binding of Rauscher oncovirus 10,000-dalton (p10) structural protein to nucleic acids. J Virol. 1978 Apr;26(1):54–60. doi: 10.1128/jvi.26.1.54-60.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Linial M. Avian oncovirus mutant (SE21Q1b) deficient in genomic RNA: characterization of a deletion in the provirus. J Virol. 1980 Nov;36(2):450–456. doi: 10.1128/jvi.36.2.450-456.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Sorge J., Ricci W., Hughes S. H. cis-Acting RNA packaging locus in the 115-nucleotide direct repeat of Rous sarcoma virus. J Virol. 1983 Dec;48(3):667–675. doi: 10.1128/jvi.48.3.667-675.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symington J., Green M., Brackmann K. Immunoautoradiographic detection of proteins after electrophoretic transfer from gels to diazo-paper: analysis of adenovirus encoded proteins. Proc Natl Acad Sci U S A. 1981 Jan;78(1):177–181. doi: 10.1073/pnas.78.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteegen R. J., Oroszlan S. Effect of chemical modification and fragmentation on antigenic determinants of internal protein p30 and surface glycoprotein gp70 of type C retroviruses. J Virol. 1980 Mar;33(3):983–992. doi: 10.1128/jvi.33.3.983-992.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Vrana K. E., Churchill M. E., Tullius T. D., Brown D. D. Mapping functional regions of transcription factor TFIIIA. Mol Cell Biol. 1988 Apr;8(4):1684–1696. doi: 10.1128/mcb.8.4.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]