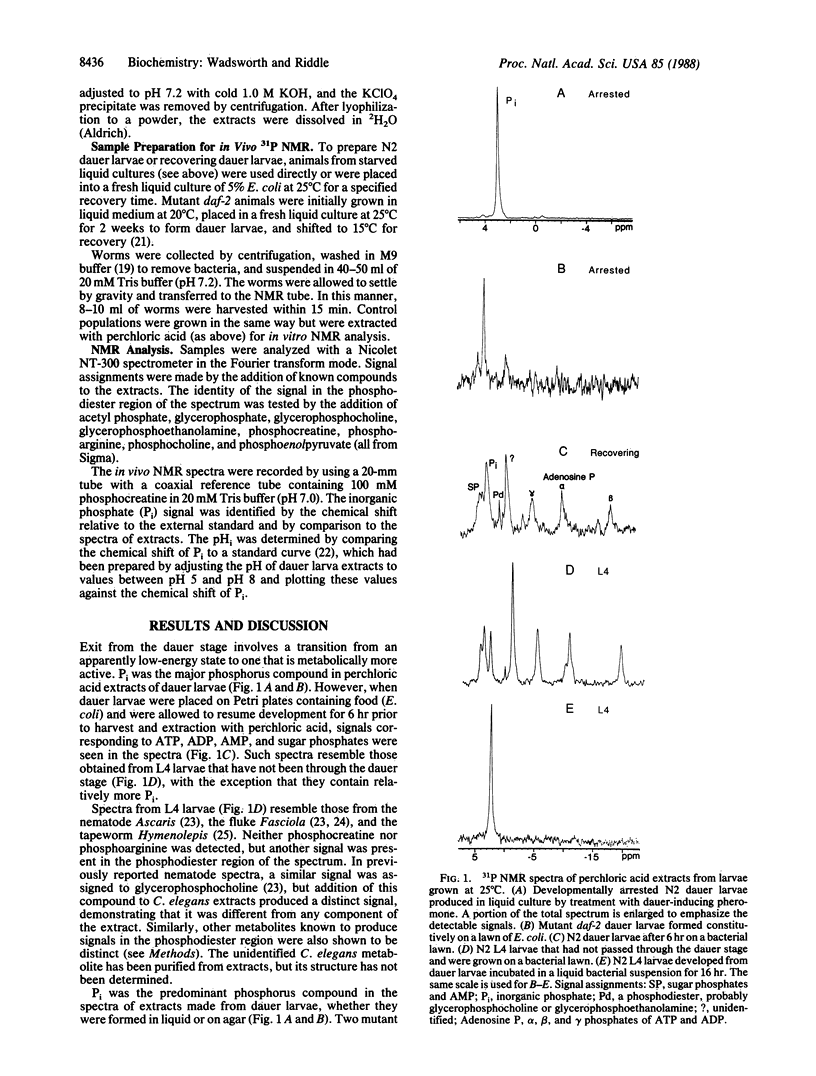
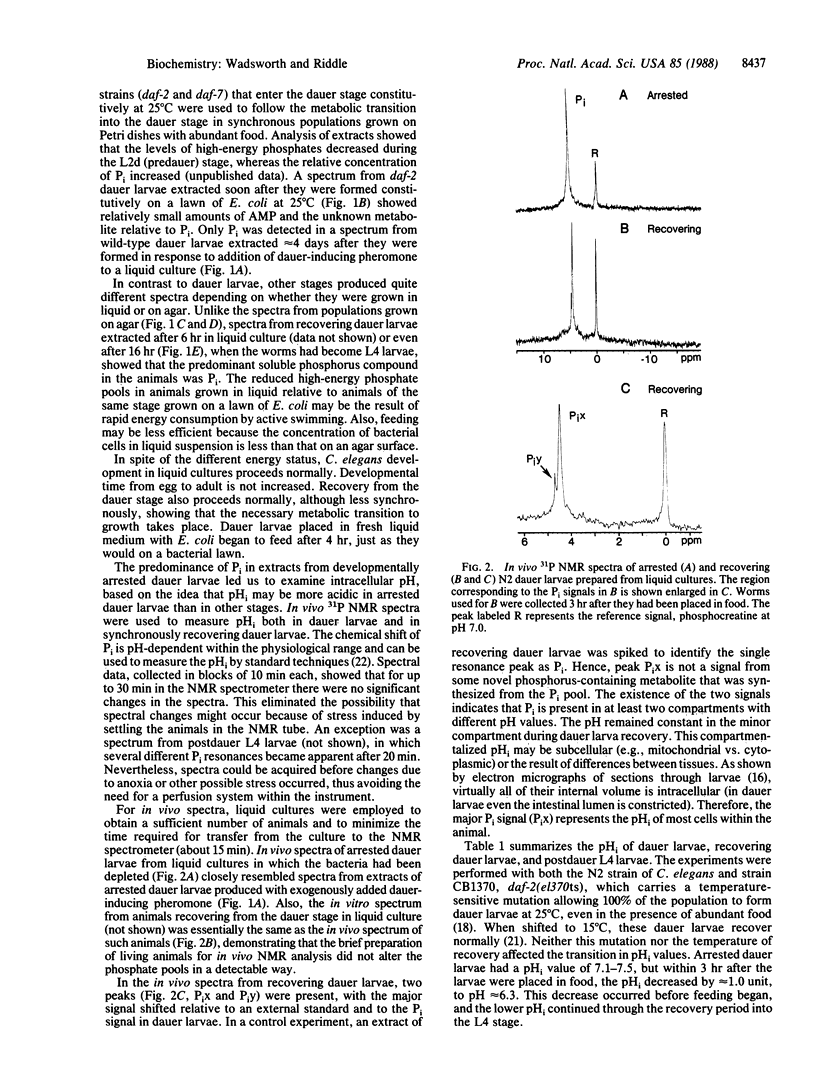
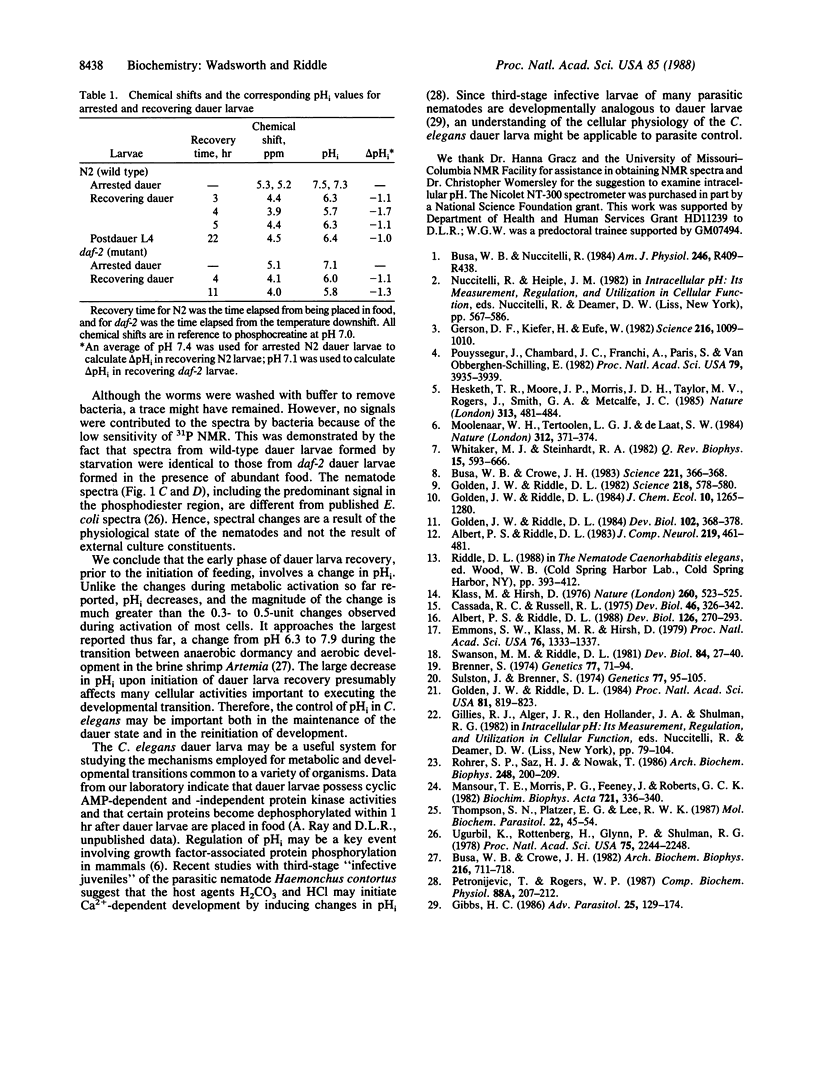
Abstract
During recovery from the developmentally arrested, nonfeeding dauer stage of the nematode Caenorhabditis elegans, metabolic activation is accompanied by a decrease in intracellular pH (pHi). Phosphorus-31 nuclear magnetic resonance (31P NMR) analyses of perchloric acid extracts show that inorganic phosphate predominates in dauer larvae, whereas ATP and other high-energy metabolites are abundant within 6 hr after dauer larvae have been placed in food to initiate development. Although metabolic activation has been associated with an alkaline pHi shift in other organisms, in vivo 31P NMR analysis of recovering dauer larvae shows a pHi decrease from approximately 7.3 to approximately 6.3 within 3 hr after the animals encounter food. This shift occurs before feeding begins, and it coincides with, or soon follows, the developmental commitment to recover from the dauer stage, suggesting that control of pHi may be important in the regulation of larval development in nematodes.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert P. S., Riddle D. L. Developmental alterations in sensory neuroanatomy of the Caenorhabditis elegans dauer larva. J Comp Neurol. 1983 Oct 1;219(4):461–481. doi: 10.1002/cne.902190407. [DOI] [PubMed] [Google Scholar]
- Albert P. S., Riddle D. L. Mutants of Caenorhabditis elegans that form dauer-like larvae. Dev Biol. 1988 Apr;126(2):270–293. doi: 10.1016/0012-1606(88)90138-8. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busa W. B., Crowe J. H. Intracellular pH Regulates Transitions Between Dormancy and Development of Brine Shrimp (Artemia salina) Embryos. Science. 1983 Jul 22;221(4608):366–368. doi: 10.1126/science.221.4608.366. [DOI] [PubMed] [Google Scholar]
- Busa W. B., Crowe J. H., Matson G. B. Intracellular pH and the metabolic status of dormant and developing Artemia embryos. Arch Biochem Biophys. 1982 Jul;216(2):711–718. doi: 10.1016/0003-9861(82)90261-2. [DOI] [PubMed] [Google Scholar]
- Busa W. B., Nuccitelli R. Metabolic regulation via intracellular pH. Am J Physiol. 1984 Apr;246(4 Pt 2):R409–R438. doi: 10.1152/ajpregu.1984.246.4.R409. [DOI] [PubMed] [Google Scholar]
- Cassada R. C., Russell R. L. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev Biol. 1975 Oct;46(2):326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- Emmons S. W., Klass M. R., Hirsh D. Analysis of the constancy of DNA sequences during development and evolution of the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1333–1337. doi: 10.1073/pnas.76.3.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerson D. F., Kiefer H., Eufe W. Intracellular pH of mitogen-stimulated lymphocytes. Science. 1982 May 28;216(4549):1009–1010. doi: 10.1126/science.6281887. [DOI] [PubMed] [Google Scholar]
- Gibbs H. C. Hypobiosis in parasitic nematodes--an update. Adv Parasitol. 1986;25:129–174. [PubMed] [Google Scholar]
- Golden J. W., Riddle D. L. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science. 1982 Nov 5;218(4572):578–580. doi: 10.1126/science.6896933. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Riddle D. L. A pheromone-induced developmental switch in Caenorhabditis elegans: Temperature-sensitive mutants reveal a wild-type temperature-dependent process. Proc Natl Acad Sci U S A. 1984 Feb;81(3):819–823. doi: 10.1073/pnas.81.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J. W., Riddle D. L. The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev Biol. 1984 Apr;102(2):368–378. doi: 10.1016/0012-1606(84)90201-x. [DOI] [PubMed] [Google Scholar]
- Hesketh T. R., Moore J. P., Morris J. D., Taylor M. V., Rogers J., Smith G. A., Metcalfe J. C. A common sequence of calcium and pH signals in the mitogenic stimulation of eukaryotic cells. Nature. 1985 Feb 7;313(6002):481–484. doi: 10.1038/313481a0. [DOI] [PubMed] [Google Scholar]
- Klass M., Hirsh D. Non-ageing developmental variant of Caenorhabditis elegans. Nature. 1976 Apr 8;260(5551):523–525. doi: 10.1038/260523a0. [DOI] [PubMed] [Google Scholar]
- Mansour T. E., Morris P. G., Feeney J., Roberts G. C. A 31P-nmr study of the intact liver fluke Fasciola hepatica. Biochim Biophys Acta. 1982 Dec 30;721(4):336–340. doi: 10.1016/0167-4889(82)90087-8. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Tertoolen L. G., de Laat S. W. Phorbol ester and diacylglycerol mimic growth factors in raising cytoplasmic pH. Nature. 1984 Nov 22;312(5992):371–374. doi: 10.1038/312371a0. [DOI] [PubMed] [Google Scholar]
- Petronijevic T., Rogers W. P. The physiology of infection with nematodes: the role of intracellular pH in the development of the early parasitic stage. Comp Biochem Physiol A Comp Physiol. 1987;88(2):207–212. doi: 10.1016/0300-9629(87)90471-3. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Chambard J. C., Franchi A., Paris S., Van Obberghen-Schilling E. Growth factor activation of an amiloride-sensitive Na+/H+ exchange system in quiescent fibroblasts: coupling to ribosomal protein S6 phosphorylation. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3935–3939. doi: 10.1073/pnas.79.13.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer S. P., Saz H. J., Nowak T. 31P-NMR studies of the metabolisms of the parasitic helminths Ascaris suum and Fasciola hepatica. Arch Biochem Biophys. 1986 Jul;248(1):200–209. doi: 10.1016/0003-9861(86)90417-0. [DOI] [PubMed] [Google Scholar]
- Sulston J. E., Brenner S. The DNA of Caenorhabditis elegans. Genetics. 1974 May;77(1):95–104. doi: 10.1093/genetics/77.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson M. M., Riddle D. L. Critical periods in the development of the Caenorhabditis elegans dauer larva. Dev Biol. 1981 May;84(1):27–40. doi: 10.1016/0012-1606(81)90367-5. [DOI] [PubMed] [Google Scholar]
- Thompson S. N., Platzer E. G., Lee R. W. In vivo 31P NMR spectrum of Hymenolepis diminuta and its change on short-term exposure to mebendazole. Mol Biochem Parasitol. 1987 Jan 2;22(1):45–54. doi: 10.1016/0166-6851(87)90068-5. [DOI] [PubMed] [Google Scholar]
- Ugurbil K., Rottenberg H., Glynn P., Shulman R. G. 31P nuclear magnetic resonance studies of bioenergetics and glycolysis in anaerobic Escherichia coli cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2244–2248. doi: 10.1073/pnas.75.5.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker M. J., Steinhardt R. A. Ionic regulation of egg activation. Q Rev Biophys. 1982 Nov;15(4):593–666. doi: 10.1017/s0033583500003760. [DOI] [PubMed] [Google Scholar]


