Abstract
Eradication of residual malignancies and metastatic tumors via a systemic approach is the key for successfully treating cancer and increasing the cancer patient survival. Systemic administration of IL12 protein in an acute large dose is effective but toxic. Systemic administration of IL12 gene by persistently expressing a low level of IL12 protein may reduce the systemic toxicity, but only eradicates IL12 sensitive tumors. Here, we discovered that sequential administration of IL12 and IL27 encoding DNA, referred to as sequential IL12-IL27 gene therapy, not only eradicated IL12 sensitive tumors from 100% of mice but also eradicated the highly malignant 4T1 tumors from 33% of treated mice in multiple independent experiments. This IL12-IL27 sequential gene therapy is not only superior to IL12-IL12 sequential gene therapy for eliminating tumors, but also for inducing CTL activity, increasing T cell infiltration into tumors, and yielding a large number of tumor-specific IFNγ positive CD8 T cells. Notably, depletion of either T- or NK-cells during the IL27 treatment phase reverses tumor eradication, suggesting an NK-cell requirement for this sequential gene therapy-mediated tumor eradication. Both reversal of the administration sequence and co-administration of IL12 and IL27 impaired the tumor eradication in 4T1 tumor bearing mice. This IL12-IL27 sequential gene therapy, via sequential administration of IL12 and IL27 encoding plasmid DNA into tumor-bearing mice through intramuscular electroporation, provides a simple but effective approach for eliminating inaccessible residual tumors.
Keywords: IL12, IL27, electroporation, gene therapy, tumors, T cells
Introduction
Interleukin (IL)-12, IL23 and IL27 are the three known members of the IL12 cytokine family. IL12 is the first member of this family discovered in the early 1990’s (1) and is one of the most effective cytokines for inhibiting tumor growth (2–4) among many other cytokines, including IL2, IL4, IL7, IL8, TNFα, and GMCSF (5–10). Daily systemic administration of recombinant IL12 protein generates a significant inhibitory effect on the metastatic growth of B16F10 melanoma and the established RENCA and CT26 tumors (11–13). However, because a large dose of IL12 protein must be used due to the short protein half-life, systemic delivery of IL12 protein is associated with severe toxicity in several experimental animal studies and in initial early-stage human trials (14–16). IL12 gene therapy has demonstrated greater efficacy and less toxicity than recombinant IL12 protein treatment in the RENCA tumor model (17), but eradication of aggressive tumors by systemic IL12 gene therapy alone cannot be achieved (18, 19).
IL27 is a novel member of the IL12 cytokine family. Similar to IL12, IL27 is a heterodimeric cytokine composed of p28 and EBI3 subunits (20–22). EBI3 and p28 are very similar to the p40 and p35 subunits of IL12, respectively. At the molecular level, IL27 activates Stat1-5 in naïve CD4 T cells via interaction with the cognate heterodimer receptor composed of wsx1 and Glycoprotein 130 (23–27). In naïve T cells, IL27 activates Stat1, resulting in the induction of T-bet and IL12Rβ2, which is crucial for Th1 polarization (25). At the cellular level, IL12 induces clonal proliferation of naïve and activated T cells, but not memory T cells. At the immunological level, IL27 plays a role in inducing Th1 response (21, 28), inflammatory and anti-inflammatory responses (29–40). Moreover, stable expression of IL27 in tumor cells inhibits tumor development and induces T cell-dependent anti-tumor immune memory (35, 41, 42), but it is unknown whether systemic IL27 treatment can trigger anti-tumor immune response and eliminate tumors.
Here, we discovered that sequential administration of IL12 and IL27 encoding DNA and systemic expression of these two cytokines not only eradicates the immunogenic colon CT26 tumors in 100% of mice, but also eradicates highly malignant breast 4T1 tumors in 33% of mice. More importantly, this sequential gene therapy induces a stronger anti-tumor T cell immune response. Either reversing the administration sequence of IL12 and IL27 or co-administration of these two cytokine genes impairs the tumor eradication found by sequential administration of IL12 and IL27. This novel sequential gene therapy sheds light on developing a simple and effective systemic electroporation gene therapy for eradicating systemic microscopic malignancy.
Materials and Methods
Gene constructs
The gene clones IL12 and IL27 were used in this study. The IL12 gene construct was obtained from Valentis, Inc. via a MTA. IL12 consists of two protein encoding subunits, p35 and p40. Both p35 and p40 subunits were driven by CMV promoter and terminated by a bGh polyadenylation signal. This construct was used in our previous study via intratumoral administration via electroporation (45). IL27 is a generous gift from Dr. Masatoshi Tagawa (Chiba Cancer Center Research Institute, Japan) (41). To increase the level of gene expression, we have sub-cloned the IL27 encoding fragment into the same vector used previously for IL12.
Animal procedures
All the animal procedures including tumor transplantation, tumor volume monitoring, gene administration, bleeding, and mice euthanization were approved by the IACUC at Louisiana State University. Six- to eight-week-old female Balb/c mice, weighing 18–20 g, from the in-house animal breeding facility were used for this study. The subcutaneous tumor model was generated by subcutaneously inoculating CT26 colon tumor cells (2×105 in a 30-μL volume per mouse) into Balb/C mice. The adenocarcinoma 4T1 model was generated by subcutaneously inoculating 4T1 tumor cells (1×105 in a 30-μL per mouse) into Balb/C mice. Both tumor cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (Life Technologies, Rockville, MD). Tumor dimensions were measured with calipers, and volume was calculated from the following formula: V= (π/8)a×b2, where V = tumor volume, a = maximum tumor diameter, and b = diameter at 90° to ‘a’ (43). Using the protocols described previously, IL12 and control plasmid DNA (10 μg in a volume of 30 μl per mouse) were injected into hind limb tibialias muscles via electroporation (44). The electroporation parameters for intramuscular injection are 350 V/cm and 20 ms pulse duration for 2 pulses (44). Both hind limb tibialis muscles were used for the first and the second DNA administration via electroporation. The first administration was initiated on day 4 or 11 to test the effectiveness of this therapy. The second administration was performed 10 days thereafter. Tumor growth and tumor eradication were monitored every 3 days. The indicated rabbit polyclonal antibodies or murine monoclonal antibodies were administered into mice (50 μg per mouse once every three days) via intraperitoneal administration for depleting the target cells. The monoclonal antibodies (Abs) used for depletion were made commercially available through Taconic, Inc.
Blood was obtained via a cheek bleeding method—tightly grabbing the mouse neck and poking the skin on the cheek with a 16 gauge needle, the bleeding will stop immediately upon releasing the fingers from the mouse neck. Blood was collected at the indicated time points displayed in the Figures, and serum was separated from the coagulated blood cells by centrifugation at 200x g. Serum was used for detecting IL12, IFNγ and IL27 expression using ELISA kits purchased from R&D system (Minneapolis, MN).
Detection of IFNγ, IL12 and IL27 expression using ELISA kits
Each collected serum sample (50 μl per mouse) was transferred into a single well of each cytokine assay plate supplied by the manufacturer (R&D System), followed by washing and binding with the HRP-conjugated antibodies. The HRP substrate was added to each well for triggering the color change, and the enzyme stop reaction buffer was added 30 minutes later for stopping the HRP activity. The color intensity representing the level of gene expression was determined in the plate reader at 405 nm. A standardized column for each cytokine (ranging from 0 to 500 pg per well in a 2-fold escalated dilution) was set in each assay plate to convert the light absorbance from each sample into the weight (pg) per mL of serum. All the reagents were included in each ELISA kit purchased from the manufacturer (R&D System).
Generation wsx1 polyclonal antibody for depletion study
Mouse IL27 receptor wsx1 construct was purchased from Open Biosystems (Huntsville, AL). Wsx1 encoding DNA was amplified from the purchased wsx1 clone via polymerase chain reaction (PCR) using forward and reverse primers Svm198F: 5′-CGGATCCATGAACCGGCTCCGGGTT-3′ and SVM199R: 5′-TCAGACTAGAAGGCCCAGCTC-3′, respectively. The amplified DNA fragment was cloned into pRSETA vector (Invitrogen, Inc.) for expressing wsx1 recombinant protein in a bacteria host. The bacteria host used in this study was BL21 DE3 plysS and the wsx1 protein was purified using ProBond Purification System under denaturing conditions (Invitrogen, Carlsbad, CA).
The column purified wsx1 protein was further purified with SDS-PAGE to remove any contaminated proteins. The SDS-PAGE-separated wsx1 protein was sliced off the gel using a sharp razor blade, and the sliced protein-gel was homogenized by a bead-beater for 5 minutes at the maximum speed to become gel slurry. Prior to immunizing the rabbits (2.5 kg per rabbit) with the homogenized protein-gel slurry, 100 μg homogenized protein-gel slurry was mixed with 1 mL Complete Freund adjuvant by syringe thoroughly and injected subcutaneously. The second and third boosts were performed on weeks 4 and 5 following the initial priming. The Incomplete Freund adjuvant was used to mix with the same amount of wsx1 protein gel homogenate for the boosting. One week after the third immunization, blood was collected and Western blot analysis was performed to confirm the antibody binds to wsx1. Once confirmed, blood was collected on day 10 following the final boost for purifying the anti-wsx1 antibody from the serum. Antibody was purified using a Sepharose protein-A column by a contracted service. The purified wsx1 antibody was used for blocking IL27 signaling by binding with wsx1, which is achieved by administering 50 μg of the antibody per mouse.
Immunostaining of T cell infiltration and CTL activity assay
Immunostaining of T cell infiltration was performed on frozen tumor sections. The procedures for frozen-block preparation, tissue sectioning, and immunostaining were the same as described previously (44–47). Tumor samples were collected 10 days after the final administration to evaluate the T cell numbers via immunostaining. The primary antibody applied to the sections was anti-CD8 (1:400, Santa Cruz Biotechnology, Santa Cruz, CA).
The same fluorescence-based CTL assay method as described previously was used to determine tumor-specific T and NK cell cytolytic activity against target tumor cells (48). To determine NK cell cytolytic activity in vitro, T cells were depleted by administering both anti-CD4 (GK1.5) and -CD8 T (2.43) cell antibodies (50 μg per mouse) into the sequential gene therapy-treated mice via intraperitoneal injection one day prior to euthanizing mice. To determine the cytolytic activity of T cells against tumor cells in vitro, NK cell-depleted spleen cells were used.
ELISPOT assay to determine the tumor-specific IFNγpositive CD8 T cells from the cured mice
To determine the acute induction of tumor specific CD8 T cells by tumor antigen stimulation, the IL12-IL27 sequential gene therapy-cured mice were challenged with tumor cells for 3 days prior to euthanization for analysis of tumor-reactive IFNγ-secreting CD8 T cells. To avoid the effect of IFNγ-secreting NK and CD4 T cells on accurately determining the number of IFNγ positive CD8 T cells, mice were administered anti-NK1.1 (PK136) and anti-CD4 T cell antibodies (GK1.5) one day prior to euthanization. Lymphocyte cells were isolated from Lymph nodes by smearing the tissue and pushing through a 70 nm strainer. A total of 50 μg per mouse was administered via intraperitoneal injection, and flow cytometry was performed to confirm that the primary collected cells were CD8 T cells the next day when mice were euthanized. The isolated CD8 T cells were incubated without and with mitomycin C-treated target tumor cells (CT26) in the IFNγ capturing ELISPOT plate purchased from R&D System. After incubating overnight, the plate was washed and the IFNγ spots were detected using immunostaining following the manufacture’s instruction. The image of IFNγ positive spots was captured via Kodak image station 440 (Rochester, NY).
Statistical Analysis
Tumor volume, CD8+ T cell infiltration, and gene expression were the primary outcomes measured. Survival analysis and tumor volumes were performed with the Mann-Whitney U test and a One-Way Repeated Measures ANOVA, respectively. Others were analyzed by the two-sided Student’s t test. P values less than .05 were considered statistically significant.
Results
Elimination of highly aggressive tumors by IL12 and IL27 sequential gene therapy is administration sequence specific
Our laboratory has made a surprise discovery that administration of IL12-encoding plasmid DNA induces IL27 expression in mice, and depletion of IL12-induced IL27 impairs the IL12-mediated anti-tumor efficacy (unpublished data). Based on this innovative observation, we hypothesize that an increased expression of IL27 in the IL12-treated mice may enhance the IL12-mediated anti-tumor efficacy. To test this hypothesis, plasmid DNA encoding IL12 and IL27 were sequentially and concomitantly administered into the hind limb muscles of 4T1 tumor-bearing mice via electroporation, where the therapeutic cytokine protein can be manufactured and secreted into the blood circulation for inhibiting tumor growth located in other organs via inhibition of angiogenesis and activation of anti-tumor immune responses (18, 19, 47). The 4T1 tumor model was used because this model was described as a highly malignant tumor, equaling the clinical grade IV malignancy of breast cancer (49). There is no effective systemic delivery approach available to eliminate this tumor.
Intriguingly, sequential administration of IL12 and IL27 encoding plasmid DNA with a 10-day interval drastically inhibited tumor growth (Fig. 1, 2A) and prolonged survival (Fig. 2B). This approach is referred to as IL12-IL27 sequential gene therapy because the tumor regression is entirely dependent on the administration sequence (IL12 followed by IL27 after 10 days). A reversed administration sequence failed to eradicate tumors, as did the co-administration of both gene-encoding plasmid DNA (Fig. 1). Neither IL12 nor IL27 treatment alone was successful in eradicating tumors (Fig. 1).
FIGURE 1. Images of tumor eradication and strong inhibition of tumor growth by IL12-IL27 sequential gene therapy.
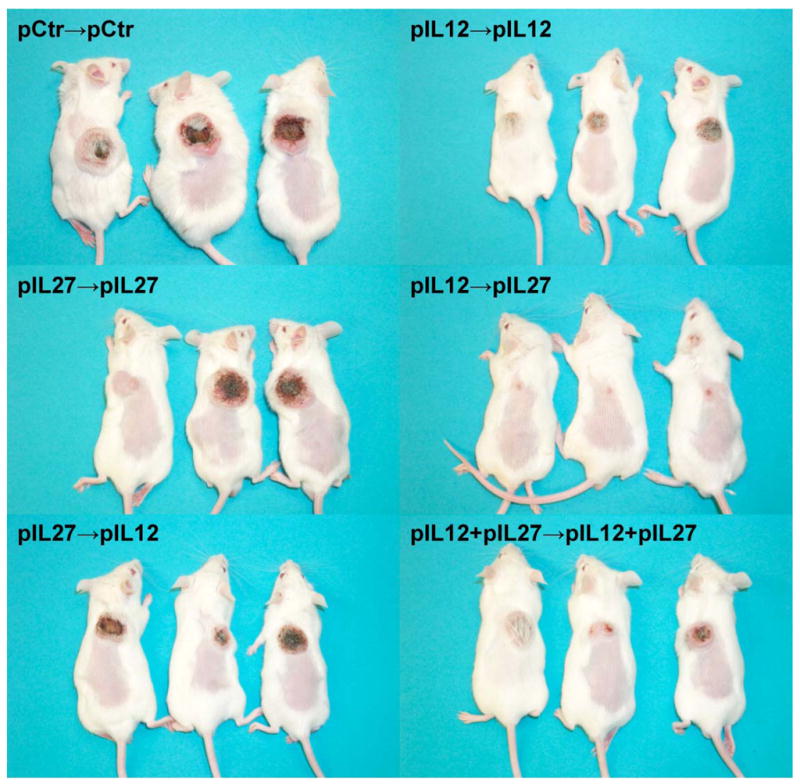
pCtr, pIL12, and pIL27 represent control, IL12, and IL27 encoding plasmid DNA, respectively. pCtr-pCtr indicates that both the first and the second administration were control plasmid DNA. The same definition applied to pIL12-pIL12, pIL27-pIL27, pIL27-pIL12, and pIL12+pIL27-pIL12+pIL27. A total of 10 μg plasmid DNA was used for each administration with a 10 day interval between two administrations. The first administration was performed on day 4 after inoculation of tumor cells. The picture was taken 18 days after the final (second) intramuscular injection.
FIGURE 2. 4T1 Tumor growth inhibition and mouse survival by IL12-IL27 sequential gene therapy.
The same dose, number of administrations, and treatment combinations described in Fig. 1 legend applied to this Figure. A. Inhibition of tumor growth by different IL12 and IL27 sequential gene therapy combinations (n=5). Arrows labeled 1st and 2nd represent the first and the second treatment times, with the first administration performed on day 4 after inoculation of tumor cells. The p- value indicates the significant difference between IL12-IL27 sequential gene therapy and the other treatment groups. B. Kaplan-Meier survival curves for IL12-IL12 and IL12-IL27 sequential gene therapy (n=9). p<0.05 for IL12-IL12 vs. IL12-IL27 treatments.
The IL12-IL27 sequential gene therapy eliminates tumors at a higher percentage than IL12 alone in an IL12 sensitive tumor model
CT26 colon tumors were known to be immunogenic and sensitive to IL12 therapy. Using muscle-based sequential administrations by which IL12 encoding plasmid DNA alone is given a total of two times at a 10-day interval (IL12-IL12), 60–75% of tumors were eradicated in both an early-treatment model (Fig. 3A) and a late-treatment model (Fig. 3C). Compared to IL12-IL12 sequential gene therapy, IL12-IL27 sequential gene therapy is more effective as tumors were eradicated in 100% of CT26 tumor-bearing mice in both early- and late-treatment models (Figs. 3B, 3D) with a trend toward earlier tumor resolution. Neither trend (cure rate nor time-to-complete-response) achieved statistical significance in this sensitive tumor model. However, administration of an antibody that binds to the IL27 receptor, wsx1, significantly reversed IL12-IL27 sequential gene therapy-caused tumor eradication in 80% of the mice (Fig. 3F) when compared to IL12-IL27 sequential gene therapy alone (p<0.05, Fig. 3E). Administration of anti-wsx1 antibody resulted in aggressive tumor growth as observed in the control group receiving control DNA (Fig. 3E), further illustrating the significance of the exogenous IL27 in causing tumor regression.
FIGURE 3. Comparison of CT26 tumor regression by IL12-IL12 and IL12-IL27 sequential gene therapy.

The same dose, administration number and Schedule for IL12-IL12 and IL12-IL27 treatment were used as described in Fig. 1 legend applied to this Figure. The first treatment for the early treatment model was initiated on day 4 (3A, 3C), whereas the first treatment for the late-treatment model was initiated on day 11 (3B, 3D). A, C. Eradication of tumors by IL12-IL12 sequential gene therapy. B, D. Eradication of tumors by IL12-IL27 sequential gene therapy. E. Aggressive tumor growth after administering control plasmid DNA. F. Impairing IL12-IL27 sequential gene therapy-mediated tumor eradication by administering anti-wsx1 antibody. p<0.05 for IL12-IL27 vs (IL12-IL27)+anti-wsx1 antibody.
A higher level of IL27 expression at the later phase of treatment is associated with the eradication of highly malignant 4T1 tumors by the sequential IL12-IL27 gene therapy
One of the major mechanisms for IL12-mediated inhibition of tumor growth is to trigger a high level of IFNγ expression in T and NK cells (50). It was reported that a synergistic induction of IFNγ was found when both IL12 and IL27 were present in T cells (22, 24, 51). Therefore, one possible mechanism for the enhanced tumor eradication by the IL12-IL27 sequential gene therapy is the synergistic induction of IFNγ. To test this assumption, the expression of IFNγ on the indicated dates after the first and the second administration was compared between the two treatment groups: IL12-IL12 vs. IL12-IL27 gene therapy. No enhanced expression of IFNγ was found in the IL12-IL27 treatment group (Fig. 4A) and similar levels of IL12 and IFNγexpression were found in these two treatment groups (Fig. 4A, 4B). As anticipated, an increased level of IL27 in the later phase was detected by the IL12-IL27 gene therapy (Fig. 4C). This result suggests that the increased IL27 expression by IL12-IL27 sequential therapy during the late treatment phase may directly contribute to the observed 4T1 tumor regression, which was not obtained by the IL12-IL12 gene therapy in this model (Fig. 1). Early administration of IL27, either alone or in combination with IL12, increases the level of IL27 expression, however, it is detrimental to the IL12 mediated anti-tumor effect, suggesting that IL27 is mainly required in the late phase of treatment.
FIGURE 4.

Kinetics of cytokine expression during the IL12-IL12 and IL12-IL27 sequential gene therapy. The same treatment dose and administration number described in Fig. 1 were used in this Figure. Blood was collected on days 1, 4, and 8 after the first and the second administration. D1, D4, and D8 were the days after the first and the second administration. 1st and 2nd indicates the first and the second administrations at an interval 10 days between two treatments. Ctr-Ctr, administration of control plasmid DNA for both administrations. A, B, and C. Expression kinetics of IL12, IFNγ and IL27. P>0.05 for the level of IL12 and IFNγ between IL12-IL12 and IL12-IL27 treatments on the same blood collection dates; p<0.05 for the level of IL27 expression after the second treatment between the same two treatment groups. P<0.001 for the cytokines between control groups and the treatment groups.
The sequential IL12-IL27 gene therapy induces a higher level of tumor-specific T cell response than the IL12-IL12 gene therapy
One of the main IL12 functions is to activate NK cells, inducing NK cell-mediated tumor cell death. Since IL27 induces IL12Rβ2 expression and may sensitize IL12 response (24–26, 35, 41), we were interested in knowing whether IL27 enhances the NK cell-mediated anti-tumor effect. Therefore, the cytolytic activities of NK cells, isolated from both IL12-IL12- and IL12-IL27-treated mice, were compared against non-specific tumor target cells. To avoid the possible effect from T cells, T cells were depleted by using anti-CD8 depletion antibody one day prior to euthanizing mice. A similar level of NK cell activity was detected from both IL12-IL12 and IL12-IL27 therapies (Figs. 5A and 5B), suggesting that the discrepancy in tumor eradication between these two treatments was not due to different levels of NK activities.
FIGURE 5.
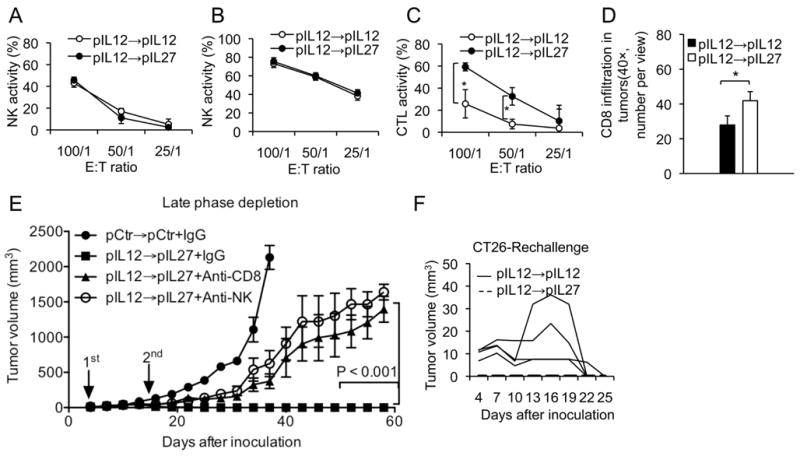
Comparison of the anti-tumor immune responses between IL12-IL12 and IL12-IL27 gene therapy. The same dose, treatment schedule and sequence described in Figure 1 were used in this experiment. Ten days after the final (the second) administration, anti-tumor immune responses between these two treatments were analyzed. The first administration was performed on day 11 after inoculation of tumor cells. A. NK cell cytolytic activity using homologous tumor cells and NK cell depleted spleen cells. B. NK cell cytolytic activity using Yac1 cells. C. Difference in T cell cytolytic activity (CTL) between IL12-IL12- and IL12-IL27-treated mice. CT26 tumor cells were used as target cells. *, represent significant difference at p<0.01. D. Difference in infiltration of T cells in CT26 tumors between IL12-IL12 and IL12-IL27 sequential gene therapy (p=0.036). E. Effect of depletion of CD8 T and NK cells on IL12-IL27 sequential gene therapy mediated anti-tumor efficacy. P<0.001 between injection of depletion antibodies against CD8 T cells or NK cells and injection of murine IgG on the indicated days. Antibodies were administered twice a week at a dose of 50 μg per mouse for each administration. Arrows indicates the plasmid DNA administrgation time. F. Kinetics of challenged tumor growth between the IL12-IL12 and IL12-IL27-cured mice.
Others found that expression of IL27 in tumor cells induced T cell-dependent anti-tumor effects (35, 41). However, systemic expression of IL27 via intramuscular administration of IL27 encoding plasmid DNA through electroporation yielded minimum inhibition of tumor growth (Fig. 1A), suggesting systemic expression of IL27 alone is not sufficient to induce any strong anti-tumor immune response. Likewise, our previous study indicates that systemic expression of IL12 alone induces a weak T cell response (52). We were interested to know whether the increased expression of IL27 in the later phase (IL27 treatment phase) potentiated IL12-mediated T cell dependent anti-tumor immune response. To test the T cell response by the sequential IL12-IL27 gene therapy, the tumor specific CTL activity was determined. A much higher level of CTL activity was detected from the IL12-IL27 treated mice, compared to IL12-IL12 treated mice (Fig. 5C). Moreover, IL12-IL27 sequential gene therapy also induced more infiltration of CD8 T cells into tumors than did the IL12-IL12 treatment (Fig. 5D, p<0.05).
To confirm that cytotoxic T cells did truly contribute for the increased anti-tumor efficacy, CD8 T cells were deleted or reduced from the sequential gene therapy-treated mice using depletion anti-CD8 antibody 2.43. The depletion antibody was administered concomitantly with IL27 plasmid DNA during the late phase of treatment. As expected, administration of anti-CD8 antibody abrogated the sequential IL12-IL27 gene therapy mediated tumor eradication, illustrating the significant role of cytotoxic T cells in tumor eradication (Fig. 5E). Anti-CD4 T cell antibody was not included in this experiment because a preliminary study concludes that depletion of CD4 T cells has no effect on IL12-IL27 sequential gene therapy-mediated anti-tumor efficacy (data not shown). Unexpectedly, administration of anti-NK antibody (PK137, ATCC) during the late phase also reversed the tumor eradication, indicating that NK or NKT cells also contributes to the sequential gene therapy-mediated anti-tumor effect (Fig. 5E). The identical NK cytolytic activities derived from both IL12-IL12 and IL12-IL27 sequential gene therapies suggests that NK cells are critical; however, they are not the key elements engendering the enhanced anti-tumor activity (Fig. 1).
To determine whether the strong IL12-IL27 immune gene therapy also induces a strong anti-tumor immune memory, the cured mice from both treatment groups, IL12-IL12 and IL12-IL27, were challenged with the tumor cells three months following the tumor disappearance. Interestingly, no tumor incidence was detected from the IL12-IL27-cured mice, suggesting the presence of a strong immune response. Tumor incidence was found in IL12-IL12-cured mice but these tumors were slowly regressed three weeks after the challenge, suggesting that the IL12-IL12 gene therapy also induced relatively weak anti-tumor immune response (Fig. 5F). The same challenge study was also performed in the mice six months after eradicating the aggressive 4T1 tumors by IL12-IL27 gene therapy. Tumors developed in 2 out of 5 challenged mice, but those two-developed tumors disappeared after 3 weeks, illustrating that this treatment yielded a long duration of anti-tumor memory against highly malignant 4T1 tumor cells. Since IL12-IL12 could not eradicate the aggressive 4T1 tumors, this challenge study could not be performed in IL12-IL12 treatment group.
The above result suggests that IL12-IL27-cured mice may produce an acute induction of activated CD8 T cells to immediately remove the challenged tumor cells but the IL12-IL12-cured mice may have only a slow response. To test this possibility, the IFNγ-secreting CD8 T cells were identified from these two groups of mice following stimulation of mice with tumor cells. To avoid the detection of the IFNγ positive CD4 and NK cells, the cognate neutralization antibodies were administered two days prior to euthanizing mice. A much higher number of IFNγ positive CD8 T cells were detected from IL12-IL27-cured mice than IL12-IL12-cured mice (Fig. 6). The presence of a larger number of IFNγ positive CD8 T cells was a good indicator for the acute induction of tumor specific CD8 T cells by the IL12-IL27 gene therapy.
FIGURE 6.

Induction of anti-tumor immune memory by IL12-IL27 sequential gene therapy. CT26 tumor model was used for this study in vivo and the spleen cells collected from the treated mice were used for ELISPOT assay, detecting the difference in induction of acute tumor-specific IFNγ positive CD8 cells between IL12-IL12 and IL12-IL27 sequential gene therapy. To ensure the effector cells were primarily CD8 T cells, both NK and CD4 T cells were depleted using neutralization antibody one day prior to euthanizing mice. E and T represent effector and target cells. Two treatments were performed (see detail in Fig. 1 legend with the first treatment on day 11 after inoculation of tumor cells). A. IFNγ positive spots from different incubations. B. Spot number as counted under dissecting microscope. ***, represents significant difference at a p<0.001.
Discussion
Microscopic tumors are the primary cause of tumor recurrence after surgery, chemotherapy, radiation or a combination of treatments. Metastatic tumors are the primary cause of tumor patient mortality. Developing a therapeutic approach for treating tumor recurrence and metastatic tumors is crucial for reducing mortality of cancer patients and controlling tumors. One ideal approach from an immune therapy point of view is to induce anti-tumor immunity that inhibits tumor metastasis and recurrence. Previous studies performed by ourselves and others have applied electroporation to administer IL12 plasmid DNA into tumors for completely eradicating tumors from 40–80% of mice (19, 45), but this approach cannot be used for treating the residual microscopic tumors after the standard treatments because tumors are too small to be detected for performing an intratumoral gene administration. Therefore, administration of IL12 plasmid DNA into muscle via electroporation was performed, in which IL12 is expressed in the injected muscles but circulated into other organs via blood circulation. This approach, referred to as systemic approach, does not need the detection of the residual tumor location, and we have demonstrated that two sequential administrations of IL12-encoding plasmid DNA can eliminate immunogenic CT26 tumors in 60–75% of treated mice (Fig. 3). However, this approach failed to eradicate aggressive tumors (18, 52).
In this study, we illustrated that sequentially systemic expression of IL12 and IL27 via intramuscular DNA electroporation completely eradicates highly malignant 4T1 tumors from 33% of treated mice. The 4T1 tumors in the non-tumor eradicated mice are severely inhibited (Figs. 1 and 2). Eradication of 4T1 tumor is significant because this mammary derived tumor model is known to equal clinical grade IV tumors (49) because no other known systemic treatments can completely regress this highly aggressive tumor, and even the aggressive intra-tumoral treatment with a combination of IL12 and bleomycin can only lead to tumor eradication from 40–60% of the treated mice (53). Therefore, the muscle-based IL12-IL27 sequential gene therapy described here is an important approach for treating microscopic tumors.
Very intriguingly, this simple IL12-IL27 sequential gene therapy-mediated tumor eradication is administration sequence-specific because all the other possible combinations, including the reversed administration sequence and co-administration, failed to eradicate tumors (Fig. 1). The fact that IL27 has to be administered at a later point after administering IL12 for inducing a greater anti-tumor T cell response may challenge the current view that IL27 is essential for the initial Th1 responses (21, 54–56). Of course, one possible explanation for the late requirement of IL27 as shown in this study is that the IL12-induced expression of endogenous IL27 is sufficient for the initial enhancement of IL12-mediated anti-tumor immune response. This is most likely true because depletion of IL27 in the initial phase prior to IL27 administration did impair tumor eradication by the IL12-IL12 sequential gene therapy in a CT26 tumor model (unpublished data). Likewise, overexpression of IL27 in the early phase of treatment may inhibit the anti-tumor program initiated by the early phase IL12 treatment, as concomitant administration of IL27 and IL12 in the early phase abrogated tumor eradication (Fig. 2). Therefore, the level of IL27 required at different treatment stages varies, providing the proper levels of IL27 and IL12 at different stages can greatly enhance the anti-tumor immune response and cause tumor eradication. The underlying mechanism by which IL27 enhances IL12-mediated tumor eradication is characterized by a compounding enhancement of the T cell response, including an acute induction of a larger number of tumor-specific CD8 T cells upon exposure to tumor antigen (Fig. 6), a higher level of CTL activity, and a larger number of CD8 T cell infiltration into the tumors (Figs. 5 and 6). The augmented T cell accumulation in tumor sites by IL12-IL27 gene therapy, as compared to IL12-IL12 gene therapy, explained why both treatments yield the similar amount of IFNγ in the blood but a higher level of IFNγ in the tumor antigen-stimulated T cells.
In regard to the mechanism, NK cells are also critical because depletion of NK cells reverses the tumor eradication exhibited by the IL12-IL27 treatment. This NK cell effect is most likely induced by IL12 but not by IL27, as similar NK lytic activity was obtained between IL12-IL12 and IL12-IL27 treatments (Fig. 5). IL12 induces a Th1 response and inhibits a Th2 response. In theory, the B cell role may not be critical for IL12 treatment. However, IL12-secretive allogeneic HER-2+ cell vaccine induces anti-HER2 antibody response, which plays a role in inhibiting spontaneous tumor development in the NeuT mice because the absence of B-cells reveres the effect (57). In a clinical study, intratumoral injection of IL12 correlates to B cell infiltration (58). In a HER-2/neu transgenic mouse model, IL12 enhanced the antibody production via her2 vaccination(59). The effect of IL27 on B cells, however, remains unknown. For this reason, B cell function, such as induction of anti-tumor antibody, was examined by IL12-IL12, IL12-IL27, and control DNA-control treatments. To our surprise, control DNA via electroporation alone induces a strong tumor specific antibody response, which is greater than or commensurate with the IL12 mediated antibody response (data not shown). This result excludes the possible role of B cells as the primary cells for IL12-IL27 sequential gene therapy-mediated tumor eradication. Regardless, this work will inspire a thorough study of this simple therapeutic approach and the underlying mechanism to make this approach more effective for treating systemic malignancy.
Abbreviations
- IL12-IL27
IL12 administration followed by IL27 administration 10 days after
- RENCA
renal carcinoma
- EBI3
Epstein-barr virus-induced gene 3
- MTA
material transfer agreement
- bGh
bovine growth hormone
Footnotes
This work was supported by the grant NIH/NCI RO1CA098928
References
- 1.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–4027. [PubMed] [Google Scholar]
- 2.Burke F. Cytokines (IFNs, TNF-alpha, IL-2 and IL-12) and animal models of cancer. Cytokines Cell Mol Ther. 1999;5:51–61. [PubMed] [Google Scholar]
- 3.Golab J, Zagozdzon R. Antitumor effects of interleukin-12 in pre-clinical and early clinical studies (Review) Int J Mol Med. 1999;3:537–544. doi: 10.3892/ijmm.3.5.537. [DOI] [PubMed] [Google Scholar]
- 4.Rakhmilevich AL, Janssen K, Turner J, Culp J, Yang NS. Cytokine gene therapy of cancer using gene gun technology: superior antitumor activity of interleukin-12. Hum Gene Ther. 1997;8:1303–1311. doi: 10.1089/hum.1997.8.11-1303. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe Y, Kuribayashi K, Miyatake S, Nishihara K, Nakayama E, Taniyama T, Sakata T. Exogenous expression of mouse interferon gamma cDNA in mouse neuroblastoma C1300 cells results in reduced tumorigenicity by augmented anti-tumor immunity. Proc Natl Acad Sci U S A. 1989;86:9456–9460. doi: 10.1073/pnas.86.23.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colombo MP, Modesti A, Parmiani G, Forni G. Local cytokine availability elicits tumor rejection and systemic immunity through granulocyte-T-lymphocyte cross-talk. Cancer Res. 1992;52:4853–4857. [PubMed] [Google Scholar]
- 7.Forni G, Giovarelli M, Cavallo F, Consalvo M, Allione A, Modesti A, Musiani P, Colombo MP. Cytokine-induced tumor immunogenicity: from exogenous cytokines to gene therapy. J Immunother. 1993;14:253–257. doi: 10.1097/00002371-199311000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Gansbacher B, Zier K, Daniels B, Cronin K, Bannerji R, Gilboa E. Interleukin 2 gene transfer into tumor cells abrogates tumorigenicity and induces protective immunity. J Exp Med. 1990;172:1217–1224. doi: 10.1084/jem.172.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pardoll DM. Paracrine cytokine adjuvants in cancer immunotherapy. Annu Rev Immunol. 1995;13:399–415. doi: 10.1146/annurev.iy.13.040195.002151. [DOI] [PubMed] [Google Scholar]
- 10.Mackensen A, Lindemann A, Mertelsmann R. Immunostimulatory cytokines in somatic cells and gene therapy of cancer. Cytokine Growth Factor Rev. 1997;8:119–128. doi: 10.1016/s1359-6101(96)00052-4. [DOI] [PubMed] [Google Scholar]
- 11.Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M, Wolf SF, Gately MK. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993;178:1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nastala CL, Edington HD, McKinney TG, Tahara H, Nalesnik MA, Brunda MJ, Gately MK, Wolf SF, Schreiber RD, Storkus WJ, et al. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994;153:1697–1706. [PubMed] [Google Scholar]
- 13.Tannenbaum CS, Tubbs R, Armstrong D, Finke JH, Bukowski RM, Hamilton TA. The CXC chemokines IP-10 and Mig are necessary for IL-12-mediated regression of the mouse RENCA tumor. J Immunol. 1998;161:927–932. [PubMed] [Google Scholar]
- 14.Sarmiento UM, Riley JH, Knaack PA, Lipman JM, Becker JM, Gately MK, Chizzonite R, Anderson TD. Biologic effects of recombinant human interleukin-12 in squirrel monkeys (Sciureus saimiri) Lab Invest. 1994;71:862–873. [PubMed] [Google Scholar]
- 15.Cohen J. IL-12 deaths: explanation and a puzzle. Science. 1995;270:908. doi: 10.1126/science.270.5238.908a. [DOI] [PubMed] [Google Scholar]
- 16.Tare NS, Bowen S, Warrier RR, Carvajal DM, Benjamin WR, Riley JH, Anderson TD, Gately MK. Administration of recombinant interleukin-12 to mice suppresses hematopoiesis in the bone marrow but enhances hematopoiesis in the spleen. J Interferon Cytokine Res. 1995;15:377–383. doi: 10.1089/jir.1995.15.377. [DOI] [PubMed] [Google Scholar]
- 17.Tan J, Newton CA, Djeu JY, Gutsch DE, Chang AE, Yang NS, Klein TW, Hua Y. Injection of complementary DNA encoding interleukin-12 inhibits tumor establishment at a distant site in a murine renal carcinoma model. Cancer Res. 1996;56:3399–3403. [PubMed] [Google Scholar]
- 18.Hanna E, Zhang X, Woodlis J, Breau R, Suen J, Li S. Intramuscular electroporation delivery of IL-12 gene for treatment of squamous cell carcinoma located at a distant site. Ca Gene Ther. 2001;8:1–7. doi: 10.1038/sj.cgt.7700287. [DOI] [PubMed] [Google Scholar]
- 19.Lucas ML, Heller L, Coppola D, Heller R. IL-12 plasmid delivery by in vivo electroporation for the successful treatment of established subcutaneous B16. F10 melanoma. Mol Ther. 2002;5:668–675. doi: 10.1006/mthe.2002.0601. [DOI] [PubMed] [Google Scholar]
- 20.Chen Q, Ghilardi N, Wang H, Baker T, Xie MH, Gurney A, Grewal IS, de Sauvage FJ. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida H, Hamano S, Senaldi G, Covey T, Faggioni R, Mu S, Xia M, Wakeham AC, Nishina H, Potter J, Saris CJ, Mak TW. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15:569–578. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 22.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 23.Villarino AV, Huang E, Hunter CA. Understanding the pro- and anti-inflammatory properties of IL-27. J Immunol. 2004;173:715–720. doi: 10.4049/jimmunol.173.2.715. [DOI] [PubMed] [Google Scholar]
- 24.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, Yoshida H. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 25.Hibbert L, Pflanz S, De Waal Malefyt R, Kastelein RA. IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J Interferon Cytokine Res. 2003;23:513–522. doi: 10.1089/10799900360708632. [DOI] [PubMed] [Google Scholar]
- 26.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2003;100:15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 28.Artis D, Villarino A, Silverman M, He W, Thornton EM, Mu S, Summer S, Covey TM, Huang E, Yoshida H, Koretzky G, Goldschmidt M, Wu GD, de Sauvage F, Miller HR, Saris CJ, Scott P, Hunter CA. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol. 2004;173:5626–5634. doi: 10.4049/jimmunol.173.9.5626. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg R, Zohar Y, Wildbaum G, Geron Y, Maor G, Karin N. Suppression of ongoing experimental autoimmune encephalomyelitis by neutralizing the function of the p28 subunit of IL-27. J Immunol. 2004;173:6465–6471. doi: 10.4049/jimmunol.173.10.6465. [DOI] [PubMed] [Google Scholar]
- 30.Owaki T, Asakawa M, Kamiya S, Takeda K, Fukai F, Mizuguchi J, Yoshimoto T. IL-27 suppresses CD28-mediated [correction of medicated] IL-2 production through suppressor of cytokine signaling 3. J Immunol. 2006;176:2773–2780. doi: 10.4049/jimmunol.176.5.2773. [DOI] [PubMed] [Google Scholar]
- 31.Villarino AV, Stumhofer JS, Saris CJ, Kastelein RA, de Sauvage FJ, Hunter CA. IL-27 limits IL-2 production during Th1 differentiation. J Immunol. 2006;176:237–247. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- 32.Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 33.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 34.Hamano S, Himeno K, Miyazaki Y, Ishii K, Yamanaka A, Takeda A, Zhang M, Hisaeda H, Mak TW, Yoshimura A, Yoshida H. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19:657–667. doi: 10.1016/s1074-7613(03)00298-x. [DOI] [PubMed] [Google Scholar]
- 35.Hisada M, Kamiya S, Fujita K, Belladonna ML, Aoki T, Koyanagi Y, Mizuguchi J, Yoshimoto T. Potent antitumor activity of interleukin-27. Cancer Res. 2004;64:1152–1156. doi: 10.1158/0008-5472.can-03-2084. [DOI] [PubMed] [Google Scholar]
- 36.Honda K, Nakamura K, Matsui N, Takahashi M, Kitamura Y, Mizutani T, Harada N, Nawata H, Hamano S, Yoshida H. T helper 1-inducing property of IL-27/WSX-1 signaling is required for the induction of experimental colitis. Inflamm Bowel Dis. 2005;11:1044–1052. doi: 10.1097/01.mib.0000191611.05466.1f. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Gran B, Zhang GX, Rostami A, Kamoun M. IL-27 subunits and its receptor (WSX-1) mRNAs are markedly up-regulated in inflammatory cells in the CNS during experimental autoimmune encephalomyelitis. J Neurol Sci. 2005;232:3–9. doi: 10.1016/j.jns.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 38.Miyazaki Y, Inoue H, Matsumura M, Matsumoto K, Nakano T, Tsuda M, Hamano S, Yoshimura A, Yoshida H. Exacerbation of experimental allergic asthma by augmented Th2 responses in WSX-1-deficient mice. J Immunol. 2005;175:2401–2407. doi: 10.4049/jimmunol.175.4.2401. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt C, Giese T, Ludwig B, Mueller-Molaian I, Marth T, Zeuzem S, Meuer SC, Stallmach A. Expression of interleukin-12-related cytokine transcripts in inflammatory bowel disease: elevated interleukin-23p19 and interleukin-27p28 in Crohn’s disease but not in ulcerative colitis. Inflamm Bowel Dis. 2005;11:16–23. doi: 10.1097/00054725-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O’Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 41.Chiyo M, Shimozato O, Yu L, Kawamura K, Iizasa T, Fujisawa T, Tagawa M. Expression of IL-27 in murine carcinoma cells produces antitumor effects and induces protective immunity in inoculated host animals. Int J Cancer. 2005;115:437–442. doi: 10.1002/ijc.20848. [DOI] [PubMed] [Google Scholar]
- 42.Salcedo R, Stauffer JK, Lincoln E, Back TC, Hixon JA, Hahn C, Shafer-Weaver K, Malyguine A, Kastelein R, Wigginton JM. IL-27 mediates complete regression of orthotopic primary and metastatic murine neuroblastoma tumors: role for CD8+ T cells. J Immunol. 2004;173:7170–7182. doi: 10.4049/jimmunol.173.12.7170. [DOI] [PubMed] [Google Scholar]
- 43.Puisieux I, Odin L, Poujol D, Moingeon P, Tartaglia J, Cox W, Favrot M. Canarypox virus-mediated interleukin 12 gene transfer into murine mammary adenocarcinoma induces tumor suppression and long-term antitumoral immunity. Hum Gene Ther. 1998;9:2481–2492. doi: 10.1089/hum.1998.9.17-2481. [DOI] [PubMed] [Google Scholar]
- 44.Li S, Xia X, Zhang X, Suen J. Regression of tumors by IFN-alpha electroporation gene therapy and analysis of the responsible genes by cDNA array. Gene Ther. 2002;9:390–397. doi: 10.1038/sj.gt.3301645. [DOI] [PubMed] [Google Scholar]
- 45.Li S, Zhang X, Xia X. Regression of tumor growth and induction of long-term antitumor memory by interleukin 12 electro-gene therapy. J Natl Cancer Inst. 2002;94:762–768. doi: 10.1093/jnci/94.10.762. [DOI] [PubMed] [Google Scholar]
- 46.Li S, Xia X, Mellieon FM, Liu J, Steele S. Candidate genes associated with tumor regression mediated by intratumoral IL-12 electroporation gene therapy. Mol Ther. 2004;9:347–354. doi: 10.1016/j.ymthe.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 47.Li S, Zhang X, Xia X, Zhou L, Breau R, Suen J, Hanna E. Intramuscular electroporation delivery of IFN-alpha gene therapy for inhibition of tumor growth located at a distant site. Gene Ther. 2001;8:400–407. doi: 10.1038/sj.gt.3301418. [DOI] [PubMed] [Google Scholar]
- 48.Torrero MN, Xia X, Henk W, Yu S, Li S. Stat1 deficiency in the host enhances interleukin-12-mediated tumor regression. Cancer Res. 2006;66:4461–4467. doi: 10.1158/0008-5472.CAN-05-3554. [DOI] [PubMed] [Google Scholar]
- 49.Pulaski BA, Ostrand-Rosenberg S. Reduction of established spontaneous mammary carcinoma metastases following immunotherapy with major histocompatibility complex class II and B7.1 cell-based tumor vaccines. Cancer Res. 1998;58:1486–1493. [PubMed] [Google Scholar]
- 50.Wigginton JM, Gruys E, Geiselhart L, Subleski J, Komschlies KL, Park JW, Wiltrout TA, Nagashima K, Back TC, Wiltrout RH. IFN-gamma and Fas/FasL are required for the antitumor and antiangiogenic effects of IL-12/pulse IL-2 therapy. J Clin Invest. 2001;108:51–62. doi: 10.1172/JCI10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morishima N, Owaki T, Asakawa M, Kamiya S, Mizuguchi J, Yoshimoto T. Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J Immunol. 2005;175:1686–1693. doi: 10.4049/jimmunol.175.3.1686. [DOI] [PubMed] [Google Scholar]
- 52.Li S, Zhang L, Torrero M, Cannon M, Barret R. Administration route- and immune cell activation-dependent tumor eradication by IL12 electrotransfer. Mol Ther. 2005;12:942–949. doi: 10.1016/j.ymthe.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 53.Torrero MN, Henk WG, Li S. Regression of high-grade malignancy in mice by bleomycin and interleukin-12 electrochemogenetherapy. Clin Cancer Res. 2006;12:257–263. doi: 10.1158/1078-0432.CCR-05-1514. [DOI] [PubMed] [Google Scholar]
- 54.Hunter CA, Villarino A, Artis D, Scott P. The role of IL-27 in the development of T-cell responses during parasitic infections. Immunol Rev. 2004;202:106–114. doi: 10.1111/j.0105-2896.2004.00213.x. [DOI] [PubMed] [Google Scholar]
- 55.Brombacher F, Kastelein RA, Alber G. Novel IL-12 family members shed light on the orchestration of Th1 responses. Trends Immunol. 2003;24:207–212. doi: 10.1016/S1471-4906(03)00067-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robinson DS, O’Garra A. Further checkpoints in Th1 development. Immunity. 2002;16:755–758. doi: 10.1016/s1074-7613(02)00331-x. [DOI] [PubMed] [Google Scholar]
- 57.De Giovanni C, Nicoletti G, Landuzzi L, Astolfi A, Croci S, Comes A, Ferrini S, Meazza R, Iezzi M, Di Carlo E, Musiani P, Cavallo F, Nanni P, Lollini PL. Can Res. 2004;64:4001–4009. doi: 10.1158/0008-5472.CAN-03-2984. [DOI] [PubMed] [Google Scholar]
- 58.van Herpen CM, van der Voort R, van der Laak JA, Klasen IS, de Graaf AO, van Kempen LC, de Vries IJ, Boer TD, Dolstra H, Torensma R, van Krieken JH, Adema GJ, De Mulder PH. Intratumoral rhIL-12 administration in head and neck squamous cell carcinoma patients induces B cell activation. Int J Cancer. 2008;123:2354–2361. doi: 10.1002/ijc.23756. [DOI] [PubMed] [Google Scholar]
- 59.Nanni P, Nicoletti G, De Giovanni C, Landuzzi L, Di Carlo E, Cavallo F, Pupa SM, Rossi I, Colombo MP, Ricci C, Astolfi A, Musiani P, Forni G, Lollini PL. Combined allogeneic tumor cell vaccination and systemic interleukin 12 prevents mammary carcinogenesis in HER-2/neu transgenic mice. J Exp Med. 2001;194:1195–1205. doi: 10.1084/jem.194.9.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]



