Abstract
The c-Jun N-terminal kinase (JNK) mediates stress-induced apoptosis and the cytotoxic effect of anticancer therapies. Paradoxically, recent clinical studies indicate that elevated JNK activity in human breast cancer is associated with poor prognosis. Here we show that overexpression of a constitutively active JNK in human breast cancer cells did not cause apoptosis, but actually induced cell migration and invasion, a morphological change associated with epithelial-mesenchymal transition (EMT), expression of mesenchymal-specific markers vimentin and fibronectin, and activity of AP-1 transcription factors. Supporting this observation, mouse mammary tumor cells that have undergone EMT showed upregulated JNK activity, and the EMT was reversed by JNK inhibition. Sustained JNK activity enhanced insulin receptor substrate-2-mediated ERK activation, which in turn increased c-Fos expression and AP-1 activity. In addition, hyperactive JNK attenuated the apoptosis of breast cancer cells treated by the chemotherapy drug paclitaxel, which is in contrast to the requirement for inducible JNK activity in response to cytotoxic chemotherapy. Blockade of ERK activity diminished hyperactive JNK-induced cell invasion and survival. Our data suggest that the role of JNK changes when its activity is elevated persistently above the basal levels associated with cell apoptosis, and that JNK activation may serve as a marker of breast cancer progression and resistance to cytotoxic drugs.
Keywords: AP-1, breast cancer, epithelial-mesenchymal transition, ERK, IRS-2, JNK
Introduction
The c-Jun N-terminal kinase (JNK), also called the stress-activated protein kinase (SAPK), belongs to the mitogen-activated protein kinase (MAPK) family, which also includes the extracellular signal-regulated kinase (ERK) and p38 MAPK (1, 2). JNK is activated by two dual-specificity kinases, MKK4 and MKK7, which phosphorylate the conserved Thr-Pro-Tyr motif in its kinase domain. JNK is stimulated by environmental stresses, mitogens, and oncogenes. One of the most extensively studied functions of JNK is its induction of apoptosis via release of mitochondrial cytochrome c under stress conditions (3, 4).
Once activated, JNK can translocate to the nucleus where it regulates transcription factors such as c-Jun, ATF-2, Elk-1, p53, and c-Myc. Less is known about the cytoplasmic targets of JNK. It has been shown that Ras-induced transformation requires c-Jun and is suppressed by mutation of the JNK phosphorylation sites on c-Jun (5, 6). Similarly, the transforming capability of other oncogenes such as Met and Bcr-Abl depends on JNK (7, 8), as does invasive epidermal neoplasia triggered by NF-κB deficiency and Ras activation (9).
Studies using mouse embryonic fibroblasts have demonstrated a requirement for JNK in UV and TNF-α induced apoptosis (1, 2). JNK can also sensitize breast cancer cells to apoptosis induced by anti-tumor agents (10, 11), and this effect may depend on the cell cycle (12). Interestingly, emerging evidence has indicated that JNK can also contribute to cell survival. For example, JNK1 and JNK2 double-null mouse embryos exhibit increased apoptosis within the forebrain (13), and JNK is required for extracelluar matrix-elicited survival signaling (14). In addition, the pro-apoptotic protein BAD can be inactivated by JNK (15). It has been postulated that cell signaling context may define the role of JNK in apoptosis or survival (16).
Much attention has been focused on the role of JNK in anticancer agent-induced apoptosis. If JNK activity is required for stress-induced apoptosis of cancer cells, then higher or sustained activity of JNK might be assumed to favor spontaneous apoptosis or growth inhibition. However, recent studies of human tumor specimens, including breast cancer, demonstrated a correlation between elevated JNK activity and worse clinical outcome (17–21). This surprising finding is the basis for our hypothesis that a sustained increase in JNK activity may promote human breast cancer progression. In the present study, we investigated the role of hyperactive JNK in breast cancer cell models. We found that hyperactive JNK enhances the invasion and survival of breast cancer cells by increasing ERK signaling.
Materials and Methods
Materials
All general experiment materials and chemicals were from Sigma (St. Louis, MO) unless otherwise noted. The small-molecule inhibitors SP600125 and U0126 were purchased from Calbiochem (La Jolla, CA). All cell culture and transfection reagents were purchased from Invitrogen (Carlsbad, CA). Dunn chambers and cell invasion chambers were purchased from Hawksley (Lancing, UK) and BD Biosciences (Bedford, MA), respectively. A dominant negative c-Fos (A-Fos) vector was provided by Charles Vinson(Laboratory of Biochemistry, National Cancer Institute, NIH).
Cell culture
MDA-MB-468 breast cancer cells were obtained from the Breast Center at Baylor College of Medicine. Mouse breast cancer cell lines 67NR, 168FAR, 4T07, and 4T1 were provided by Fred Miller (Wayne State University). Cells were routinely maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 50 IU/ml of penicillin, 50 μg/ml of streptomycin, and 10 μg/ml insulin. Cells were kept at 37°C in a humidified incubator with 5% CO2. After treatment with paclitaxel or signaling inhibitors, cells were washed twice with ice-cold PBS and then lysed in 200 μl of lysis buffer, which contained50 mM Tris-HCl (pH 7.4), 1% Nonidet P-40, 2 mM EDTA, 100 mM NaCl, 10% glycerol, and a fresh protease inhibitor cocktail (Roche, Indianapolis, IN). Then cells were left on ice for 30 min, and the lysates were clarified by centrifugation at 14,000 × g for 15 min at 4°C. Protein concentration of the supernatant was measured by BCA detection reagents (Pierce, Rockford, IL). The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) cell proliferation assay was performed as described previously (22). Cells were plated at a density of 104 in 24-well plates. Spectrophotometrical absorbance was obtained at a wavelength of 570 nm.
Transfection
To increase cellular JNK activity, we utilized a constitutively active JNK, SAPKβ-MKK7. This chimeric protein of SAPKβ and its upstream activator MKK7 was a gift from Ulf Rapp (University of Wurzburg, Wurzburg, Germany) (23). MDA-MB-468 cells were stably transfected with SAPKβ-MKK7 in pcDNA3.1 using Lipofectamine 2000 (Invitrogen), and 600 μg/ml G418 was used to select stable clones. For simplicity of interpretation, effects of this constitutively active JNK are reported here for pooled stable transfectants or two representative clones. A lentiviral construct pLKO-Puro (Sigma) expressing a mouse JNK2 shRNA (GCGGACTCAACTTTC ACTGTTCT) was stably transduced into 4T1 cells, which were then selected with 5 μg/ml puromycin. This shRNA is upstream of the α/β and p46/p54 splicing sites, thus targeting all JNK2 isoforms. A shRNA which does not match any known human coding cDNA was used as an experimental control. In the siRNA experiments, IRS-2, c-Jun, and c-Fos siRNA was obtained from Dharmacon (Lafayette, CO). A final concentration of 100 nM was used in the transfection. Two days after transfection, cells were subjected to invasion assays. A dominant-negative JNK (APF) mutant, provided by Tse-Hua Tan (Baylor College of Medicine), was transiently transfected into cells. After 2 d of culture, cell lysates were harvested and then immunoblotted for IRS-2.
Quantitative RT-PCR
Total RNA was isolated with RNeasy Midi kit (Qiagen, Valencia, CA). SYBR green QRT-PCR was conducted using vimentin primers (forward 5′-CAACCTGGCCGAGGACAT-3′, reverse 5′-ACGCATTGTCAACATCCTGTCT-3′) and fibronectin primers (forward 5′-CCGCCGAAT GTAGGACAAGA-3′, reverse 5′-TGCCAACAGGATGACATGAAA-3′). Reverse transcriptions of vimentin and fibronectin mRNA were performed in 96-well optical plates using Superscript II reverse transcriptase. All RNA samples were first treated with deoxyribonuclease I to eliminate residual genomic DNA. The plates were incubated at 50°C for 30 min followed by 10 min at 72°C. Then real-time quantitative PCR was conducted in an ABI PRISM 7700 Sequence Detector (PE Applied Biosystems). The plates were incubated at 94°C for 1 min, followed by 40cycles at 94°C for 12 sec and 60°C for 30 sec. Quantitative RT-PCR was performed in triplicate for each sample. Vimentin and fibronectin mRNA data were normalized by the β-actin mRNA value.
Immunoblotting and immunoprecipitation
Total protein (40 μg) was separated by 8% SDS-PAGE and transferred to a nitrocellulose membrane overnight at 4°C. The remaining steps were conducted according to a standard immunoblotting protocol. Briefly, the membrane was blocked with PBS plus 0.1% Tween-20 (PBST) containing 5% nonfat milk for 1 h, and then incubated with a 1:1000 dilution of anti-JNK, anti-p-JNK, PARP (Cell Signaling), vimentin, fibronectin (BD Biosciences), or anti-β-actin (Sigma) antibodies in blocking solution at 4°C for 12 h. After the primary antibody incubation, the membrane was again washed with PBST three times (5 min each) and then incubated with a horseradish peroxidase (HRP)-linked secondary antibody (Amersham, Piscataway, NJ) at a dilution of 1:4000 in blocking solution. The membrane was washed and bands were visualized by chemiluminescence assays. For immunoprecipitation, cell lysates (500 μg) were pre-cleared by protein-G agarose beads (Zymed Laboratory, San Francisco, CA) and then incubated with specific antibodies at a 1:100 dilution overnight at 4°C. The beads were washed with the above lysis buffer three times and resuspended in protein sample buffer before the immunoprecipitated protein was subjected to immunoblotting.
Apoptosis assay
Cells were maintained in culture medium. For flow cytometry analysis of DNA content, paclitaxel-treated cells (0.01 μM) were collected by trypsinization and washed with cold PBS. Then the cells were fixed in 70% ethanol and stored overnight at 4°C. The fixed cells were washed twice and resuspended in PBS containing 100 μg/ml RNase A and 50 μg/ml propidium iodide. After an hour of incubation at room temperature, the cells were analyzed by flow cytometry using a BD FACSCalibur. The cytotoxicity assay was performed according to the instruction manual (Promega). Briefly, cells were grown in 96-well plates. A non-membrane-permeable fluorogenic substrate peptide (bis-alanyl-alanyl-phenylalanyl-rhodamine 110) was added to the culture. The number of dead cells was determined by the activity of tripeptidyl peptidase released from cytoplasm during complete cell membrane breakdown. The released peptidase cleaved the labeled extracellular peptide to generate fluorescence that was measured by a microplate reader. To visualize apoptotic cells, propidium iodide (5 μg/ml) and SYTO-13 green fluorescent nucleic acid dye (1 μM) (Invitrogen) were added to the culture medium. After 15 min, cells were examined under a fluorescent microscope using excitation at 488 nm. PI produces red staining of necrotic or late apoptotic cells, whereas SYTO-13 produces green staining of live cells and early apoptotic cells.
AP-1 activity assay
Cells were collected and kept in ice-cold hypotonic buffer (20 mM Hepes, pH 7.5, 5 mM NaF, 10 μM Na2MoO4, 0.1 mM EDTA) for 15 min. Then NP-40 (0.5% final) was added and suspension was vortexed vigorously for 10 seconds. After centrifugation, the nuclear pellet was resuspended in extraction buffer (20 mM Hepes pH 7.9, 0.4 M NaCl, 1 mM EDTA). Supernatant (nuclear extract) was retained after a second centrifugation. The binding assay was performed according to the instruction manual. Samples (10 μg) were added to 96-well plates coated with an oligonucleotide that contains the AP-1 consensus site 5′-TGAGTCA-3′. After 1 h incubation at room temperature, primary antibodies of distinct AP-1 components were added; subsequent addition of HRP-conjugated secondary antibody produced a sensitive colorimetric readout quantified by spectrophotometry at the 450-nm wavelength. An AP-1-luciferase reporter construct (pGL3-AP-1), provided by Powel Brown (Baylor College of Medicine), was also used to detect AP-1 activity. The plasmid and a β-galactosidase vector were transiently transfected into cells. Then the ERK inhibitor U0126 was added and cells were harvested after 24 h. Luciferase activity was measured and normalized by β-galactosidase activity.
Cell migration and invasion assay
Cell migration was measured using the Dunn chamber assay (Hawksley, Lancing, UK). Briefly, 2 × 104 cells were plated on a Dunn chamber cover slip, which was later inverted over the two wells in the center of the chamber filled with serum-free medium. The outer well contained DMEM with 10% serum as a chemoattractant. A paintbrush was used to wax the coverslips onto the chamber. After overnight incubation, more cells migrated into the annular bridge between the inner and outer walls. Cell migration ability was represented by an increase of cell number after overnight incubation in the bridge region. Cells were counted in 5 different areas. For detecting cell invasion in vitro, Boyden chamber inserts were coated with a thin layer of Matrigel basement membrane matrix. Briefly, 2 × 104 cells were plated on the top of the inserts, which were then transferred into a 24-well plate. Each well contained DMEM with 10% serum as a chemoattractant. After 16 h incubation, cells remaining on the upper surface of the chambers were removed with cotton swabs. Cells on the lower surface of the inserts were fixed and stained with the HEMA3 kit (Fisher, Middletown, VA). The membrane was then mounted onto a microscope slide and the migrating cells were counted in 5 different areas using a light microscope.
Human apoptosis protein array
To compare the levels of apoptosis-related proteins under different treatment conditions, a human apoptosis protein array (R&D Systems, Minneapolis, MN) was used according to the manufacturer’s instructions. Briefly, protein lysates (400 μg) from control or CA-JNK-expressing MDA-MB-468 cells were loaded onto an array membrane that had been blocked with PBST plus 5% non-fat milk for 1 h. The membrane was incubated overnight at 4°C, washed three times for 5 min each with PBST, and then incubated with a horseradish peroxidase (HRP)-linked secondary antibody at a dilution of 1:4000 in blocking solution. After the membrane was washed, bands were visualized by chemiluminescence assays. Densitometry of protein dot signals was obtained. The average density of duplicate spots representing each apoptosis-related protein indicated its relative levels. To compare the spot density from different membranes, relative density was used (relative density = mean density of each protein/mean density of positive control × 100%). Protein expression levels in control MDA-MB-468 were compared with those in CA-JNK-expressing cells.
Results
Constitutive JNK activity induces migration and invasion
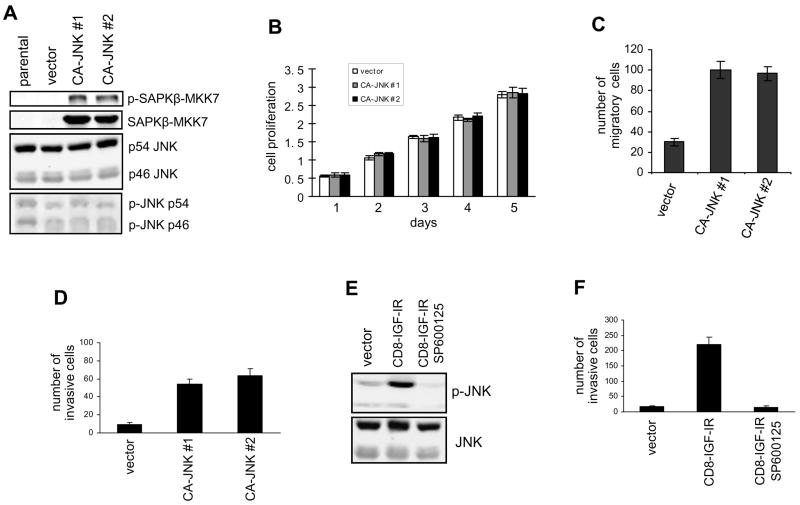
To explore the role of JNK in breast cancer progression, we asked whether increasing JNK activity would alter breast cancer cell functions. For this purpose, we ectopically expressed a constitutively active JNK, SAPKβ-MKK7 (CA-JNK), a fusion protein of JNK and its upstream activator MKK7, in MDA-MB-468 human breast cancer cells (23). We previously used this cell line to show that JNK signaling is induced and utilized by growth factors to regulate cell functions (24). Of note, effects of this constitutively active JNK are described here for pooled or two representative stable transfectants. Immunoblotting with an anti-p-JNK antibody (Thr183/Tyr185) demonstrated persistent phosphorylation of CA-JNK (90 kD) at the Thr-Pro-Tyr motif of JNK under normal growth conditions (Fig. 1A), which indicates constitutive activation of this fusion protein. As expected, levels of total and phosphorylated endogenous JNK (p46 and p54) were not altered in vector- and CA-JNK-expressing cells. As illustrated in Fig. 1B, ectopic expression of hyperactive JNK did not affect the proliferation of MDA-MB-468 cells. In addition, caspase-3 staining and flow cytometry analysis demonstrated that expression of CA-JNK did not induce spontaneous cell apoptosis or alter cell cycle progression in MDA-MB-468 cells (data not shown).
Figure 1.
Hyperactive JNK induces migration and invasion. (A) SAPKβ-MKK7 (CA-JNK, 90 kD) was stably expressed in MDA-MB-468 cells. Its expression was examined by immunoblotting with anti-JNK and anti-p-JNK antibodies. Endogenous JNK (46 and 54 kD) was used as a control. (B) Cell proliferation of MDA-MB-468 cells expressing CA-JNK or the vector was measured using the MTT assay. (C) The Dunn chamber migration assay was conducted with 10% serum medium as a chemoattractant. Migration ability is presented as the increased number of MDA-MB-468 cells detected in the annular bridge region. Each bar represents mean ± SD of samples measured in duplicate. (D) MDA-MB-468 cells expressing CA-JNK or the vector were assayed for their ability to invade a Matrigel matrix coated on the upper-surface of chambers. (E) JNK activation in MCF-10A cells transfected with CD8-IGF-IR or the vector was examined by immunoblotting. The inhibitor SP600125 (5 μM) was used to block JNK activity. (F) The transwell invasion assay was conducted using control and CD8-IGF-IR MCF-10A cells. The effect of SP600125 on invasion was tested.
Because JNK is required for cell movement (25), we used the Dunn chamber migration assay to assess whether sustained JNK activity leads to enhanced cell motility. As shown in Fig. 1C, overexpression of CA-JNK significantly potentiated the migration of MDA-MB-468 breast cancer cells. In addition, hyperactive JNK also rendered MDA-MB-468 cells more invasive (Fig. 1D), as demonstrated by the transwell invasion assay. The increase of cell migration and invasion by CA-JNK was abolished using the small-molecule JNK inhibitor SP600125 (Supplementary Fig. 1)
Insulin-like growth factors (IGFs) are critically involved in breast cancer progression. Previously we reported that a constitutively active type I IGF receptor (CD8-IGF-IR) causes transformation of MCF-10A human mammary epithelial cells accompanied by a dramatic increase in cell invasion (26). Thus we explored whether sustained JNK activity could be induced by overexpression of CD8-IGF-IR. Western blot analysis demonstrated that levels of phosphorylated JNK were persistently elevated in CD8-IGF-IR-transformed mammary epithelial cells, while levels of total JNK were unchanged (Fig. 1E). Furthermore, the transwell invasion assay showed that blocking JNK activity with a widely used small-molecule JNK inhibitor SP600125 abolished the increase of cell invasion by CD8-IGF-IR (Fig. 1F), whereas the ERK inhibitor U0126 had a much less profound effect (Supplementary Fig. 2), suggesting that sustained JNK activity is involved in the IGF-IR effect on breast cancer cell invasion. In summary, these data demonstrate that hyperactive JNK can potentiate cell migration and invasion without eliciting cell apoptosis.
Constitutive JNK activity induces partial EMT
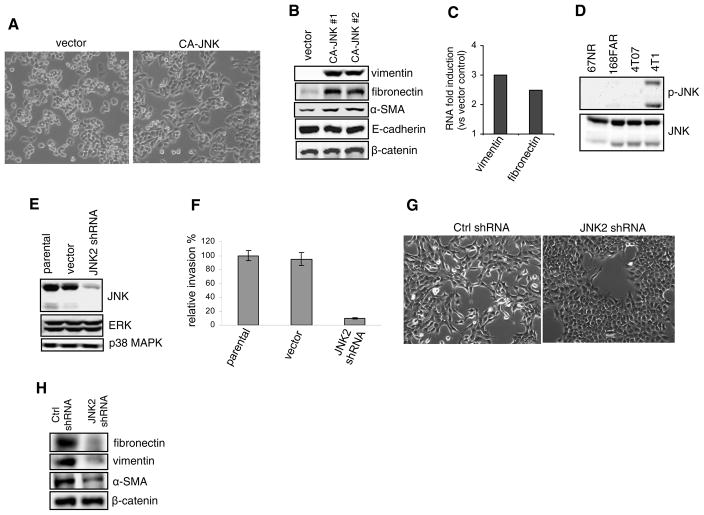
Epithelial-mesenchymal transition (EMT) is a complex process associated with alterations in epithelial cell junctions, changes in cell morphology, reorganization of the cell cytoskeleton, expression of fibroblastic markers, and enhancement of cell motility and invasiveness (27). We found that ectopic expression of CA-JNK caused MDA-MB-468 cells to partially shed their cuboidal morphology and acquire instead a more elongated shape, to some extent reminiscent of mesenchymal cells (Fig. 2A). To examine whether mesenchymal markers were induced, we performed immunoblotting. As presented in Fig. 2B, expression of vimentin and fibronectin was dramatically upregulated by CA-JNK and levels of α-smooth muscle actin were moderately but consistently elevated, whereas N-cadherin was not detected in control cells or stable transfectants (data not shown). In contrast, there were no significant changes in levels of epithelial cell-specific proteins such as E-cadherin and β-catenin. This suggests that constitutive JNK activity can partially program the EMT process by orchestrating the expression of specific mesenchymal markers.
Figure 2.
Sustained JNK leads to partial EMT. (A) Phase-contrast images of MDA-MB-468 cells expressing the vector or CA-JNK. (B) Immunoblotting of mesenchymal (vimentin, fibronectin, and α-smooth muscle actin) and epithelial (E-cadherin and β-catenin) markers in control and CA-JNK-overexpressing MDA-MB-468 cells. (C) qRT-PCR analysis of vimentin and fibronectin expression. Data represent their expression in CA-JNK-expressing MDA-MB-468 cells compared with control cells. (D) Immunoblotting of p-JNK and total JNK was conducted using cell lysates from mouse mammary tumor sublines 67NR, 168FAR, 4T07, and 4T1. (E) The lentiviral vector expressing mouse JNK2 shRNA was stably transduced into 4T1 cells. Knockdown of total JNK was analyzed by immunoblotting. (F) Control and JNK2 shRNA 4T1 cells were tested using transwell invasion assays. Relative invasion is presented as percent of invading JNK2 shRNA cells over the vector control. (G) Morphologies of 4T1 cells stably transduced by control or JNK2 shRNA are shown. (H) Immunoblotting of mesenchymal (vimentin, fibronectin, and α-smooth muscle actin) and epithelial (β-catenin) markers in control and JNK2-knockdown 4T1 cells. N-cadherin and E-cadherin were not detected (data not shown).
To ascertain whether the increase of vimentin and fibronectin occurs via a transcriptional mechanism, we performed quantitative RT-PCR. As expected, vimentin and fibronectin RNA levels were increased by 3.0 and 2.5 fold respectively in MDA-MB-468 cells expressing CA-JNK as compared with the control cells (Fig. 2C).
To confirm that JNK may be involved in EMT, we also exploited four mouse breast cancer cell lines (67NR, 168FAR, 4T07, and 4T1) derived from a mammary tumor in a wildtype mouse (28)., Of these four cell lines, only 4T1 cells can spontaneously metastasize to lungs and other organs when transplanted into the mammary glands of mice, providing a model of stage IV breast cancer. 4T1 cells reportedly have undergone EMT (29). In our study, immunoblotting showed similar total JNK levels among the four cell lines, but only 4T1 cells possessed sustained JNK activation (Fig. 2D). Because JNK2 was found to be the dominant JNK isoform in 4T1 cells (data not shown), we stably transduced a JNK2 shRNA lentiviral construct into 4T1 cells. Total JNK levels and cell invasion were dramatically reduced in these JNK2 shRNA-expressing cells (Fig. 2, E and F), which was further substantiated by the blockade of 4T1 cell invasion with SP600125 (Supplementary Fig. 3). JNK2 knockdown caused fibroblast-like 4T1 cells to become cobblestone-like (Fig. 2G) and reduced the expression of fibroblast markers, particularly fibronectin and vimentin (Fig. 2H). Moreover, ectopic expression of CA-JNK in weakly invasive 67NR mouse breast cancer cells enhanced cell invasion (Supplementary Fig. 4). Collectively, these data further support a role of JNK in the regulation of EMT.
Hyperactive JNK upregulates AP-1 activity
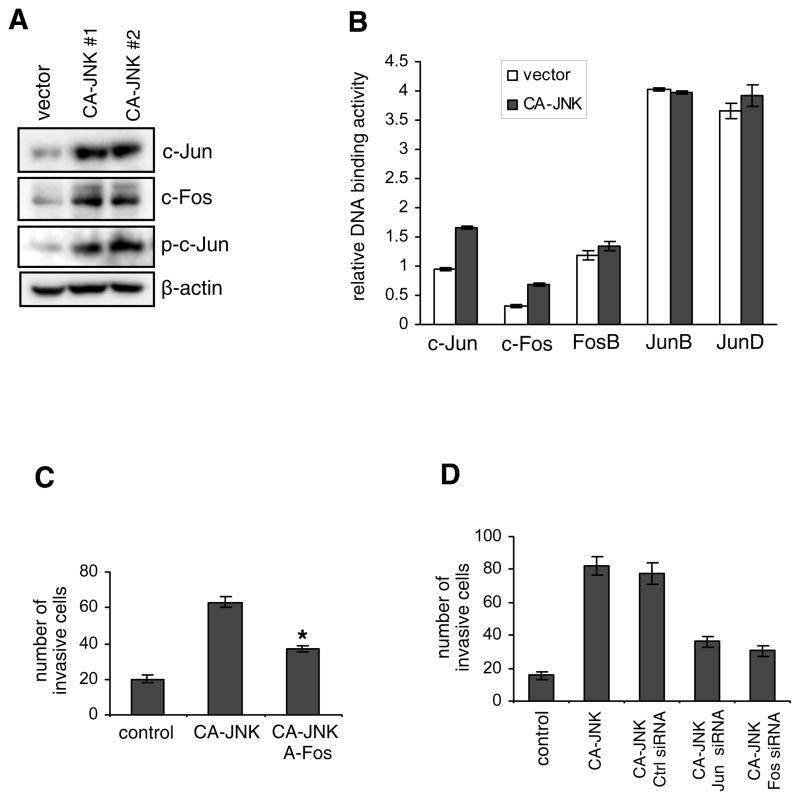
Because JNK is an activator of AP-1, we postulated that AP-1 activity would be upregulated in breast cancer cells with constitutive JNK activity. Thus, we performed western blotting of the AP-1 components c-Jun and c-Fos. As illustrated in Fig. 3A, total levels of c-Jun and c-Fos were markedly elevated by expression of CA-JNK. Phosphorylation of c-Jun at Ser73 was also increased. To confirm that AP-1 activity was increased in CA-JNK-expressing breast cancer cells, we isolated nuclear proteins and tested the binding of different AP-1 components to the consensus oligonucleotide 5′-TGAGTCA-3′ using ELISA. As demonstrated in Fig. 3B, DNA binding capacity increased for c-Jun and c-Fos, but not for FosB, JunB, and JunD. Next, we examined whether the enhanced AP-1 activity contributed to cell invasion induced by hyperactive JNK. We ectopically expressed a dominant negative c-Fos (A-Fos) in CA-JNK-overexpressing cells (30). As illustrated in Fig. 3C, inhibition of AP-1 by A-Fos impaired cell invasion. Cell migration and expression of vimentin and fibronectin were also decreased by A-Fos overexpression (data not shown). In consistence, inhibition of AP-1 by c-Jun or c-Fos siRNA also impeded cell invasion induced by hyperactive JNK (Fig. 3D). Taken together, these data suggest that JNK may increase cell migration and invasion in part by upregulating AP-1 activity.
Figure 3.
Constitutively active JNK upregulates AP-1 activity. (A) Immunoblotting of AP-1 components c-Jun and c-Fos, and phosphorylated c-Jun in control and CA-JNK-overexpressing MDA-MB-468 cells. (B) Binding of different AP-1 components to the consensus AP-1 site 5′-TGAGTCA-3′ was assessed by ELISA. The values represent mean ± SD of two independent experiments, each performed in triplicate. (C) A-Fos or the empty vector was transiently transfected into CA-JNK-overexpressing MDA-MB-468 cells. Cell invasion was measured by the Boyden chamber assay. * P < 0.05 vs the empty vector. (D) c-Jun, c-Fos, or control siRNA was transiently transfected into CA-JNK MDA-MB-468 cells, followed by the cell invasion assay after 48 h.
Hyperactive JNK induces ERK activation
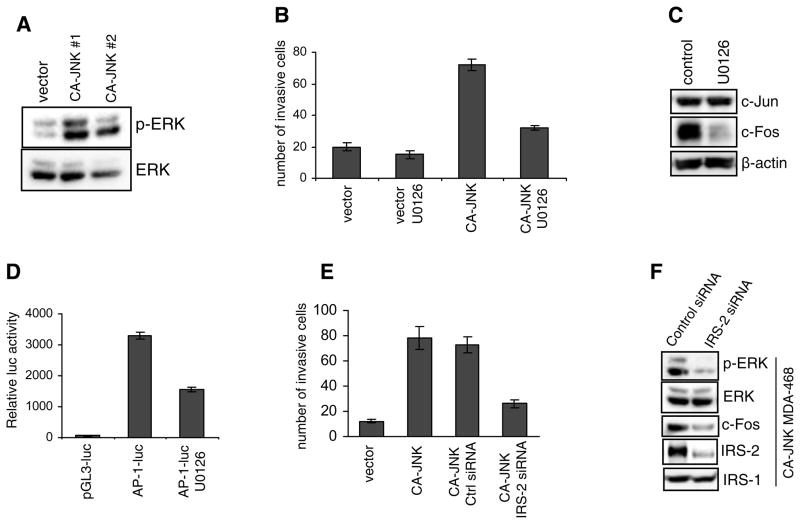
Because both ERK and JNK are potently activated by EGF in MDA-MB-468 cells (24), and ERK is involved in cell migration, invasion, and EMT (31), we speculated that hyperactive JNK might modulate ERK activation. To address this question, we compared phosphorylated ERK levels in control and CA-JNK-expressing MDA-MB-468 cells using immunoblotting. As illustrated in Fig. 4A, expression of the hyperactive JNK dramatically elevated levels of ERK phosphorylation, but did not change total ERK levels.
Figure 4.
Sustained JNK activates ERK. (A) Immunoblotting of total and phosphorylated levels of ERK in control and CA-JNK-overexpressing MDA-MB-468 cells. (B) The transwell invasion assay was conducted using control and CA-JNK cells. The inhibitor U0126 was used to block ERK activity. Each bar represents mean ± SD of samples measured in triplicate. (C) Expression of c-Jun and c-Fos was examined in CA-JNK-expressing cells treated with U0126 for 24 h. (D) CA-JNK-expressing cells were transfected with an AP-1-luc reporter construct and then treated with U0126 for 24 h, followed by luciferase assays. A β-galactosidase vector was used for normalization. (E) CA-JNK-expressing MDA-MB-468 cells were transfected with control siRNA or IRS-2 siRNA for 48 h. Cells were trypsinized and subjected to the transwell invasion assay. (F) CA-JNK-expressing cells were transfected with control or IRS-2 siRNA for 72 h. Levels of phosphorylated ERK and c-Fos were examined by immunoblotting. The IRS-2 homog IRS-1 was used as a control for confirming IRS-2 siRNA specificity.
Next we tested whether enhanced ERK activation could affect CA-JNK-induced cell invasion. To this end, we used the small-molecule inhibitor U0126 to block ERK activity and performed Boyden chamber transwell invasion assays. As illustrated in Fig. 4B, ERK inhibition largely suppressed cell invasion elicited by CA-JNK, suggesting that increased ERK activation mediates the effects of hyperactive JNK on breast cancer cell invasion.
It is well established that ERK can upregulate c-Fos transcription (32). To investigate whether increased ERK activity was involved in the induction of AP-1 by hyperactive JNK, we pretreated CA-JNK-expressing MDA-MB-468 cells with the ERK inhibitor U0126. Immunoblotting demonstrated that ERK inhibition suppressed the c-Fos increase but did not affect c-Jun expression (Fig. 4C). To further establish the role of ERK in the regulation of AP-1 by hyperactive JNK, we transiently transfected the CA-JNK-expressing cells with an AP-1-luciferase reporter construct and then treated the cells with U0126. As illustrated in Fig. 4D, ERK inhibition reduced the AP-1-driven luciferase activity.
Previously we showed that the EGF/JNK/AP-1 pathway upregulates a critical signaling scaffold protein IRS-2 in MDA-MB-468 cells (24). In the present study, we found that CA-JNK induced IRS-2 expression in MDA-MB-468 cells, which was abolished by the JNK inhibitor SP600125 or a dominant-negative JNK (APF) mutant (supplementary Fig. 5). Notably, IRS-2 levels were elevated in 4T1 mouse breast cancer cells (Supplementary Fig. 6A), which possess constitutively active JNK (see Fig. 2D). Overexpression of IRS-2 enhanced the invasion of weakly invasive 67NR mouse breast cancer cells (Supplementary Fig. 6B). IRS-2 is essential for breast cancer cell migration and invasion (33, 34). In support of this notion, IRS-2 knockdown by siRNA impaired the invasion abilities of both 4T1 cells (Supplementary Fig. 6C) and CA-JNK-expressing MDA-MB-468 cells (Fig. 4E). In addition to playing critical roles in insulin and IGF signaling, IRS-2 is involved in cytokine, growth hormone, and integrin signaling. A well-characterized feature of the activated IRS proteins is their association with Grb2 (35), leading to activation of the Ras/Raf/ERK pathway. To examine whether IRS-2 was involved in the elevation of ERK activity elicited by hyperactive JNK, we used siRNA to knockdown IRS-2 (24). Immunoblotting indicated that suppression of IRS-2 expression in CA-JNK-expressing cells reduced the levels of ERK phosphorylation and c-Fos but did not affect total ERK levels (Fig. 4F). Taken together, our data indicate that JNK induce breast cancer cell invasion by increasing ERK/AP-1 signaling via IRS-2.
Sustained JNK activity reduces cell sensitivity to the chemotherapy agent paclitaxel
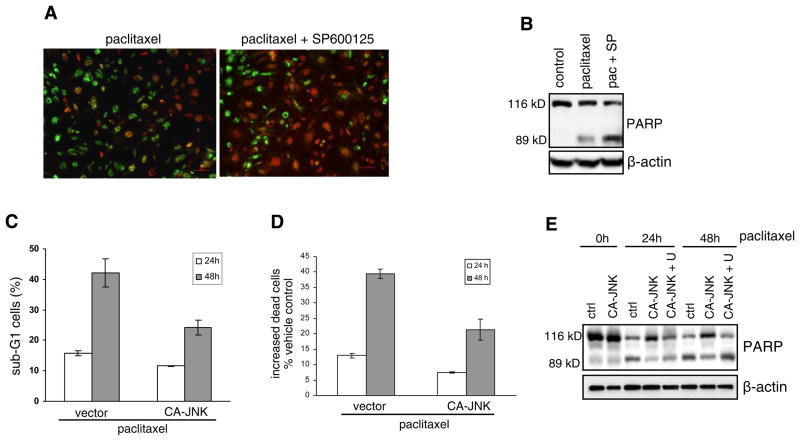
JNK elicits anticancer drug-elicited cell apoptosis when it is slowly activated over a long time course (10, 11). JNK can also mediates cell survival when it is activated in a rapid and transient fashion by growth factors (36). Thus, hyperactive JNK may be assumed to trigger apoptosis. Interestingly, after 4T1 cells, which have constitutively active JNK, were treated with the chemotherapy drug paclitaxel in the presence or absence of the JNK inhibitor SP600125, propidium iodide and SYTO-13 double staining showed that JNK blockade increased paclitaxel-induced apoptosis (Fig. 5A). In addition, immunoblotting showed that SP600125 increased levels of the 89 kD cleaved fragment of nuclear poly (ADP-ribose) polymerase (PARP), one of the main cleavage targets of caspases, in paclitaxel-treated 4T1 cells (Fig. 5B).
Figure 5.
Constitutive JNK activity reduces cell sensitivity to paclitaxel. (A) 4T1 cells were treated with 0.01 μM paclitaxel for 48 h in the presence or absence of the JNK inhibitor SP600125. Then cells were stained with PI (red staining for necrotic and late apoptotic cells) and SYTO-13 (green staining for live and early apoptotic cells). Fluorescent images are shown. (B) 4T1 cells were treated with 0.01 μM paclitaxel (pac) in the presence or absence of 10 μM SP600125 (SP). Immunoblotting of PARP is shown. The 89 kD cleaved product is an indicator of cell apoptosis. (C) Cells were treated with 0.01 μM paclitaxel for 24 h or 48 h, and then stained with PI. The DNA content was analyzed by flow cytometry. The data represent percent of total cells. Each experiment was repeated twice. (D) Paclitaxel-induced dead cells in comparison to vehicle-treated control were analyzed by a cytotoxicity assay. Relative number of dead cells is associated with and thus reflected by measured activity of tripeptidyl peptidase released from cytoplasm due to membrane breakdown. Each bar represents mean ± SD of samples measured in triplicate. (E) PARP cleavage was examined by immunoblotting. U0126 (5 μM) was used to block ERK activity.
As aforementioned, CA-JNK did not enhance spontaneous apoptosis. To further investigate whether hyperactive JNK potentiates breast cancer cell survival, we treated control and CA-JNK-expressing MDA-MB-468 cells with paclitaxel and examined apoptosis using both sub-G1 flow cytometry analysis and fluorescence cytotoxicity assays. In stark contrast to the well-known function of basal JNK activity, hyperactive JNK activation reduced cell apoptosis induced by paclitaxel (Fig. 5C and D). Immunoblotting demonstrated that CA-JNK reduced levels of the 89 kD PARP in MDA-MB-468 cells (Fig. 5E). In addition, the ERK inhibitor U0126 impaired the effect of CA-JNK on PARP degradation (Fig. 5E), suggesting that increased ERK activation mediates the effect of hyperactive JNK on cell survival.
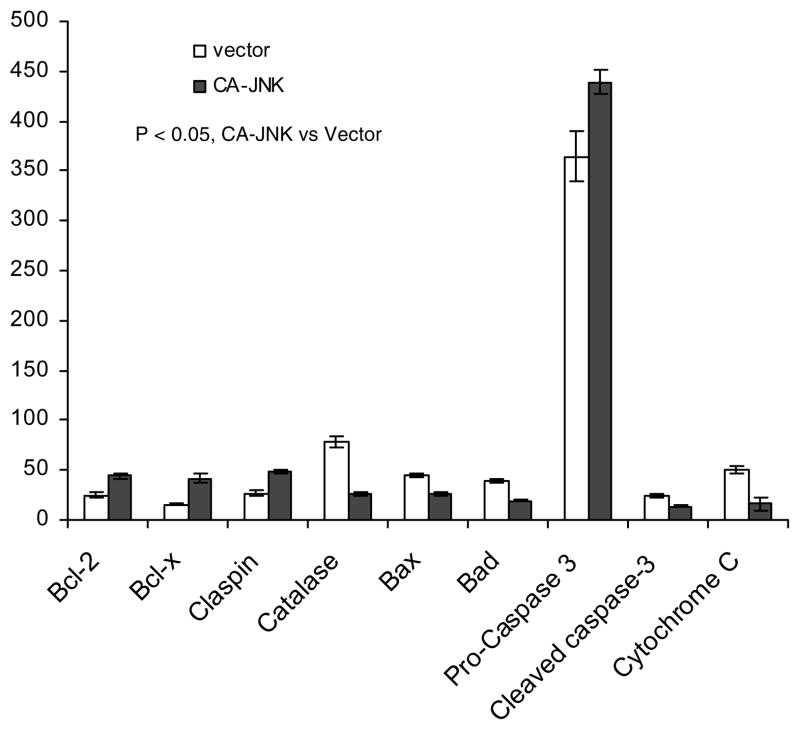
Next we conducted an apoptosis/survival protein antibody array analysis with control and CA-JNK-expressing MDA-MB-468 cells. Immunoblotting of the array showed that survival proteins such as Bcl-2, Bcl-XL, and claspin were up-regulated by CA-JNK, while apoptosis proteins such as Bax, Bad, and cytochrome C were downregulated (Fig. 6). Overexpression of the redox protein catalase has also been shown to promote apoptosis (37, 38), as prolonged removal of intracellular reactive oxygen species is detrimental to cell functions. In summary, these data suggest that constitutive JNK activity in breast cancer cells inhibits apoptosis induced by cytotoxic drugs.
Figure 6.
Anti- and pro-apoptotic proteins are regulated by sustained JNK activity. Cell lysates from control and CA-JNK-expressing MDA-MB-468 cells were analyzed using protein antibody arrays of pro- or anti-apoptotic proteins. Relative densitometry (see materials and methods) was used to compare levels of the proteins. The data represent mean ± SD of two independent experiments. P < 0.05, control versus CA-JNK.
Discussion
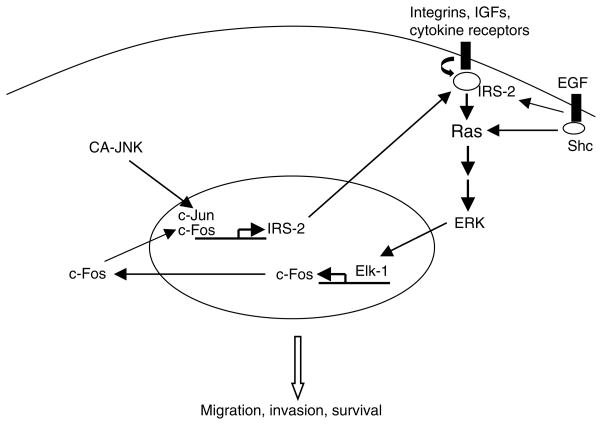
The present study shows that persistent JNK activity does not spontaneously induce apoptosis. Instead, it enhances cell migration and invasion by increasing AP-1 and ERK activity. In our in vitro models, overexpression of JNK in human breast cancer cells was associated with partial induction of EMT and decreased sensitivity to the anticancer drug paclitaxel; this effect was mediated by ERK signaling. Recent reports have shown that elevated JNK activation contributes to the pathogenesis and progression of human brain tumors, prostate carcinoma, and osteosarcoma (17–19). Two clinical studies also show that levels of phosphorylated JNK correlate with breast cancer metastasis and decreased overall survival (20, 21). Furthermore, increased JNK activity has been associated with acquired tamoxifen resistance in breast cancer (39, 40). Although JNK is known to have anti- and pro-apoptotic functions, depending on the signaling network and stimuli (41), the role of JNK signaling in breast cancer response to chemotherapy is poorly understood. Our studies reveal a novel positive feedback mechanism by which hyperactive JNK activity, unlike basal JNK activity, may promote tumor progression via activating IRS-2/ERK signaling (Fig. 7).
Figure 7.
Illustration of the signaling pathways activated by hyperactive JNK.
We found that hyperactive JNK elicited partial EMT with a concomitant increase of ERK and AP-1 in breast cancer cells. It is well known that hyperactivation of ERK mitogenic stimulation typically results in induction of EMT (31, 42). TGF-β reported induces EMT in human keratinocytes and mouse tracheal epithelial cells by mechanisms that involve JNK (43, 44). Both JNK and ERK are upstream of AP-1 induction. In addition to the c-Jun phosphorylation at Ser63 and Ser73, AP-1 activity can also be potentiated via increase of c-Fos expression by ERK-mediated TCF/Elk-1 phosphorylation (32, 45). Jun can act as an effector of both JNK and ERK pathways during development of Drosophila (46). Our data in breast cancer cells supports a model in which hyperactive JNK activates the ERK pathway and thereby stimulates c-Fos expression; c-Jun expression may be directly induced by JNK, as c-Jun is positively autoregulated by itself after its phosphorylation by JNK (47). Consequently, high AP-1 activity leads to expression of vimentin and fibronectin (48, 49).
How might JNK upregulate ERK? Previously, Chen et al. found that the phosphorylation of ERK and AP-1 DNA binding were concomitantly inhibited in JNK2(−/−) mice (50). One explanation is that IRS-2 mediates the JNK effect on ERK. The IRS network of upstream and downstream signaling may place IRS proteins in a central position to integrate and coordinate multiple signaling pathways (51, 52). As is well known, IRS-2 and its homolog IRS-1 coordinate the signaling pathways elicited by insulin, IGFs, and cytokines. Interestingly, IRS-1 and IRS-2, despite their structural and functional similarities, are not completely interchangeable in terms of their mediation of IGF-stimulated gene expression and cell cycle progression (53), as reflected by the distinct phenotypes in respective knockout and MMTV-IRS transgenic mice (54–56). IRS-2 is required for breast cancer cell migration, invasion, and survival (33). Interestingly, recent work suggests that IRS-2 but not its homolog IRS-1 may contribute to ERK signaling (53, 57). We have also shown that transgenic mice with IRS-2 overexpression in the mammary gland develop mammary tumors with high ERK activation (22). IRS-2 may serve as a link between the JNK and ERK pathways.
Another interesting finding in our study is that hyperactive JNK attenuated the apoptosis of breast cancer cells treated with the chemotherapy drug paclitaxel. This suggests that the role of JNK changes when its activity/expression increases above the basal levels associated with apoptosis. It has been proposed that the opposing roles of JNK in apoptosis and survival are determined by the time course of JNK activation (58): prolonged JNK activation is required for apoptotic signaling and is sufficient for apoptosis (59), whereas transient JNK activation caused by TNF and other growth factors contributes to survival (60). However, our data suggest that sustained JNK activation can induce cell survival, and this JNK effect may be mediated by IRS-2/ERK activation. IRS-2 null mammary tumor cells were more apoptotic in response to growth factor deprivation than their wildtype counterparts (33). One surprising finding is that hyperactive JNK increases Bcl-2 survival protein and decreases apoptosis-promoting proteins such as Bax and Bad. Inhibition of Bcl-2 and activation of Bax have been proposed to mediate the effect of JNK on cell death (61). Thus, constitutively active JNK and transiently induced JNK play opposing roles in cell survival regulation. How hyperactive JNK regulates Bcl-2 family protein expression merits further investigation.
Recently, it has been found that hepatocyte death is associated with compensatory proliferation of surviving hepatocytes (62), which may imply a novel mechanism of cancer therapeutic resistance, i.e., therapy-elicited apoptosis of tumor cells with basal JNK activity may release mitogens that induce persistent JNK activation in neighboring cells to promote growth and invasion. In summary, our findings identify a novel mechanism of cross-talk between the JNK and ERK signaling pathways. JNK activation may serve as a marker of breast cancer progression and might also be exploited as novel therapeutic targets.
Supplementary Material
Acknowledgments
Grant support: This work was supported by the Susan G. Komen Breast Cancer Foundation grant BCTR0601346 (X. Cui), NIH SPORE P50CA58183 career development award (X. Cui), and NIH grant R01CA94118 (A. Lee).
We thank Dr. Ulf Rapp for providing SAPKβ-MKK7 vector, Dr. Charles Vinson for A-Fos vector, Dr. Fred Miller for 4T1 mouse breast cancer cells, Dr. Tse-Hua Tan for JNK (APF) mutant construct, and Dr. Hyun-Jung Kim for technical assistance.
References
- 1.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103(2):239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 2.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410(6824):37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 3.Tournier C, Hess P, Yang DD, et al. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288(5467):870–4. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 4.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27(48):6245–51. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson R, Spiegelman B, Hanahan D, Wisdom R. Cellular transformation and malignancy induced by ras require c-jun. Mol Cell Biol. 1996;16(8):4504–11. doi: 10.1128/mcb.16.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behrens A, Jochum W, Sibilia M, Wagner EF. Oncogenic transformation by ras and fos is mediated by c-Jun N-terminal phosphorylation. Oncogene. 2000;19(22):2657–63. doi: 10.1038/sj.onc.1203603. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues GA, Park M, Schlessinger J. Activation of the JNK pathway is essential for transformation by the Met oncogene. Embo J. 1997;16(10):2634–45. doi: 10.1093/emboj/16.10.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickens M, Rogers JS, Cavanagh J, et al. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science. 1997;277(5326):693–6. doi: 10.1126/science.277.5326.693. [DOI] [PubMed] [Google Scholar]
- 9.Zhang JY, Green CL, Tao S, Khavari PA. NF-kappaB RelA opposes epidermal proliferation driven by TNFR1 and JNK. Genes Dev. 2004;18(1):17–22. doi: 10.1101/gad.1160904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakshatri H, Rice SE, Bhat-Nakshatri P. Antitumor agent parthenolide reverses resistance of breast cancer cells to tumor necrosis factor-related apoptosis-inducing ligand through sustained activation of c-Jun N-terminal kinase. Oncogene. 2004;23(44):7330–44. doi: 10.1038/sj.onc.1207995. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Wieder R. All-trans retinoic acid potentiates Taxotere-induced cell death mediated by Jun N-terminal kinase in breast cancer cells. Oncogene. 2004;23(2):426–33. doi: 10.1038/sj.onc.1207040. [DOI] [PubMed] [Google Scholar]
- 12.Mingo-Sion AM, Marietta PM, Koller E, Wolf DM, Van Den Berg CL. Inhibition of JNK reduces G2/M transit independent of p53, leading to endoreduplication, decreased proliferation, and apoptosis in breast cancer cells. Oncogene. 2004;23(2):596–604. doi: 10.1038/sj.onc.1207147. [DOI] [PubMed] [Google Scholar]
- 13.Kuan CY, Yang DD, Samanta Roy DR, Davis RJ, Rakic P, Flavell RA. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22(4):667–76. doi: 10.1016/s0896-6273(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 14.Almeida EA, Ilic D, Han Q, et al. Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH(2)-terminal kinase. J Cell Biol. 2000;149(3):741–54. doi: 10.1083/jcb.149.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu C, Minemoto Y, Zhang J, et al. JNK suppresses apoptosis via phosphorylation of the proapoptotic Bcl-2 family protein BAD. Mol Cell. 2004;13(3):329–40. doi: 10.1016/s1097-2765(04)00028-0. [DOI] [PubMed] [Google Scholar]
- 16.Lamb JA, Ventura JJ, Hess P, Flavell RA, Davis RJ. JunD mediates survival signaling by the JNK signal transduction pathway. Mol Cell. 2003;11(6):1479–89. doi: 10.1016/s1097-2765(03)00203-x. [DOI] [PubMed] [Google Scholar]
- 17.Antonyak MA, Kenyon LC, Godwin AK, et al. Elevated JNK activation contributes to the pathogenesis of human brain tumors. Oncogene. 2002;21(33):5038–46. doi: 10.1038/sj.onc.1205593. [DOI] [PubMed] [Google Scholar]
- 18.Yang YM, Bost F, Charbono W, et al. C-Jun NH(2)-terminal kinase mediates proliferation and tumor growth of human prostate carcinoma. Clin Cancer Res. 2003;9(1):391–401. [PubMed] [Google Scholar]
- 19.Papachristou DJ, Batistatou A, Sykiotis GP, Varakis I, Papavassiliou AG. Activation of the JNK-AP-1 signal transduction pathway is associated with pathogenesis and progression of human osteosarcomas. Bone. 2003;32(4):364–71. doi: 10.1016/s8756-3282(03)00026-7. [DOI] [PubMed] [Google Scholar]
- 20.Yeh YT, Hou MF, Chung YF, et al. Decreased expression of phosphorylated JNK in breast infiltrating ductal carcinoma is associated with a better overall survival. Int J Cancer. 2006;118(11):2678–84. doi: 10.1002/ijc.21707. [DOI] [PubMed] [Google Scholar]
- 21.Davidson B, Konstantinovsky S, Kleinberg L, et al. The mitogen-activated protein kinases (MAPK) p38 and JNK are markers of tumor progression in breast carcinoma. Gynecol Oncol. 2006 doi: 10.1016/j.ygyno.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 22.Dearth RK, Cui X, Kim HJ, et al. Mammary tumorigenesis and metastasis caused by overexpression of insulin receptor substrate 1 (IRS-1) or IRS-2. Mol Cell Biol. 2006;26(24):9302–14. doi: 10.1128/MCB.00260-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rennefahrt UE, Illert B, Kerkhoff E, Troppmair J, Rapp UR. Constitutive JNK activation in NIH 3T3 fibroblasts induces a partially transformed phenotype. J Biol Chem. 2002;277(33):29510–8. doi: 10.1074/jbc.M203010200. [DOI] [PubMed] [Google Scholar]
- 24.Cui X, Kim HJ, Kuiatse I, Kim H, Brown PH, Lee AV. Epidermal Growth Factor Induces Insulin Receptor Substrate-2 in Breast Cancer Cells via c-Jun NH2-Terminal Kinase/Activator Protein-1 Signaling to Regulate Cell Migration. Cancer Res. 2006;66(10):5304–13. doi: 10.1158/0008-5472.CAN-05-2858. [DOI] [PubMed] [Google Scholar]
- 25.Huang C, Rajfur Z, Borchers C, Schaller MD, Jacobson K. JNK phosphorylates paxillin and regulates cell migration. Nature. 2003;424(6945):219–23. doi: 10.1038/nature01745. [DOI] [PubMed] [Google Scholar]
- 26.Kim HJ, Litzenburger BC, Cui X, et al. Constitutively active type I insulin-like growth factor receptor causes transformation and xenograft growth of immortalized mammary epithelial cells and is accompanied by an epithelial-to-mesenchymal transition mediated by NF-kappaB and snail. Mol Cell Biol. 2007;27(8):3165–75. doi: 10.1128/MCB.01315-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 28.Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52(6):1399–405. [PubMed] [Google Scholar]
- 29.Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Olive M, Krylov D, Echlin DR, Gardner K, Taparowsky E, Vinson C. A dominant negative to activation protein-1 (AP1) that abolishes DNA binding and inhibits oncogenesis. J Biol Chem. 1997;272(30):18586–94. doi: 10.1074/jbc.272.30.18586. [DOI] [PubMed] [Google Scholar]
- 31.Xie L, Law BK, Chytil AM, Brown KA, Aakre ME, Moses HL. Activation of the Erk pathway is required for TGF-beta1-induced EMT in vitro. Neoplasia. 2004;6(5):603–10. doi: 10.1593/neo.04241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavigelli M, Dolfi F, Claret FX, Karin M. Induction of c-fos expression through JNK-mediated TCF/Elk-1 phosphorylation. Embo J. 1995;14(23):5957–64. doi: 10.1002/j.1460-2075.1995.tb00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagle JA, Ma Z, Byrne MA, White MF, Shaw LM. Involvement of insulin receptor substrate 2 in mammary tumor metastasis. Mol Cell Biol. 2004;24(22):9726–35. doi: 10.1128/MCB.24.22.9726-9735.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson JG, Zhang X, Yoneda T, Yee D. Regulation of breast cancer cell motility by insulin receptor substrate-2 (IRS-2) in metastatic variants of human breast cancer cell lines. Oncogene. 2001;20(50):7318–25. doi: 10.1038/sj.onc.1204920. [DOI] [PubMed] [Google Scholar]
- 35.White MF. The IRS-signaling system: a network of docking proteins that mediate insulin and cytokine action. Recent Prog Horm Res. 1998;53:119–38. [PubMed] [Google Scholar]
- 36.Chen YR, Tan TH. The c-Jun N-terminal kinase pathway and apoptotic signaling (review) Int J Oncol. 2000;16(4):651–62. doi: 10.3892/ijo.16.4.651. [DOI] [PubMed] [Google Scholar]
- 37.Brown MR, Miller FJ, Jr, Li WG, et al. Overexpression of human catalase inhibits proliferation and promotes apoptosis in vascular smooth muscle cells. Circ Res. 1999;85(6):524–33. doi: 10.1161/01.res.85.6.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wedgwood S, Black SM. Induction of apoptosis in fetal pulmonary arterial smooth muscle cells by a combined superoxide dismutase/catalase mimetic. Am J Physiol Lung Cell Mol Physiol. 2003;285(2):L305–12. doi: 10.1152/ajplung.00382.2002. [DOI] [PubMed] [Google Scholar]
- 39.Schiff R, Reddy P, Ahotupa M, et al. Oxidative stress and AP-1 activity in tamoxifen-resistant breast tumors in vivo. J Natl Cancer Inst. 2000;92(23):1926–34. doi: 10.1093/jnci/92.23.1926. [DOI] [PubMed] [Google Scholar]
- 40.Johnston SR, Lu B, Scott GK, et al. Increased activator protein-1 DNA binding and c-Jun NH2-terminal kinase activity in human breast tumors with acquired tamoxifen resistance. Clin Cancer Res. 1999;5(2):251–6. [PubMed] [Google Scholar]
- 41.Lin A, Dibling B. The true face of JNK activation in apoptosis. Aging Cell. 2002;1(2):112–6. doi: 10.1046/j.1474-9728.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- 42.Ellenrieder V, Hendler SF, Boeck W, et al. Transforming growth factor beta1 treatment leads to an epithelial-mesenchymal transdifferentiation of pancreatic cancer cells requiring extracellular signal-regulated kinase 2 activation. Cancer Res. 2001;61(10):4222–8. [PubMed] [Google Scholar]
- 43.Santibanez JF. JNK mediates TGF-beta1-induced epithelial mesenchymal transdifferentiation of mouse transformed keratinocytes. FEBS Lett. 2006;580(22):5385–91. doi: 10.1016/j.febslet.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Alcorn JF, Guala AS, van der Velden J, et al. Jun N-terminal kinase 1 regulates epithelial-to-mesenchymal transition induced by TGF-beta1. J Cell Sci. 2008;121(Pt 7):1036–45. doi: 10.1242/jcs.019455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young MR, Nair R, Bucheimer N, et al. Transactivation of Fra-1 and consequent activation of AP-1 occur extracellular signal-regulated kinase dependently. Mol Cell Biol. 2002;22(2):587–98. doi: 10.1128/MCB.22.2.587-598.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peverali FA, Isaksson A, Papavassiliou AG, et al. Phosphorylation of Drosophila Jun by the MAP kinase rolled regulates photoreceptor differentiation. Embo J. 1996;15(15):3943–50. [PMC free article] [PubMed] [Google Scholar]
- 47.Angel P, Hattori K, Smeal T, Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988;55(5):875–85. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- 48.Sommers CL, Skerker JM, Chrysogelos SA, Bosseler M, Gelmann EP. Regulation of vimentin gene transcription in human breast cancer cell lines. Cell Growth Differ. 1994;5(8):839–46. [PubMed] [Google Scholar]
- 49.Tamura K, Chen YE, Lopez-Ilasaca M, et al. Molecular mechanism of fibronectin gene activation by cyclic stretch in vascular smooth muscle cells. J Biol Chem. 2000;275(44):34619–27. doi: 10.1074/jbc.M004421200. [DOI] [PubMed] [Google Scholar]
- 50.Chen N, Nomura M, She QB, et al. Suppression of skin tumorigenesis in c-Jun NH(2)-terminal kinase-2-deficient mice. Cancer Res. 2001;61(10):3908–12. [PubMed] [Google Scholar]
- 51.Dearth RK, Cui X, Kim HJ, Hadsell DL, Lee AV. Oncogenic transformation by the signaling adaptor proteins insulin receptor substrate (IRS)-1 and IRS-2. Cell Cycle. 2007;6(6):705–13. doi: 10.4161/cc.6.6.4035. [DOI] [PubMed] [Google Scholar]
- 52.Gibson SL, Ma Z, Shaw LM. Divergent roles for IRS-1 and IRS-2 in breast cancer metastasis. Cell Cycle. 2007;6(6):631–7. doi: 10.4161/cc.6.6.3987. [DOI] [PubMed] [Google Scholar]
- 53.Bruning JC, Winnay J, Cheatham B, Kahn CR. Differential signaling by insulin receptor substrate 1 (IRS-1) and IRS- 2 in IRS-1-deficient cells. Mol Cell Biol. 1997;17(3):1513–21. doi: 10.1128/mcb.17.3.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tamemoto H, Kadowaki T, Tobe K, et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature. 1994;372(6502):182–6. doi: 10.1038/372182a0. [DOI] [PubMed] [Google Scholar]
- 55.Araki E, Lipes MA, Patti ME, et al. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372(6502):186–90. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- 56.Withers DJ, Gutierrez JS, Towery H, et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391(6670):900–4. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- 57.Huang C, Thirone AC, Huang X, Klip A. Differential contribution of insulin receptor substrates 1 versus 2 to insulin signaling and glucose uptake in l6 myotubes. J Biol Chem. 2005;280(19):19426–35. doi: 10.1074/jbc.M412317200. [DOI] [PubMed] [Google Scholar]
- 58.Traverse S, Seedorf K, Paterson H, Marshall CJ, Cohen P, Ullrich A. EGF triggers neuronal differentiation of PC12 cells that overexpress the EGF receptor. Curr Biol. 1994;4(8):694–701. doi: 10.1016/s0960-9822(00)00154-8. [DOI] [PubMed] [Google Scholar]
- 59.Chen YR, Wang X, Templeton D, Davis RJ, Tan TH. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J Biol Chem. 1996;271(50):31929–36. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- 60.Sluss HK, Barrett T, Derijard B, Davis RJ. Signal transduction by tumor necrosis factor mediated by JNK protein kinases. Mol Cell Biol. 1994;14(12):8376–84. doi: 10.1128/mcb.14.12.8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19(2):142–9. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 62.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121(7):977–90. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.