Abstract
KIT activation, through binding of its ligand, stem cell factor (SCF), is crucial for normal mast cell growth, differentiation, and survival. Furthermore, KIT may also contribute to mast cell homing and cytokine generation. Activating mutations in KIT lead to the dysregulated mast cell growth associated with the myeloproliferative disorder mastocytosis. We investigated the potential of down-regulating such responses through mast cell inhibitory receptor activation. Here, we report that the B cell-associated ITIM (immunoreceptor tyrosine-based inhibitory motif)-containing inhibitory receptor, CD72, is expressed in human mast cells. Ligation of CD72 with the agonistic antibody, BU40, or with recombinant human CD100 (rCD100), its natural ligand, induced the phosphorylation of CD72 with a resulting increase in its association with the tyrosine phosphatase SHP-1 (SH2 domain containing phosphatase-1). This, in turn, resulted in an inhibition of KIT-induced phosphorylation of Src family kinases and extracellular-regulated kinases (ERK1/2). As a consequence of these effects, KIT-mediated mast cell proliferation, chemotaxis, and chemokine production were significantly reduced by BU40 and rCD100. Furthermore, BU40 and rCD100 also down-regulated the growth of the HMC1.2 human mast cell line. Thus, targeting CD72 may provide a novel approach to the suppression of mast cell disease such as mastocytosis.
Introduction
Mast cells are cells of hematopoietic lineage which participate in both innate and acquired immune responses (1). The activation of KIT by its ligand, stem cell factor (SCF, also termed Steel factor or KIT ligand), initiates signaling cascades which are critical for mast cell growth, development, and survival (2). Furthermore, these signals also induce mast cell chemotaxis and, at least under experimental conditions, adhesion (2). Gain of function mutations in KIT lead to the dysregulated cell growth associated with the clonal accumulation of mast cells in tissues as observed in systemic mastocytosis and mast cell leukemia (3 – 5).
KIT-mediated responses in mast cells, however, can be modified by signals produced by other receptors expressed on the cell surface. For example, the ability of KIT to promote mast cell growth can be markedly enhanced by IL-3-induced ligation of the IL-3 receptor (2). In contrast, mast cells also express surface receptors including FcγRIIb, Siglecs, MAFA, signal regulatory protein α, and leukocyte Ig-like receptor B4 (formerly gp49B1), paired Ig-like receptor-B, myeloid-associated immunoglobulin-like receptor (MAIR) I, CD200 receptor, and CD300a, which have the capacity to down-regulate such activation (9 – 11). These inhibitory receptors are largely typified by immunoreceptor tyrosine-based inhibitory (ITIM) motifs within their cytosolic domains (9). ITIMs comprise the homology sequence (I/V/L/S)xYxx(L/V) (x; any residue) (12). Upon receptor ligation/activation, the tyrosines contained within these sequences become phosphorylated following the activation of receptor tyrosine kinases or Src family member tyrosine kinases (SFKs). This allows the recruitment of the non-receptor protein phosphatases, Src homology 2 domain-containing tyrosine phosphatase (SHP)-1, SHP-2, or Src homology 2 domain-containing inositol 5-phosphatase (SHIP) 1 (12). SHP-1 and SHP-2 are tyrosine phosphatases which dephosphorylate tyrosine-containing signaling molecules, thereby reversing the action of tyrosine kinases, whereas SHIP1 dephosphorylates phospatidylinositol 3,4,5 trisphosphate at the 3’ position thereby terminating PI3K (phosphatidylinositol 3-kinase)-driven signaling pathways (12). ITIM-containing receptors, thus, may have application in the management of mast cell-driven disease. However, in many cases the natural ligands for the inhibitory receptors are unknown (9) and those that are known may not be ideal for down-regulating mast cell responses. For these reasons, down-regulation of mast cell responses via inhibitory receptors has primarily been achieved using antibodies targeting the receptors.
We, therefore, wished to explore whether we could down-regulate KIT-mediated mast cell responses through an ITIM-bearing inhibitory receptor utilizing its recognized soluble ligand. One such receptor is CD72 (Lyb-2), an ITIM-containing, 45 kDa type II transmembrane protein of the C type lectin family (13) whose natural ligand has been identified as CD100 or Semaphorin 4D (Sema4D). Here we report that CD72 is expressed on human mast cells derived from CD34-positive peripheral blood cells of healthy volunteers (huMCs) and human mast cell lines. The concurrent ligation of CD72 and KIT resulted in an increase in the phosphorylation of CD72, and enhanced association between CD72 and SHP-1. This led to the suppression of the KIT-mediated phosphorylation of SFKs and ERKs, critical players in KIT-mediated huMC responses (6). Thus, ligation of CD72 reduced KIT-mediated proliferation, chemotaxis, and monocyte chemoattractant protein-1 (MCP-1 or CCL2) production in huMCs and the suppression of growth of HMC1.2 harboring the gain-of-function mutation in KIT gene. From these studies, we conclude that CD72 – CD100 interactions down-regulate KIT-mediated mast cell responses via the formation of the CD72 – SHP-1 complex. Thus, down-regulation of KIT-mediated responses through CD72 may provide a potential means for the control of mast cell-driven disorders.
Materials and Methods
Cells
Human mast cells (huMCs), derived from CD34-positive peripheral blood cells, were cultured in StemPro-34 medium with supplement (Invitrogen, Calrlsbad, CA), containing l-glutamine (2 mM), penicillin (100 units/ml), streptomycin (100 µg/ml), recombinant human SCF (100 ng/ml, Peprotech, Rocky Hill, NJ), and recombinant human IL-6 (100 ng/ml, Peprotech) as before (15). The human LAD2 mast cell line was cultured in StemPro-34 with supplement containing l-glutamine (2 mM), penicillin (100 units/ml), streptomycin (100 µg/ml), and recombinant human SCF (100 ng/ml, Peprotech) (16). HMC1.1 (expresses V560G KIT) and HMC1.2 (expresses V560G and D816V KIT) human mast cell lines were grown in IMDM medium supplemented with FBS (10%), l-glutamine (2 mM), penicillin (100 units/ml), and streptomycin (100 µg/ml) (5, 17, 18). The U937 monocytic cell line and the Raji human B cell line were purchased from American Type Culture Collection (Manassas, VA), and grown in RPMI1640 medium supplemented with FBS (10%), l-glutamine (2 mM), penicillin (100 units/ml), and streptomycin (100 µg/ml).
Recombinant CD100 and antibodies
Recombinant CD100 protein was prepared as described (19). The anti-human CD72 antibody (Ab) BU40 (monoclonal, mouse IgG) was purchased from Southern Biotechnology Associates (Birmingham, AL). The anti-CD72 Ab H-96 (rabbit polyclonal IgG), and anti SHP-1 Ab (C-19, rabbit polyclonal IgG) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phosphotyrosine Ab (4G10, mouse monoclonal IgG) was purchased from Millipore (Billerica, MA). Anti-phospho-KIT (Tyr 703) Ab (rabbit polyclonal IgG) was purchased from Invitrogen. Anti-phospho-Src (Tyr 416) and anti-phospho-ERK Ab (both Abs were rabbit polyclonal IgG) were obtained from Cell Signaling Technology (Beverly, MA). Anti-β-actin Ab (mouse monoclonal IgG) was obtained from Sigma-Aldrich (St. Louis, MO). Isotype control Abs were obtained from BD Biosciences (San Jose, CA) and the secondary Abs were peroxidase-labeled anti-rabbit or anti-mouse IgG Abs from Santa Cruz Biotechnology (Santa Cruz, CA).
Immunoblotting and immunoprecipitation
Cell lysates were prepared, the proteins separated by electrophoresis, and gels probed for immunoreactive proteins as described (20). Immunoprecipitation experiments were executed using an anti-CD72 Ab (H-96) or anti-SHP-1 Ab (C-19) as described (19). The cells were lysed with buffer containing 150 mM NaCl, 10 mM Tris-HCl (pH 8.0), 1 mM EDTA, 1mM Na3VO4, 0.5 mM PMSF, 5 µg/ml aprotinin, 5 µg/ml leupeptin, Complete protease inhibitor cocktails (Roche, Indianapolis, IN), and NP-40 (1%). The cell lysates were incubated with rabbit IgG-bound protein G-sepharose for 30 min, then incubated with anti-CD72 Ab (H-96)-bound or anti-SHP-1 Ab (C-19)-bound protein G-sepharose overnight at 4 °C with rotation.
RT-PCR
Total RNA was extracted from 5 × 105 cells (HuMCs at various times of culture, LAD2, HMC1.1, HMC1.2, U937 and Raji). Cells were lysed with Trizol (Invitrogen) and RNA was extracted using Rneasy Mini Kit (Qiagen, Valencia CA). cDNA was synthesized and amplified using SuperScript III One-Step RT-PCR System (Invitrogen). One ug of RNA was used for cDNA synthesis. A 834 bp region of CD72 gene between exon1–6 was amplified using the primers 5'-ATGGCTGAGGCCATCACCTA-3', and 5'-TGATTGTGGATAAATTTCACTGAAT-3' and a 998 bp fragment between exon 2–8 was amplified using primers 5'-ACCCAGGGGCTGATGAT-3' and 5'-CTAATCTGGAAACCTGAAAGCTG-3'. Complementary DNA synthesis and PCR amplification were performed with a DNA Engine PTC200 cycler (Bio Rad Laboratories, Hercules, CA) with the following thermal cycle protocol. 50 °C for 30 min, 94 °C for 2 min followed by 30 cycles of 94 °C for 30s, 60 °C for 1 min, 72 °C for 1 min and a final extension of 10 min at 72 °C.
Flow cytometry
For the detection of CD72 surface expression, huMCs, LAD2, HMC1.1, or HMC1.2 cells were washed with PBS, and fixed with 4 % paraformaldehyde (Sigma-Aldrich). The cells were stained with anti-human CD72 (BU40) overnight at 4 °C followed by anti-mouse IgG-FITC for 2 hours at 4 °C. The cells were then analyzed using a FACScan flow cytometer.
Cell cycle analysis
Cell cycle progression of huMCs or HMC1.2 was analyzed as described (21). Briefly, huMCs or HMC1.2 cells were cultured overnight in cytokine-free medium, and resuspended in HEPES buffer containing 0.04 % BSA (huMCs) or IMEM + 10 % FBS (HMC1.2). After 30 minutes pre-culture, 10 ng/ml SCF was added and the cells cultured for 30 min with or without control IgG, BU40, or rCD100 (10 µg/ml, respectively). The cells were fixed with 100 % ethanol, treated with RNase A (Roche), and stained with propidium iodine (Sigma-Aldrich) for 2 h at 4°C. The cells were then analyzed using a FACScan flow cytometer.
Bromodeoxyuridine (BrdU) cell proliferation assay
Cell proliferation was determined by the BrdU (an analogue of thymidine) assay which measures its incorporation into DNA. HuMCs were cultured overnight in cytokine-free StemPro-34 medium, then resupended in the same medium. HuMCs were then incubated for 24 h at 1.5 × 105 cells/100 µl in 96-well plates with PBS with 0.1% NaN3, SCF + PBS with 0.1% NaN3, SCF + control IgG (10 µg/ml), SCF + BU40 (10 µg/ml), or SCF + rCD100 (10 µg/ml) (SCF; 10 ng/ml, respectively). HMC1.2 cells were cultured overnight in IMEM without FBS, then resuspended in IMEM + 10 % FBS. HMC1.2 cells were then incubated for 24 hours at 1.5 × 105 cells/100 µl in 96-well plates with PBS with 0.1% NaN3, control IgG (10 µg/ml), BU40 (10 µg/ml), or rCD100 (10 µg/ml). Incorporation of BrdU into the huMCs or HMC1.2 cells was assessed using a BrdU cell proliferation assay kit (Calbiochem, San Diego, CA) as previously described (21).
Chemotaxis assay
Chemotaxis was assessed using transwell polycarbonate membranes (8 µm pores, Costar, Cole-Parmer, Vernon Hills, IL) as described (20). Briefly, 1 × 105 huMCs were cultured overnight in cytokine-free medium, and re-suspended in HEPES buffer containing 0.5 % BSA. PBS with 0.1% NaN3, control IgG (10 µg/ml), BU40 (10 µg/ml), or rCD100 (10 µg/ml) was added to the cell suspension (1 × 105 / 100 µl), then the cells placed in the upper chamber. The upper chambers were placed in lower chambers containing 600 µl of HEPES buffer with 0.5 % BSA for 30 min at 37 °C, then chemotaxis was assessed by placing the upper chambers in a new set of lower chambers containing the same buffer with SCF (30 ng/ml). After 4 h incubation at 37 °C, the cells migrating to the lower wells were collected and counted by microscopy.
Monocyte chemotactic protein (MCP)-1 (CCL2) secretion
To examine KIT-mediated MCP-1 (CCL2) secretion (22), huMCs were cultured overnight in cytokine-free medium, then resuspended in cytokine-free medium. One × 105 huMCs per 100 µl / well were cultured for 6 h in the presence of 10 ng/ml SCF with or without control IgG, BU40, or rCD100 (10 µg/ml, respectively). Fifty µl of the cell-free supernatants were taken for ELISA assay (human MCP-1 DuoSet ELISA system, R&D systems, Minneapolis, MN, USA) according to the manufacture’s protocol.
Degranulation assay
Degranulation from huMCs was monitored by β-hexominidase (β-hex) release as previously described (20). HuMCs were sensitized overnight in cytokine-free, supplemented StemPro cell culture medium containing human myeloma IgE (100 ng/ml, Calbiochem, EMD Bioscienes, San Diego, CA, biotinylated in the NIAID core facility), in the presence of control IgG, BU40, or rCD100 (10 µg/ml, respectively). Following rinsing in HEPES buffer containing 0.04% BSA, the cells were activated in this buffer with streptavidin (100 ng/ml, Sigma-Aldrich) with or without SCF (1 ng/ml) for 30 min. The reactions were terminated by centrifugation (3000 rpm for 5 min) at 4 °C, and the supernatants were aliquoted to 96-well plates for β-hex assay. The remaining cells were lysed by adding 0.1% Triton X-100, then also analyzed for β-hex content. Degranulation was calculated as percentages of total β-hex content found in the supernatants following challenge.
Statistical analysis
Data were expressed as the mean +/− SE. Differences between groups were examined for statistical significance using Student’s t-test (Excel: Microsoft, Redmond, WA, USA). A P value less than 0.05 indicated statistical significance.
Results
Human mast cells express CD72
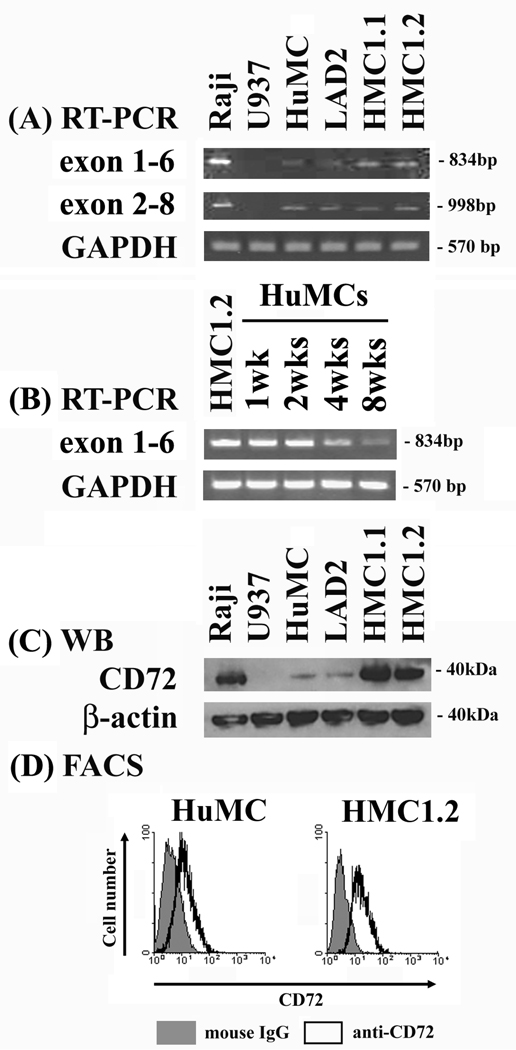
To first explore whether CD72 is expressed in human mast cells, we examined the presence of CD72 mRNA in huMCs and the HMC1.1, HMC1.2, and LAD2 human mast cell lines. Two sets of primers were designed, targeting exon1 to exon6, or exon2 to exon8, of CD72 mRNA. Raji cells (human Burkitt’s lymphoma B cell line) were used as a positive control and U937 cells as a negative control (23). As expected, mRNA for CD72 was found in the Raji cells, but not in the U937 cells (Fig. 1A). CD72 mRNA was also detected in primary cultured huMCs, and in the LAD2, HMC1.1, and HMC1.2 human mast cell lines, with the size of these transcripts being identical to that for the Raji cells (Fig. 1A). CD72 mRNA was detected at all times during the development of the huMCs cultures. The CD72 mRNA levels of 1 and 2 week old cultures were as high as that of HMC1.2 cells However the CD72 mRNA levels tended to decrease during subsequent culture periods (Fig. 1B).
Figure 1. Human mast cells and mast cell lines express CD72.
(A) RT-PCR. (B) Western blotting. (C) Flow cytometry were performed as described in Material and Methods. The Raji cells were used as a positive control and the U937 cells as a negative control.
To confirm the presence of CD72 in human mast cells, we next examined protein expression by immunoblot analysis. These studies verified that CD72 protein is present in the primary cultured huMCs and human tumor mast cell lines, as well as in the Raji control cells (Fig. 1C). The expression of CD72 in the HMC1.1 and HMC1.2 cell lines was greater than the expression levels observed in the mature (8 wk) huMCs and LAD2 cells. Again, CD72 protein was absent in the U937 negative control (Fig. 1C). To establish that CD72 was expressed at the cell surface, we examined surface staining with an anti-CD72 antibody by FACS analysis. As can be seen from Fig. 1D, surface CD72 was detected on both cell types examined; huMCs and HMC1.2 cells.
Concurrent ligation of CD72 and KIT induces CD72 phosphorylation, SHP-1 recruitment, and SHP1 phosphorylation
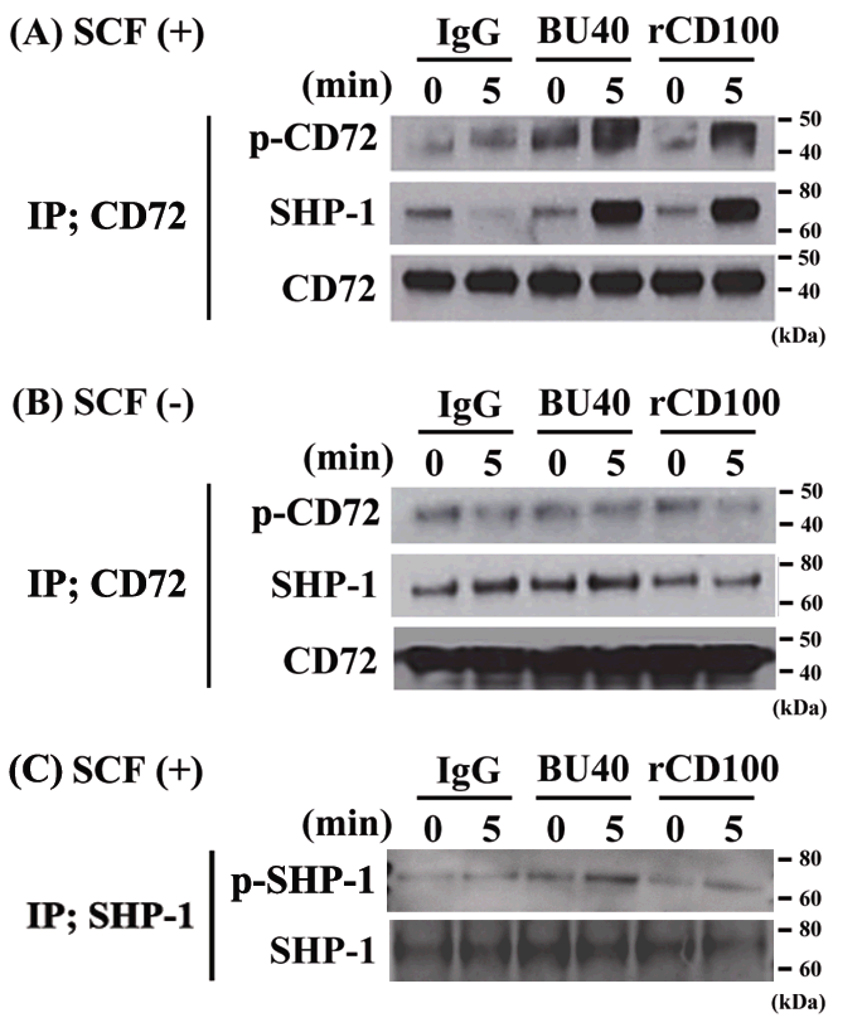
Members of the ITIM-bearing family of inhibitory receptors mediate their effects through recruitment of the inositol phosphatases SHIP1 and SHIP2 or the tyrosine phosphates SHP-1 and SHP-2 (12). CD72 contains two ITIMs, one of which binds SHP-1 and the other Grb2, when the tyrosine residues within these ITIMs are phosphorylated (24, 25). To examine whether KIT activation induced CD72 phosphorylation or dephosphorylation in mast cells, and whether the phosphorylation status of CD72 was further modified following CD72 ligation, huMCs were stimulated for 5 minutes with SCF in the absence or presence of BU40 or rCD100. Cell lysates were then prepared, CD72 immunoprecipitated with an anti-CD72 antibody, and the immunoprecipitates probed for phosphotyrosine and the association of SHP-1. Lysates were also immunoprecipitated with a control antibody to assess non-specific interactions. Using the control antibody for immunoprecipitating, we detected no increase in the tyrosine phosphorylation of CD72 or its association with SHP-1 under any of the experimental conditions described (data not shown).
Using the anti-CD72 antibody for immunoprecipitations, a slight, but detectable, constitutive tyrosine phosphorylation of CD72 was observed under resting conditions (Fig. 2A & 2B). This was associated with a similar slight, but detectable, constitutive association of SHP-1 with CD72 (Fig. 2A & 2B). Challenging the cells with SCF in the absence of BU40 or rCD100 induced minimal change in tyrosine phosphorylation of CD72 and no detectable increase in association of SHP-1 with CD72 (Fig. 2A). When the cells were challenged with SCF in the presence of BU40 or rCD100, however, there was an appreciable increase in tyrosine phosphorylation of CD72 which was linked to a marked enhancement of the association of SHP-1 with CD72 (Fig. 2A). However when the cells were challenged with BU40 or rCD100 in the absence of SCF, we could not detect a significant change in the level of the phosphorylation of CD72 or the association between CD72 and SHP-1 (Fig. 2B).
Figure 2.
The effects of BU40 or recombinant CD100 on the tyrosine phosphorylation of CD72, the association of SHP-1 with CD72 and the tyrosine phosphorylation of SHP-1 in huMCs. (A) BU40 or recombinant CD100 administration with SCF to huMCs induces the tyrosine phosphorylation of CD72 and the association with SHP-1, whereas (B) BU40 or recombinant CD100 administration in the absence of SCF did not. HuMCs were incubated for 0 or 5 min with control IgG (10 µg/ml), BU40 (10 µg/ml), or recombinant CD100 (10 µg/ml) in the presence or absence of SCF (10 ng/ml). CD72 was immunoprecipitated with anti-CD72 (H-96), and visualized with anti-phosphotyrosine 4G10, anti-SHP-1, or anti-CD72. (C) BU40 or recombinant CD100 administration with SCF (10 ng/ml) to huMCs induces the tyrosine phosphorylation of SHP-1, when incubated for 5 min. SHP-1 was immunoprecipitated with an anti-SHP-1 antibody (C-19), and visualized with anti-phosphotyrosine 4G10 or anti-SHP-1. Data are representative of three individual experiments.
Tyrosine phosphorylation of SHP-1 up-regulates the phosphatase activity of SHP-1 (26). To assess whether ligation of CD72 induced tyrosine phosphorylation of SHP-1 in addition to recruitment, huMCs were stimulated with SCF concurrently with CD72 ligation, whole cell lysates were immunoprecipitated with an anti-SHP-1 antibody, then the immunoprecipitated proteins probed with an anti-phosphotyrosine antibody. Slight tyrosine phosphorylation of SHP-1 was observed under resting conditions (Fig. 2C). However, when the cells were challenged with SCF in the presence of BU40 or rCD100, the tyrosine phosphorylated SHP-1 levels were observed to increase (Fig.2C).
Taken together, these data provide support for the conclusion that concurrent ligation of CD72 and KIT results in the activation and recruitment of SHP-1 to CD72 with the potential to down-regulate KIT-mediated responses in mast cells. We therefore next examined the effects of the CD72 agonistic antibody BU40 and rCD100 on KIT-mediated mast cell function.
CD72 ligation suppresses KIT-dependent growth of human mast cells
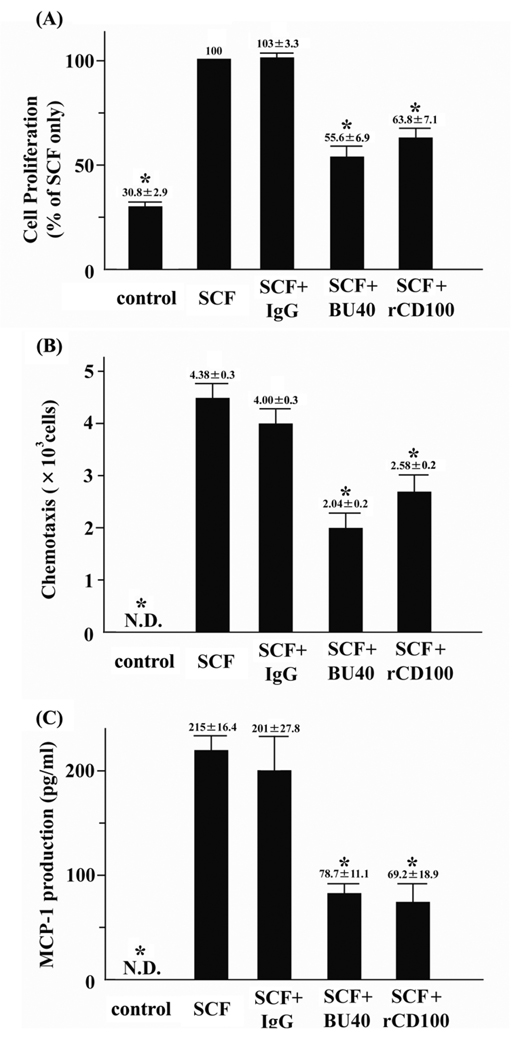
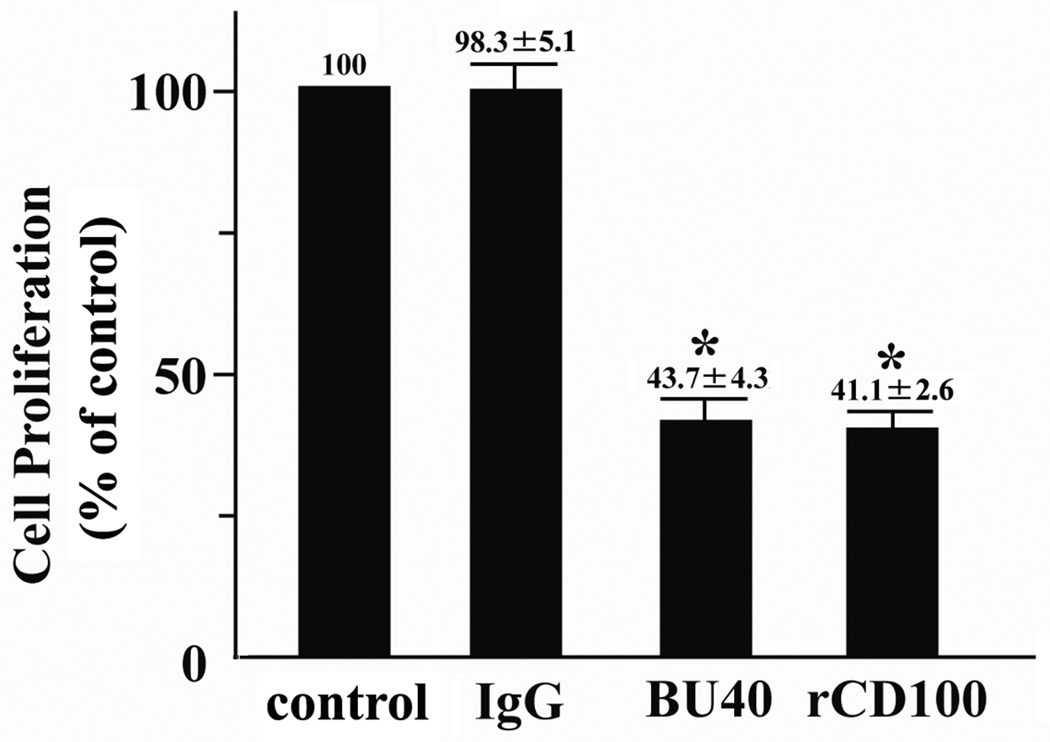
To explore the potential ability of ligated CD72 to down-regulate mast cell function, we next examined the effects of the agonistic antibody against human CD72, BU40, or rCD100, on indices of KIT-mediated growth of huMCs. To evaluate the potential role of CD72 in controlling progression through the cell cycle, cytokine-starved huMCs were cultured with SCF for 30 min with or without control IgG, BU40, or rCD100, then the cell cycle status of the cells was assessed by flow cytometry. The proportion of huMCs in G2/M + S phases following 30 min exposure to SCF was not reduced by control antibody. However, administration of either BU40 or rCD100, together with SCF, resulted in a marked reduction in the ratio of cells in the G2/M + S phases of cell cycle, indicating arrested cell growth (Table 1). To provide further support for this conclusion, we examined the abilities of BU40 and rCD100 to inhibit KIT-induced proliferation of huMCs as assessed by BrdU assay. HuMCs were cultured for 24 hours in the absence or presence of SCF + control IgG, SCF + BU40, or SCF + rCD100, then the relative growth rates determined. As in the case with cell cycle arrest, both BU40 and rCD100 significantly reduced the growth of huMCs detected in the BrdU assay (Fig. 3A), providing further evidence that ligation of CD72 induces down-regulation of KIT-mediated growth of human mast cells.
Table 1.
Ligation of CD72 with BU40 or recombinant CD100 suppresses KIT-mediated cell cycle progression of human mast cells.
After overnight culture in cytokine-free medium, huMCs were cultured for 30 min in HEPES buffer containing 0.04 % BSA (huMCs) with SCF, SCF + control IgG, SCF + BU40, or SCF + rCD100. The cells were stained with propidium iodine, and then analyzed using a FACScan flow cytometer.
| Treatment | Percentage of cells in G2/M + S phases (%) |
|---|---|
| SCF only | 6.93 ± 0.58 |
| SCF + control IgG | 7.39 ± 0.85 |
| SCF + BU40* | 3.07 ± 0.22 |
| SCF + recombinant CD100* | 3.60 ± 0.44 |
P < 0.05, when compared with the value of SCF + control IgG.
Figure 3.
The effects of BU40 or recombinant CD100 on KIT-mediated responses of huMCs. (A) The ligation of CD72 with BU40 or recombinant CD100 suppresses KIT-induced proliferation of huMCs (BrdU assay, n = 4). Human mast cells were incubated for 24 h with or without control IgG, BU40, or recombinant CD100 (10 µg/ml, respectively) in the absence or presence of SCF (10 ng/ml). HuMC proliferation was assessed using a BrdU cell proliferation assay kit according to the manufacture’s protocol. The relative values are indicated when the value of SCF + control IgG is 100. *; P < 0.05, when compared with SCF + control IgG. (B) Ligation of CD72 with BU40 or recombinant CD100 suppresses SCF-induced mast cell chemotaxis. HuMCs (1 × 105) with or without control IgG, BU40, or recombinant CD100 (10 µg/ml, respectively) were incubated in the upper chambers to assess the migration to the lower chambers containing SCF (30 ng/ml) for 4 hours (n = 3). After the incubation, cell migration to the lower wells was assed by counting by microscopy. *; P < 0.05, when compared with SCF + control IgG. (C) Ligation of CD72 with BU40 or recombinant CD100 suppresses SCF-induced MCP-1 secretion. HuMCs were incubated for 6 h with or without control IgG, BU40, or recombinant CD100 (10 µg/ml, respectively) in the absence or presence of SCF (10 ng/ml) (n = 5). The culture supernatants were used for the ELISA assay for human MCP-1. *; P < 0.05, when compared with SCF + control IgG.
CD72 ligation down-regulates KIT-induced huMC chemotaxis and MCP-1 (CCL2) production
In addition to regulating cell growth, SCF-dependent KIT activation induces mast cell chemotaxis and the production of MCP-1 (CCL2) (2). We, thus, next investigated if these parameters were down-regulated by CD72 ligation in a similar manner to that observed on cell growth. To evaluate the potential of CD72 to down-regulate SCF-induced chemotaxis (27), cytokine-starved huMCs were placed in the upper chambers of dual chemotactic wells, then the cells were allowed to migrate to the lower chambers in response to SCF for 4 h in the absence and presence of control IgG, BU40 or rCD100. From Figure 3B, it can be seen that both BU40 and rCD100 significantly reduced SCF-induced huMC chemotaxis, whereas the control IgG had little effect.
KIT activation promotes MCP-1 (CCL2) secretion from human lung mast cells even in the absence of FcεRI stimulation (22). We therefore next explored the ability of BU40 or rCD100 to inhibit SCF-induced MCP-1 secretion from huMCs. HuMCs were cultured in medium containing SCF in the absence or presence of control IgG, BU40, or rCD100 for 6 hours, then the supernatants were assayed for MCP-1 content by ELISA. As with cell growth and chemotaxis, BU40 and rCD100 significantly inhibited SCF-mediated huMC MCP-1 production (Fig. 3C).
CD72 ligation does not affect FcεRI-medated huMC degranulation but inhibits the augmentation of this response by Kit
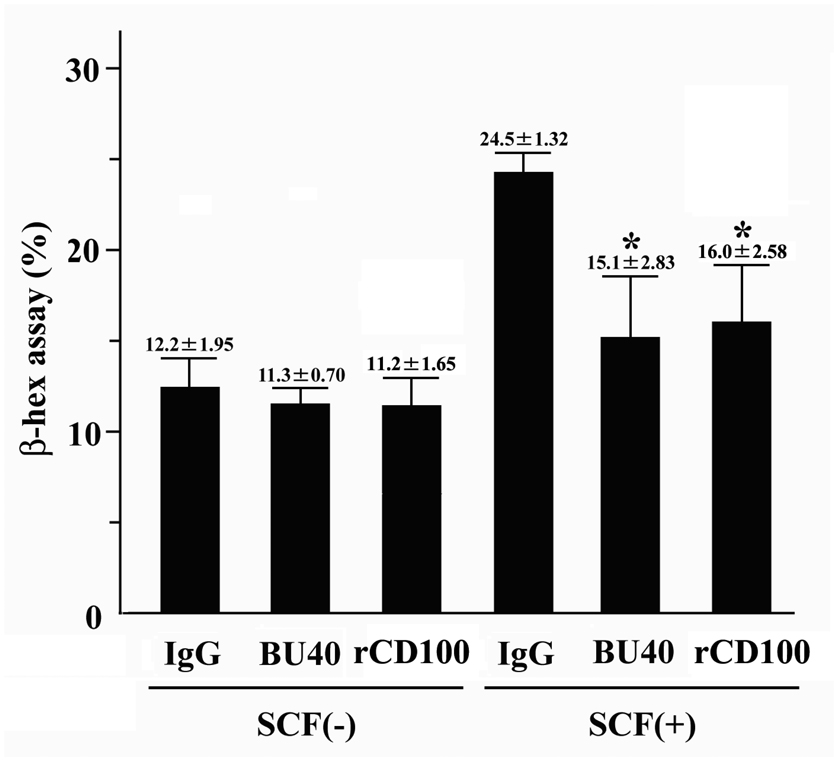
Antigen-mediated aggregation of high affinity receptors for IgE (FcεRI) triggers degranulation of mast cells (1, 2). This response can be further augmented following SCF-dependent KIT activation (6, 8). We, thus, examined whether these responses were downregulated following CD72 ligation in huMCs. Concurrent ligation of CD72 with FcεRI failed to reduce the response observed with FcεRI alone. However, CD72 ligation resulted in a significant reduction in the augmentation of this response by SCF (Fig. 4), demonstrating that the Kit-, but not FcεRI-mediated component of this response could be downregulated by CD72 ligation.
Figure 4.
The effects of BU40 or recombinant CD100 on IgE-triggered degranulation of huMCs. BU40 or recombinant CD100 administration did not inhibit IgE/streptavidin- (FcεRI-mediated) induced degranulation in the absence of SCF. However, BU40 and recombinant CD100 administration suppressed SCF-induced augmentation of IgE/streptavidin-induced degranulation of huMCs. Degranulation from huMCs was evaluated by β-hexominidase release as described in Material and Methods. HuMCs were sensitized overnight in cytokine-free medium containing biotinylated-human myeloma IgE (100 ng/ml) in the presence of control IgG, BU40, or rCD100 (10 µg/ml, respectively), and activated with streptavidin (100 ng/ml) with or without SCF (1 ng/ml) for 30 min (n = 3). *; P < 0.05, when compared with control IgG in the absence or presence of SCF.
CD72 ligation induces down-regulation of KIT-mediated phosphorylation of signaling molecules
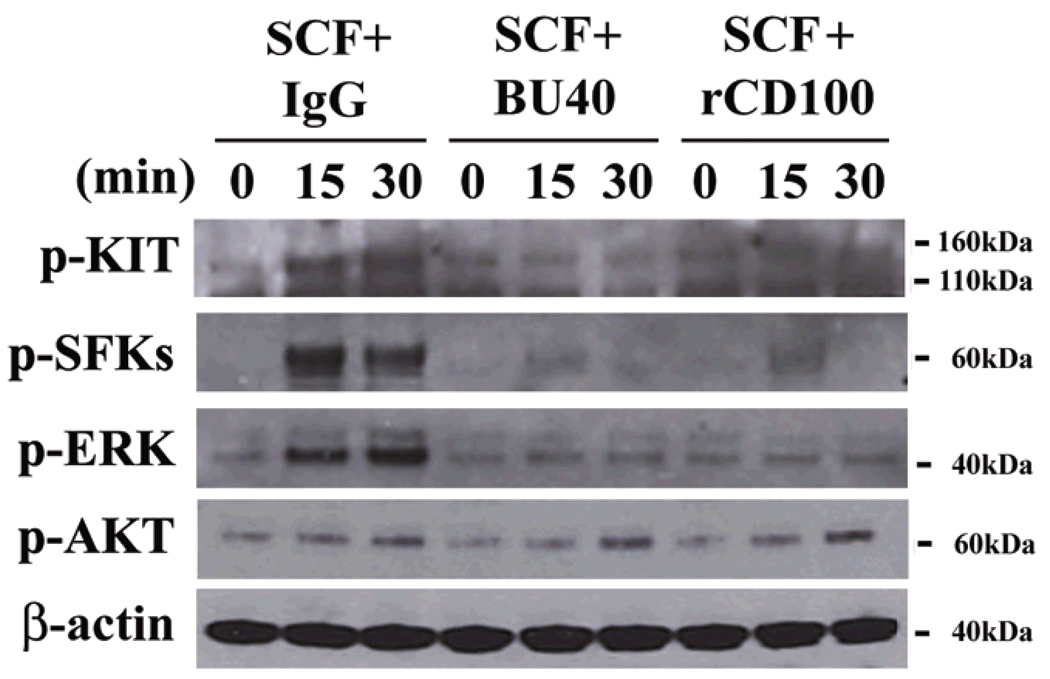
SCF-induced KIT dimerization results in KIT autophosphorylation, thereby recruiting critical signaling molecules into the receptor signaling complex where they become phosphorylated, allowing their regulation of downstream processes required for mast cell growth and function (6). Such events include recruitment and activation of Src family kinases (SFKs) and downstream signaling cascades regulated by phosphatidylinositol 3-kinase (PI3K), mitogen-activated protein kinases (MAPKs), and signal transducers and activators of transcription (Stat) 3 (6). We therefore examined whether the recruitment of SHP-1, following ligation of CD72, was associated with the reversal of these signaling pathways. HuMCs were again stimulated with SCF in the presence or absence of control IgG, BU40 or rCD100 for 15 or 30 mins, then lysates probed for phospho-KIT, phospho-AKT (a surrogate marker for PI3K activation), phospho-SFKs (recognizes both phospho-Lyn and phospho-Fyn), phospho-ERK1/2 MAPKs, and phospho-Stat3.
At both time points, it can be seen that phosphorylation of KIT, SFKs, and ERK1/2 was markedly reduced in the cells treated with BU40 and rCD100 (Fig. 5). However, there were no marked differences in the phosphorylation of either Stat3 (data not shown) or AKT (Fig. 5) following treatment of the cells with these reagents, though both proteins were phosphorylated by SCF challenge (data not shown). These data suggest that the ability of CD72 to inhibit mast cell growth and function is linked to the SHP-1-dependent down-regulation of pathways regulating and regulated by SFKs and ERK1/2, but not by PI3K/AKT and Stat3.
Figure 5.
Ligation of CD72 with BU40 or recombinant CD100 suppresses the activation of KIT signaling in huMCs. HuMCs were incubated for the indicated times with control IgG, BU40, or recombinant CD100 (10 µg/ml, respectively) in the presence of SCF (10 ng/ml). The levels of phospho-KIT, phospho-ERK, phospho-Src family kinases (Tyr 416), and β-actin were then evaluated by immunoblot analysis. Data are representative from three individual experiments.
CD72 downregulates the growth and the phosphorylation of signaling molecules in HMC1.2 cells
The HMC1.2 human mast cell line was established from a patient with mast cell leukemia (17). This cell line harbors gain-of-function mutations in KIT, thus proliferates independently of SCF (5, 18). We examined the effect of BU40 or rCD100 on mutated KIT-induced growth of HMC1.2 by cell cycle analysis and by BrdU assay, as for the huMC analysis. HMC1.2 cells were cultured without SCF for 30 min with or without control IgG, BU40, or rCD100, then the cell cycle status of the cells was assessed by flow cytometry. The proportion of HMC1.2 in G2/M + S phases following 30 min exposure to rCD100 or BU40 was not reduced compared to that exposure to control IgG (none; 23.6 +/− 1.38 %, control IgG; 23.3 +/− 1.78 %, BU40; 20.9 +/− 1.93 %, recombinant CD100; 21.5 +/− 2.36 %). We then assessed the effects of rCD100 or BU40 by BrdU assay. In contrast to the cell cycle data, rCD100 or BU40 markedly suppressed the HMC1.2 proliferation for 24 h by this assay (Fig. 6).
Figure 6.
Ligation of CD72 with BU40 or recombinant CD100 suppresses mutated KIT-driven proliferation of HMC1.2 (BrdU assay, n = 3). HMC1.2 cells were incubated for 24 h with control medium, control IgG, BU40, or recombinant CD100 (10 µg/ml, respectively). Incorporation of BrdU into the HMC1.2 cells was assessed using a BrdU cell proliferation assay kit according to the manufacture’s protocol. The relative values are indicated when the value of control IgG is 100. *; P < 0.05, when compared with control IgG.
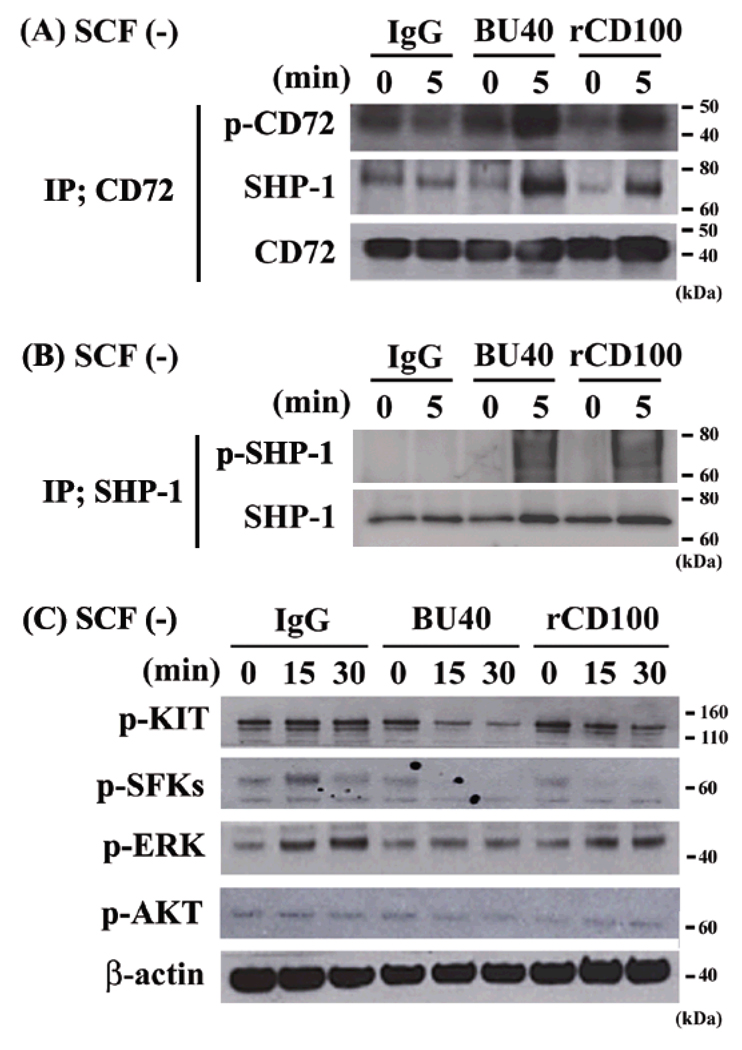
To address the negative effects of BU40 or rCD100 on HMC1.2, we evaluated the phosphorylation status of CD72 and SHP-1 by immunoprecipitation, and of signal molecules by immunoblotting. Incubation of HMC1.2 cells with BU40 or rCD100 increased the tyrosine phosphorylation of CD72 and SHP-1 and the association between CD72 and SHP-1 even in the absence of SCF stimulation (Fig. 7A – 7C). In addition, we observed the suppressive effects of BU40 or rCD100 on the mutated KIT-induced phosphorylations of KIT, SFKs and ERK (Fig. 7D). These data, which were compatible with the responses observed in the SCF-stimulated huMCs, show that the aberrant growth of the tumor cell line could also be inhibited upon CD72 ligation.
Figure 7.
The effects of BU40 or recombinant CD100 on the tyrosine phosphorylation of CD72, the association with SHP-1 with CD72, and on the activation of signaling molecules in HMC1.2 cells. BU40 or recombinant CD100 administration to HMC1.2 cells up-regulates (A) the tyrosine phosphorylation of CD72, the association with SHP-1 and (B) the tyrosine phosphorylation of SHP-1. HMC1.2 cells were incubated for 0 or 5 min with control IgG, BU40, or recombinant CD100 (10 µg/ml respectively) in the absence of SCF. CD72 was immunoprecipitated with anti-CD72 (H-96) or anti-SHP-1 (C-19), and visualized with anti-phosphotyrosine 4G10, anti-SHP-1, or anti-CD72. Data are representative from three individual experiments. (C) Ligation of CD72 with BU40 or recombinant CD100 suppresses the activation of signal molecules in HMC1.2 cells. HMC1.2 cells were incubated for the indicated time with control IgG, BU40, or recombinant CD100 (10 µg/ml respectively). The levels of phospho-KIT, phospho-ERK, phospho-Src family kinases (Tyr 416), and β-actin were then assssed by immunoblot analysis. Data are representative from three individual experiments.
Discussion
The ITIM-bearing inhibitory receptor, CD72, has been reported to be expressed on B cells, T cells, NK cells, dendritic cells, and macrophages (13, 28). Here, we now demonstrate that CD72 is also expressed on human mast cells derived from CD34-positive peripheral blood and on human mast cell lines. This was demonstrated through the presence of mRNA for CD72, and by CD72 protein expression as detected by western blot and by FACS analysis (Fig. 1A, 1B, & 1C). Messenger RNA for CD72 was observed at all stages of development of the huMC cultures examined. However, there was a trend for reduced message in the more mature cultures. Thus, in mature mast cells (8 wk culture), CD72 protein expression was substantially lower than in the HMC 1.2 cells. Regardless, the surface expression as detected by FACS was comparable. A possible explanation for this is that, in HMC1.2 cells, the protein is partly retained in the cytosol (data not shown) due to defective regulation of translocation of CD72 protein to the cell surface following transcription in the HMC1.2 cells. Differences in CD72 protein expression between the huMCs and HMC1.2 cells may be reflective of the transformed nature of the latter cell type or may reflect an immature phenotype of the transformed cells.
Our data do differ from a previous study where the expression of CD72 by FACS analysis was not detected on human mast cells derived from umbilical cord blood (29). However, in that report, the expression of mast cell function-associated antigen (MAFA), which is known to be expressed in mast cells (9), was also not detected. The difference in CD72 expression between the two reports may thus reflect different sensitivities of the antibodies used or difference of the origins of the mast cell progenitors. Nevertheless, based on the data presented herewith in, CD72 can now be added to the list of inhibitory receptors which are documented to be expressed in mast cells.
The natural ligand for CD72 is recognized to be CD100, thus, an advantage of employing CD72 to down-regulate SCF-dependent mast cell function is that this can be achieved through interaction of CD72 with its natural ligand. Indeed, in this study we observed that the cellular responses induced by an anti-CD72 antibody could be mimicked by CD100. Expression of CD100 is reported in B cells, T cells and neuronal cells (30), but to date has not been reported in mast cells. Our preliminary studies have also failed to detect CD100 expression in primary cultured human mast cells and in the HMC1.2 human mast cell line (data not shown). Regardless, it is possible that interaction between CD72-expressing mast cells and CD100-expressing immune cells may influence mast cell function, as is the case with the interaction among CD72-expressing B cells and CD100-expressing T cells (30). Associations between mast cells and neurons have also been reported (31). Thus, such events may also influence mast cell function via CD72 – CD100 interactions. Certainly, our data do provide supportive evidence that ligation of CD72 by CD100, or by means of an agonistic antibody, has the potential to modify KIT-mediated mast cell responses. In this respect, we observed that both rCD100 and an agonistic anti-CD72 antibody down-regulated the KIT dependent growth of human mast cells (Figure 3A), in addition to significantly reducing SCF-induced human mast cell chemotaxis, SCF-induced MCP-1 (CCL2) production (Figure 3B & 3C), and the SCF-enhancement of IgE-dependent degranulation (Figure 4).
Cellular responses regulated by CD72 have been primarily investigated in B cells and B cell lines (13). However, these studies have sometimes produced conflicting data (13). Although the consensus of studies report that CD72 ligation positively regulates responses in B cells by reversing the inhibitory potential of CD72 (14, 32 – 39), other studies have revealed that CD72 ligation further increases its inhibitory potential (40). Specifically, CD72 ligation has been reported to induce the proliferation of B cells (32, 33), and to positively regulate CD40-induced (14) and antigen-mediated (36) proliferation of B cells. Furthermore, CD72 ligation by an anti-CD72 antibody was reported to rescue B cell apoptosis mediated by B cell receptor (BCR) ligation and IgM hyper-crosslinking (38, 40). In contrast to these data, which imply that CD72 ligation reverses its inhibitory potential, CD72 expression in B cell line K46µm λ and incubation of splenic B cells with an anti-CD72 antibody resulted in down-modulation of BCR-mediated ERK activation and calcium mobilization (40). These data imply that CD72 ligation promotes its inhibitory potential.
These apparently conflicting data have led to the conclusion that CD72 may regulate positive and negative signaling pathways for the regulation of B cell responses and that this may, in part, be related to the stages of B cell development (13). Regardless, the ability of CD72 to regulate cellular responses is dependent on its phosphorylation status, hence its ability to recruit SHP-1, an interaction that is reported to be negatively influenced through its interaction with CD72-bound Grb2 (41). It has been proposed that, in the scenario in which CD72 ligation reverses its inhibitory activity, rCD100 or the agonistic antibody results in dissociation of CD72 from the B cell signaling complex thus reversing BCR dependent phosphorylation of CD72. In contrast, in the scenario, in which CD72 ligation induces its inhibitory activity, the agonistic anti-CD72 antibody promotes association of CD72 with the receptor signaling-complex thus allowing its phosphorylation and recruitment of SHP-1 (40).
In resting B cells, there appears to be minimal constitutive phosphorylation of CD72 (25). However, in the huMCs, we observed that there was a slight but detectible constitutive phosphorylation of CD72 and its association with SHP-1 in the resting state (Fig, 2). Hence the possibility exists that CD72 may help regulate the basal activation state of the human mast cells. Our data, furthermore, demonstrate that in these cells, ligation of CD72 with either rCD100 or the agonistic antibody BU40 concurrently with KIT activation, results in the activation of necessary events which allow CD72 to inhibit Kit-mediated signaling. Thus, in the case of mast cells, the mode of the responses elicited by rCD100 and BU40 would appear similar to that reported in the splenic B cells (40), which is suggestive of permissive phosphorylation of CD72 by KIT following CD72 ligation. This conclusion was further supported by the enhancement of tyrosine phosphorylation of CD72 observed in the CD72-immunoprecipitates from mast cells incubated with BU40 or rCD100 and triggered through KIT (Fig. 2A & 2B).
Unlike the BCR, KIT possesses inherent catalytic activity which is increased upon SCF-induced KIT dimerization. An increase in CD72 phosphorylation was not observed in cells incubated with rCD100 or BU40 in the absence of KIT activation (Fig. 2B). It is likely that the inducible phosphorylation of CD72 observed in the human mast cells is directly due to phosphorylation by KIT, contrary to the case in immature B cells. Our data further demonstrated that there was a significant increase in the association of SHP-1 with the tyrosine phosphorylated CD72 (Fig. 2A). SHP-1 has been demonstrated to down-regulate KIT signals in vivo (42) and, indeed, we observed that the phosphorylation of SHP-1, which is known to increase its phosphatase activity (26), was elevated in the huMCs (Fig. 2C) and HMC1.2 cells (Fig. 7B) following CD72 ligation. In addition to CD72, SHP-1 has also been shown to be associated with other inhibitory receptors which down-regulate KIT-mediated responses in mast cells (12). Thus, we can conclude that the ability of ligated CD72 to inhibit KIT-mediated responses in mast cells is linked to its ability to recruit SHP-1 following its phophorylation.
SHP-1 dephosphorylates regulatory tyrosine residues on critical proteins which participate in signaling cascades initiated by multiple receptors including KIT (6). We observed that CD72 phosphorylation led to the suppression of the phosphorylation of KIT, SFKs, and ERK induced by SCF challenge (Fig. 5), but not of AKT or Stat3. Both KIT and SFKs are known to be directly dephosphorylated by interactions with SHP-1 (43 – 45). Therefore, it is likely that the down-regulated phosphorylation of KIT and SFKs induced by costmulation of KIT with CD72 in mast cells was a consequence of the formation of the CD72 – SHP-1 complex. It is unclear whether SHP-1 can directly regulate the activation of ERK (46, 47). Therefore there are two possible explanations for the observed ERK dephosphorylation observed in the human mast cells in response to co-activation of KIT and CD72: (i) The suppressed activation of ERK by CD72 stimulation was mediated directly by the CD72 – SHP-1 complex or; (ii) the suppressed ERK phosphorylation was indirect due to the down-regulation of SFKs activity, as ERK phosphorylation is known to be regulated by SFKs (48).
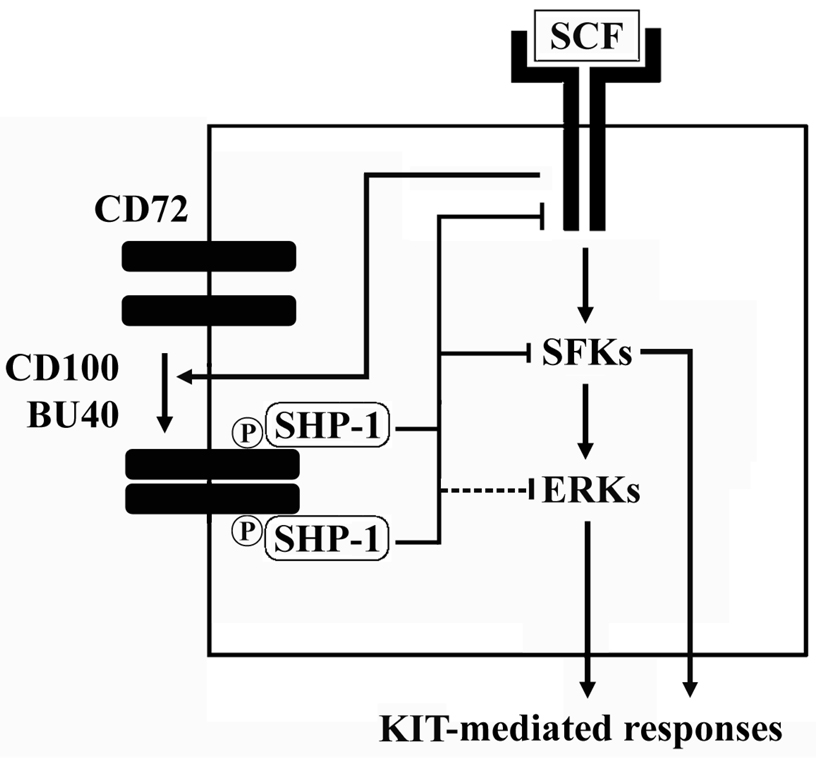
Regardless of whether direct or indirect, the down-regulation of the KIT-dependent phosphorylation of KIT, SFKs and ERK by the interaction of SHP-1 with phosphorylated CD72 would certainly account for the ability of ligated CD72 to down-regulate KIT-mediated responses in human mast cells. In this respect, in mast cells, the SFKs, Lyn and Fyn, play important roles in KIT-mediated proliferation and chemotaxis (49) and ERK participates in the process of KIT-mediated proliferation and MCP-1 production (50). The downregulation of SCF-induced phosphorylation of SFKs by CD72 would also account for the observed inhibition of SCF-enhanced degraulation following CD72 ligation (Fig. 4). The inability of ligated CD72 to decrease the degranulation response induced by FcεRI aggregation alone again points to the requirement of direct phosphorylation of CD72 by Kit to induced inhibitory responses. Taken together, from the above conclusions, we can now put together a model of how CD72 may regulate KIT-mediated human mast cell function (Fig. 8).
Figure 8.
Proposed model of the effects of CD72 – CD100 system on human mast cells. SFKs; Src family kinases, ERKs; extracellular-regulated kinases.
In summary, we have presented data which demonstrates that the ITIM-containing inhibitory receptor CD72 is expressed in human mast cells and mast cell lines. When ligated either by its natural ligand CD100 or by an anti-CD72 antibody, concurrently with activated KIT, CD72 becomes phosphorylated thereby recruiting SHP-1 resulting in dephosphorylation of critical signaling molecules resulting in down-regulation of KIT-mediated mast cell activation. The ability of ligated CD72 to also down-regulate the growth of tumor mast cells provides evidence of the potential application of CD72 in mast cell disorders such as mastocytosis.
Acknowledgments
The source of support
This work was supported by the National Institute of Allergy and Infectious Diseases Division of Intramural Research within the National Institutes of Health, USA, and by grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Abbreviations
- BrdU
bromodeoxyurudine
- CCL2
chemokine C-C motif ligand 2
- huMCs
human mast cell
- MCP1
monocyte chemoattractant protein-1
- SCF
stem cell factor
References
- 1.Kraft S, Kinet JP. New developments in FcεRI regulation, function and inhibition. Nat. Rev. Immunol. 2007;7:365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- 2.Okayama Y, Kawakami T. Development, migration, and survival of mast cells. Immunol. Res. 2006;34:97–115. doi: 10.1385/IR:34:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112:946–956. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannenbaum S, Suzuki Y, Metcalfe DD. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc. Natl. Acad. Sci. USA. 1995;92:10560–10564. doi: 10.1073/pnas.92.23.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furitsu T, Tsujimura T, Tono T, Ikeda H, Kitayama H, Koshimizu U, Sugahara H, Butterfield JH, Ashman LK, Kanayama Y, Matsuzawa Y, Kitamura Y, Kanakura Y. Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J. Clin. Invest. 1993;92:1736–1744. doi: 10.1172/JCI116761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilfillan AM, Tkaczyk C. Integrated signaling pathways for mast-cell activation. Nat. Rev. Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 7.Jensen B, Akin MC, Gilfillan AM. Pharmacological targeting of the KIT growth factor receptor: a therapeutic consideration for mast cell disorders. Br. J. Pharmacol. 2008;154:1572–1582. doi: 10.1038/bjp.2008.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilfillan AM, Peavy RD, Metcalfe DD. Amplification mechanisms for the enhancement of antigen-mediated mast cell activation. Immunol. Res. 2009;43:15–24. doi: 10.1007/s12026-008-8046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Yao Z. Mast cell and immune inhibitory receptors. Cell. Mol. Immunol. 2004;1:408–415. [PubMed] [Google Scholar]
- 10.Cherwinski HM, Murphy CA, Joyce BL, Bigler ME, Song YS, Zurawski SM, Moshrefi MM, Gorman DM, Miller KL, Zhang S, Sedgwick JD, Phillips JH. The CD200 receptor is a novel and potent regulator of murine and human mast cell function. J. Immunol. 2005;174:1348–1356. doi: 10.4049/jimmunol.174.3.1348. [DOI] [PubMed] [Google Scholar]
- 11.Bachelet I, Munitz A, Berent-Maoz B, Mankuta D, Levi-Schaffer F. Suppression of normal and malignant kit signaling by a bispecific antibody linking kit with CD300a. J. Immunol. 2008;180:6064–6069. doi: 10.4049/jimmunol.180.9.6064. [DOI] [PubMed] [Google Scholar]
- 12.Unkeless JC, Jin J. Inhibitory receptors, ITIM sequences and phosphatases. Curr. Opin. Immunol. 1997;9:338–343. doi: 10.1016/s0952-7915(97)80079-9. [DOI] [PubMed] [Google Scholar]
- 13.Wu HJ, Bondada S. CD72, a coreceptor with both positive and negative effects on B lymphocyte development and function. J. Clin. Immunol. 2009;29:12–21. doi: 10.1007/s10875-008-9264-6. [DOI] [PubMed] [Google Scholar]
- 14.Kumanogoh A, Watanabe C, Lee I, Wang X, Shi W, Araki H, Hirata H, Iwahori K, Uchida J, Yasui T, Matsumoto M, Yoshida K, Yakura H, Pan C, Parnes JR, Kikutani H. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity. 2000;13:621–631. doi: 10.1016/s1074-7613(00)00062-5. [DOI] [PubMed] [Google Scholar]
- 15.Kirshenbaum AS, Goff JP, Semere T, Foster B, Scott LM, Metcalfe DD. Demonstration that human mast cells arise from a progenitor cell population that is CD34(+), c-kit(+), and expresses aminopeptidase N (CD13) Blood. 1999;94:2333–2342. [PubMed] [Google Scholar]
- 16.Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, Rao VK, Metcalfe DD. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcεRI or FcγRI. Leuk. Res. 2003;27:677–682. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 17.Butterfield JH, Weiler D, Dewald G, Gleich GJ. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk. Res. 1988;12:345–355. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- 18.Sundström M, Vliagoftis H, Karlberg P, Butterfield JH, Nilsson K, Metcalfe DD, Nilsson G. Functional and phenotypic studies of two variants of a human mast cell line with a distinct set of mutations in the c-kit proto-oncogene. Immunology. 2003;108:89–97. doi: 10.1046/j.1365-2567.2003.01559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishida I, Kumanogoh A, Suzuki K, Akahani S, Noda K, Kikutani H. Involvement of CD100, a lymphocyte semaphorin, in the activation of the human immune system via CD72: implications for the regulation of immune and inflammatory responses. Int. Immunol. 2003;15:1027–1034. doi: 10.1093/intimm/dxg098. [DOI] [PubMed] [Google Scholar]
- 20.Kim MS, Kuehn HS, Metcalfe DD, Gilfillan AM. Activation and function of the mTORC1 pathway in mast cells. J. Immunol. 2008;180:4586–4595. doi: 10.4049/jimmunol.180.7.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kataoka TR, Nishizawa Y. Stat4 suppresses the proliferation of connective tissue-type mast cells. Lab. Invest. 2008;88:856–864. doi: 10.1038/labinvest.2008.51. [DOI] [PubMed] [Google Scholar]
- 22.Baghestanian M, Hofbauer R, Kiener HP, Bankl HC, Wimazal F, Willheim M, Scheiner O, Füreder W, Müller MR, Bevec D, Lechner K, Valent P. The c-kit ligand stem cell factor and anti-IgE promote expression of monocyte chemoattractant protein-1 in human lung mast cells. Blood. 1997;90:4438–4449. [PubMed] [Google Scholar]
- 23.Schwarting R, Castello R, Moldenhauer G, Pezzutto A, von Hoegen I, Ludwig WD, Parnes JR, Dörken B. Human Lyb-2 homolog CD72 is a marker for progenitor B-cell leukemias. Am. J. Hematol. 1992;41:151–158. doi: 10.1002/ajh.2830410303. [DOI] [PubMed] [Google Scholar]
- 24.Adachi T, Flaswinkel H, Yakura H, Reth M, Tsubata T. The B cell surface protein CD72 recruits the tyrosine phosphatase SHP-1 upon tyrosine phosphorylation. J. Immunol. 1998;160:4662–4665. [PubMed] [Google Scholar]
- 25.Wu Y, Nadler MJ, Brennan LA, Gish GD, Timms JF, Fusaki N, Jongstra-Bilen J, Tada N, Pawson T, Wither J, Neel BG, Hozumi N. The B-cell transmembrane protein CD72 binds to and is an in vivo substrate of the protein tyrosine phosphatase SHP-1. Curr. Biol. 1998;8:1009–1017. doi: 10.1016/s0960-9822(07)00421-6. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Shen K, Lu W, Cole PA. The role of C-terminal tyrosine phosphorylation in the regulation of SHP-1 explored via expressed protein ligation. J. Biol. Chem. 2003;278:4668–4674. doi: 10.1074/jbc.M210028200. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson G, Butterfield JH, Nilsson K, Siegbahn A. Stem cell factor is a chemotactic factor for human mast cells. J. Immunol. 1994;153:3717–3723. [PubMed] [Google Scholar]
- 28.Alcón VL, Luther C, Balce D, Takei F. B-cell co-receptor CD72 is expressed on NK cells and inhibits IFN-γ production but not cytotoxicity. Eur. J. Immunol. 2009;39:826–832. doi: 10.1002/eji.200838682. [DOI] [PubMed] [Google Scholar]
- 29.Kepley CL, Taghavi S, Mackay G, Zhu D, Morel PA, Zhang K, Ryan JJ, Satin LS, Zhang M, Pandolfi PP, Saxon A. Co-aggregation of FcγRII with FcεRI on human mast cells inhibits antigen-induced secretion and involves SHIP-Grb2-Dok complexes. J. Biol. Chem. 2004;279:35139–35149. doi: 10.1074/jbc.M404318200. [DOI] [PubMed] [Google Scholar]
- 30.Kumanogoh A, Kikutani H. The CD100-CD72 interaction: a novel mechanism of immune regulation. Trends Immunol. 2001;22:670–676. doi: 10.1016/s1471-4906(01)02087-7. [DOI] [PubMed] [Google Scholar]
- 31.Ito A, Hagiyama M, Oonuma J. Nerve-mast cell and smooth muscle-mast cell interaction mediated by cell adhesion molecule-1, CADM1. J. Smooth Muscle Res. 2008;44:83–93. doi: 10.1540/jsmr.44.83. [DOI] [PubMed] [Google Scholar]
- 32.Subbarao B, Mosier DE. Induction of B lymphocyte proliferation by monoclonal anti-Lyb 2 antibody. J. Immunol. 1983;130:2033–2037. [PubMed] [Google Scholar]
- 33.Subbarao B, Mosier DE. Activation of B lymphocytes by monovalent anti-Lyb-2 antibodies. J. Exp. Med. 1984;159:1796–1801. doi: 10.1084/jem.159.6.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snow EC, Mond JJ, Subbarao B. Enhancement by monoclonal anti-Lyb-2 antibody of antigen-specific B lymphocyte expansion stimulated by TNP-Ficoll and T lymphocyte-derived factors. J. Immunol. 1986;137:1793–1796. [PubMed] [Google Scholar]
- 35.Pan C, Baumgarth N, Parnes JR. CD72-deficient mice reveal nonredundant roles of CD72 in B cell development and activation. Immunity. 1999;11:495–506. doi: 10.1016/s1074-7613(00)80124-7. [DOI] [PubMed] [Google Scholar]
- 36.Li DH, Tung JW, Tarner IH, Snow AL, Yukinari T, Ngernmaneepothong R, Martinez OM, Parnes JR. CD72 down-modulates BCR-induced signal transduction and diminishes survival in primary mature B lymphocytes. J. Immunol. 2006;176:5321–5328. doi: 10.4049/jimmunol.176.9.5321. [DOI] [PubMed] [Google Scholar]
- 37.Wu HJ, Venkataraman C, Estus S, Dong C, Davis RJ, Flavell RA, Bondada S. Positive signaling through CD72 induces mitogen-activated protein kinase activation and synergizes with B cell receptor signals to induce X-linked immunodeficiency B cell proliferation. J. Immunol. 2001;167:1263–1273. doi: 10.4049/jimmunol.167.3.1263. [DOI] [PubMed] [Google Scholar]
- 38.Nomura T, Han H, Howard MC, Yagita H, Yakura H, Honjo T, Tsubata T. Antigen receptor-mediated B cell death is blocked by signaling via CD72 or treatment with dextran sulfate and is defective in autoimmunity-prone mice. Int. Immunol. 1996;8:867–875. doi: 10.1093/intimm/8.6.867. [DOI] [PubMed] [Google Scholar]
- 39.Shi W, Kumanogoh A, Watanabe C, Uchida J, Wang X, Yasui T, Yukawa K, Ikawa M, Okabe M, Parnes JR, Yoshida K, Kikutani H. The class IV semaphorin CD100 plays nonredundant roles in the immune system: defective B and T cell activation in CD100-deficient mice. Immunity. 2000;13:633–642. doi: 10.1016/s1074-7613(00)00063-7. [DOI] [PubMed] [Google Scholar]
- 40.Adachi T, Wakabayashi C, Nakayama T, Yakura H, Tsubata T. CD72 negatively regulates signaling through the antigen receptor of B cells. J. Immunol. 2000;164:1223–1229. doi: 10.4049/jimmunol.164.3.1223. [DOI] [PubMed] [Google Scholar]
- 41.Baba T, Fusaki N, Aoyama A, Li DH, Okamura RM, Parnes JR, Hozumi N. Dual regulation of BCR-mediated growth inhibition signaling by CD72. Eur. J. Immunol. 2005;35:1634–1642. doi: 10.1002/eji.200425775. [DOI] [PubMed] [Google Scholar]
- 42.Paulson RF, Vesely S, Siminovitch KA, Bernstein A. Signaling by the W/Kit tyrosine kinase is negatively regulated in vivo by the tyrosine phosphatase SHP-1. Nat. Genet. 1996;13:309–315. doi: 10.1038/ng0796-309. [DOI] [PubMed] [Google Scholar]
- 43.Rivera J, Olivera A. Src family kinases and lipid mediators in control of allergic inflammation. Immunol. Rev. 2007;217:255–268. doi: 10.1111/j.1600-065X.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- 44.Hibbs ML, Harder KW. The duplicitous nature of the Lyn tyrosine kinase in growth factor signaling. Growth Factors. 2006;24:137–149. doi: 10.1080/08977190600581327. [DOI] [PubMed] [Google Scholar]
- 45.Kozlowski M, Larose L, Lee F, Le DM, Rottapel R, Siminovitch KA. SHP-1 binds and negatively modulates the c-kit receptor by interaction with tyrosine 569 in the c-kit juxtamembrane domain. Mol. Cell. Biol. 1998;18:2089–2099. doi: 10.1128/mcb.18.4.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie ZH, Zhang J, Siraganian RP. Positive regulation of c-Jun N-terminal kinase and TNF-γ production but not histamine release by SHP-1 in RBL-2H3 mast cells. J. Immunol. 2000;164:1521–1528. doi: 10.4049/jimmunol.164.3.1521. [DOI] [PubMed] [Google Scholar]
- 47.You M, Zhao Z. Positive effects of SH2 domain-containing tyrosine phosphatase SHP-1 on epidermal growth factor- and interferon-γ-stimulated activation of STAT transcription factors in HeLa cells. J. Biol. Chem. 1997;272:23376–23381. doi: 10.1074/jbc.272.37.23376. [DOI] [PubMed] [Google Scholar]
- 48.Ueda S, Mizuki M, Ikeda H, Tsujimura T, Matsumura I, Nakano K, Daino H, Honda ZZ, Sonoyama J, Shibayama H, Sugahara H, Machii T, Kanakura Y. Critical roles of c-kit tyrosine residues 567 and 719 in stem cell factor-induced chemotaxis: contribution of src family kinase and PI3-kinase on calcium mobilization and cell migration. Blood. 2002;99:3342–3349. doi: 10.1182/blood.v99.9.3342. [DOI] [PubMed] [Google Scholar]
- 49.O'Laughlin-Bunner B, Radosevic N, Taylor ML, Shivakrupa C, DeBerry D, Metcalfe D, Zhou M, Lowell C, Linnekin D. Lyn is required for normal stem cell factor-induced proliferation and chemotaxis of primary hematopoietic cells. Blood. 2001;98:343–350. doi: 10.1182/blood.v98.2.343. [DOI] [PubMed] [Google Scholar]
- 50.Wong CK, Tsang CM, Ip WK, Lam CW. Molecular mechanisms for the release of chemokines from human leukemic mast cell line (HMC)-1 cells activated by SCF and TNF-α: roles of ERK, p38 MAPK, and NF-κB. Allergy. 2006;61:289–297. doi: 10.1111/j.1398-9995.2006.00972.x. [DOI] [PubMed] [Google Scholar]