Abstract
Vascular smooth muscle cell (VSMC) proliferation remains a major cause of veno-arterial graft failure. We hypothesised that exposure of venous SMCs to arterial pressure would increase KLF5 expression and that of cell cycle genes. Porcine jugular veins were perfused at arterial or venous pressure in the absence of growth factors. The KLF5, c-myc, cyclin-D and cyclin-E expression were elevated within 24 h of perfusion at arterial pressure but not at venous pressure. Arterial pressure also reduced the decline in SM-myosin heavy chain expression. These data suggest a role for KLF5 in initiating venous SMCs proliferation in response to arterial pressure.
Keywords: Bypass grafting, Intimal hyperplasia, KLF5, Cell cycle, Smooth muscle cells, Organ culture
Abbreviations: CABG, coronary artery bypass grafting; LIMA, left internal mammary artery; VSMC, vascular smooth muscle cell; SM-MHC, smooth muscle myosin heavy chain; FHL1, four and a half LIM domain 1; KLF5, Krüppel-like transcription factor 5
Introduction
The saphenous vein is a commonly used conduit in bypass grafting. Whilst saphenous vein grafts have patency rates of approximately 85% at 1 year by 10 years this has fallen to between 30% and 50% [1]. Intimal hyperplasia is the main cause of vein graft failure after the first month [2] making it important to understand the mechanisms that regulate vascular smooth muscle cell (VSMC) proliferation. One factor that may contribute to intimal hyperplasia in veno-arterial grafts is the exposure of venous SMCs to arterial pressure. The involvement of this factor is supported by the different gene expression profiles of venous and arterial SMCs. Furthermore, exposure of cultured venous SMCs to stretch has been shown to regulate cell proliferation [3]. However, there are likely to be marked differences between the responses of cells that have been dispersed in culture to those that remain in the intact vessel. Therefore, the effects of pressure on VSMC phenotype and proliferation in the vessel remain to be determined.
The phenotype of proliferating VSMCs differs from that of quiescent cells in the vessel media [4]. To proliferate, the cells must express cell cycle genes as well as the transcription factors that activate their expression. One transcription factor that is important in VSMC proliferation is the Krüppel-like transcription factor 5, KLF5 [5], which activates a number of cell cycle genes including cyclin-D [6]. KLF5 expression has been shown to be elevated in response to stent induced vascular injury [5] and in rabbit models of grafting [7]. VSMCs in lesions also show altered expression of contractile proteins with reduced expression of the smooth muscle contractile proteins and their replacement with non-muscle equivalents [8] a process that has been associated with KLF5 expression [9].
Previous studies have used perfusion systems to simulate the exposure of veins to arterial pressure. These studies have identified changes in the expression of tissue factor [10] and the matrix metalloproteinases [11] and modification of vascular remodelling [12]. However, the effect of arterial pressure on VSMC phenotype and the cell cycle remains uninvestigated. In part this may is because of the presence of foetal calf serum in the perfusate exposing the cells to growth factors that would modify gene expression and initiate proliferation independent of mechanical stimulation. In this study, we tested the novel hypothesis that exposure of veins to arterial pressure induces KLF5 expression and enhances SMC proliferation. Thus, we examined the effect of exposing veins to arterial and venous pressure in a perfusion system for 48 h on gene expression. The veins were cultured in the absence of exogenous growth factors to identify changes dependent on perfusion pressure.
Materials and methods
Perfusion apparatus. Block dissections of the carotid sheath of adult pigs were obtained from Cheale Meats, Essex, UK, and stored in cold DMEM medium containing amphotericin-B, gentamicin, penicillin and streptomycin for transport to the laboratory. The internal jugular veins were dissected with aseptic technique and kept in cold DMEM as above. The veins were tied to universal 3-way taps with surgical ligatures and connected to each other in parallel with 4 vein segments in each experiment. Each vein piece used was taken from a separate animal.
The veins were transferred to a specially produced aluminium chamber with built-in adaptors for the 3-way taps to be connected to quarter inch cardio-pulmonary bypass circuit tubing and connectors for removable gas exchange membranes. The tubing was connected to a cardio-pulmonary bypass pump with De Bakey roller pumps (Sorin Group, Milan, Italy) and an aneroid manometer (Tycos) between the pump and the inlet of the perfusion chamber. DMEM with the above antibiotics was used for both perfusing the veins and to maintain the veins in the chamber. Oxygen and carbon dioxide were supplied separately through their own dedicated gas exchange membranes in the chamber. The design of the connections was such that the perfusate from the veins drained into the chamber allowing mixing of the maintenance medium in order to ensure maximum oxygen delivery. The entire chamber was kept in a water bath at 37 °C. The veins were then perfused under arterial (80–85 mmHg) or venous (5 mmHg) pressure.
For each replicate experiment samples exposed to venous or arterial pressure were obtained by removing the vein from the apparatus at 0 (control), 12, 24, 36 or 48 h. Five millimeters of vein adjacent to each 3-way tap was discarded to avoid tissue responding to damage due to the ligature. All fibrous tissue was removed from the remainder of the sample which was placed into RNA later and frozen. All samples were stored at −80 °C.
RNA preparation and quantitative real-time PCR. RNA was extracted from tissue by pulverising the tissue under liquid nitrogen. The RNA was quantified using a nanodrop spectrophotometer and 125 ng of RNA was reverse transcribed using superscript II following the manufacturer’s recommendations (Invitrogen). Quantitative PCR was performed as previously described [13] using primers designed to recognise particular porcine genes which were identified by BLAST searching of the non-redundant (NR) and expressed sequenced tag (EST) databases. Where possible, primers were designed to amplify across exon–exon boundaries. Primer sequences are provided in Table 1. PCR products were cloned and sequenced to confirm the validity of the amplification reaction. Quantitative PCR reactions were performed using SYBR green and analysed as described previously [13].
Table 1.
Primer sequences used in this study.
| SM22α for | GGTCTGGCTGAAGAATGGCGTGATTTT |
| SM22α rev | CTGAGCCACCTGCTCCATTTGCTTG |
| SMα–Actin for | GGAGCGTGGCTACTCCTTCGTGA |
| SMα–Actin rev | CGTCAGGCAGCTCGTAGCTCTTCT |
| 18S for | GTAACCCGTTGAACCCCATT |
| 18S rev | CCATCCAATCGGTAGTAGCG |
| FHL1C R | GGGAGGACTTCTACTGCGTGACTTG |
| FHL1C F | ATGCCAGGGCTGATCCTGGTAAG |
| Pig myocardin F1 | CCGCCTGCATTCCATGAGCAAAG |
| Pig myocardin R1 | CCTCTCTGCACTGGAGGCTTGGAGTATG |
| KLF5 R1 | CACCCTGCCAGTTAATTCCCAAAAC |
| KLF5 F2 | CCCAGGTGCACTTGTAGGGCTTCTC |
| NM-MHC for | CAATAAAGCTCTGGACCGGACCAAAC |
| NM-MHC rev | GTCGAGGCCGAAGTCGATGAAGTTC |
| SM-MHC for | CAGGCGAGTCTGGAGCTGGGAAAAC |
| SM-MHC rev | CCACGATGTAACCCGTGACGTCAAAG |
| Cyclin D2 for | CCCCACGACTTCATCGAGCACATT |
| Cyclin d2 rev | GCTGTTGAGCAGCACGACCTCAATC |
| c-myc for | GCCCACGACTCGCTCCTCTGAAAG |
| c-myc rev | GGTGGGCAGCAACTCGAATTTCTTC |
| c-fos for | GTCCCCAGAAGAAGAAGAGAAAAGGAGAATC |
| c-fos rev | CCCACTCAGATCAAGGGAAGCCAC |
| cyclin E2 for | GTGACGGTCATCTCCTGGCTAAATC |
| cyclin E2 rev | CCCATTCCAAACCTGAGGCTTTC |
Statistical analysis. Data were analysed using an unpaired students T test to determine significance. Correlations were performed using the spearman rank correlation.
Results
Arterial pressure increases KLF5 expression
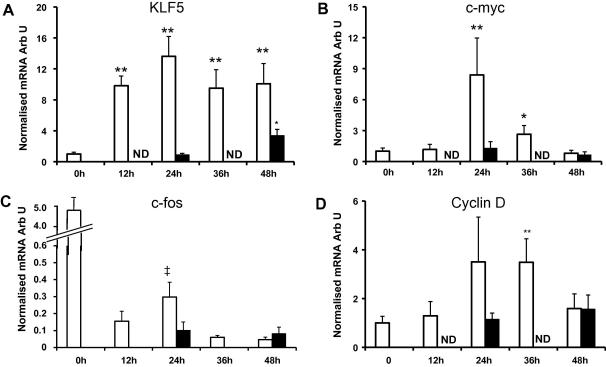
Quantitative PCR for KLF5 showed a greater than 10-fold increase in the expression of KLF5 within 12 h of the exposure to arterial pressure that remained elevated throughout the experiment. KLF5 expression did not increase in veins exposed to venous pressure until after 48 h of perfusion and this increase was much smaller (4-fold) than in the veins perfused at arterial pressure (Fig. 1A).
Fig. 1.
Expression of genes associated with smooth muscle phenotype. Quantitative real-time PCR analysis for (A) KLF5, (B) c-myc, (C) c-fos and (D) cyclin-D obtained as described in Materials and methods. Data are presented as average fold change ± SEM from control normalised to 18S RNA. (Arterial pressure, n = 7 for KLF5 and 5 for the cell cycle genes, venous pressure n = 4.) Open bars: arterial pressure, solid bars: venous pressure. (∗p < 0.05, ∗∗p < 0.01 compared to t = 0.)
Arterial pressure increases cell cycle gene expression
Analysis of the mRNA for c-myc and c-fos showed that expression of c-myc was markedly elevated 24 h after the exposure of veins to arterial pressure. c-myc expression then declined but remained elevated at 36 h (Fig. 1B). There was no change in c-myc in veins perfused at venous pressure. At all time points during the perfusion the expression of c-fos was markedly reduced compared to c-fos expression 0 h (Fig. 1C). However, within the perfusion period the kinetics of expression of c-fos was similar to c-myc, i.e. elevated at 24 h compared to 12 h and 36 h. c-fos expression is normally low or undetectable in VSMCs in vessels but is elevated by mechanical treatment [14] making it likely that the preparation of the vessels for perfusion activated c-fos expression giving an anomalously high value for c-fos at 0 h and that the activation of c-fos observed at 24 h reflects an increase in c-fos in response to the exposure to arterial pressure.
Quantitative PCR also identified an increase in cyclin-D and cyclin-E expression in response to arterial pressure. Cyclin-D expression was elevated 3-fold after 24 and 36 h of exposure to arterial pressure but this was significant only for the 36 h time point. Cyclin-E expression was also elevated at 24 h (4-fold) then declined to 2-fold over baseline by 48 h (data not shown). Veins exposed to venous pressure showed a much smaller increase in cyclin-E at 24 h but by 48 h the expression of cyclin-E had increased 8-fold.
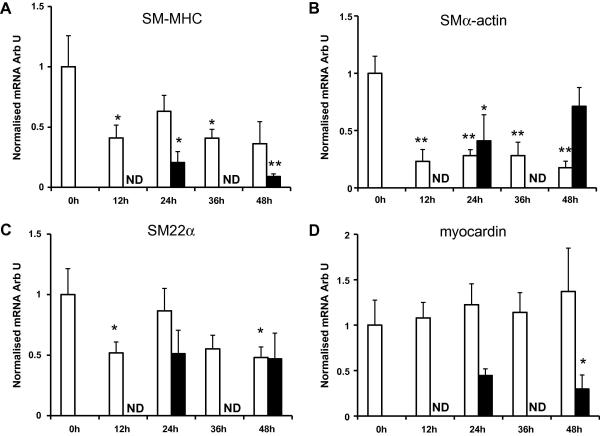
Effects of pressure on smooth muscle cell phenotype
The expression of SM-MHC, SM α-actin and SM22α (markers of VSMC phenotype) was reduced by the perfusion process irrespective of perfusion pressure. At arterial pressure the expression of SM-MHC (Fig. 2A) and SM22α (Fig. 2C) were reduced 50% within 12 h and then remained stable. SM α-actin expression (Fig. 2B) was reduced by 80% over the same time period. At venous pressure SM-MHC expression fell by 90% within 48 h and SM22α expression fell by 50% over the same time period. SMα-actin levels were also reduced at 24 h but increased over the subsequent 24 h (Fig. 2). The expression of myocardin an important transcription coactivator in VSMCs [15] did not change in response to perfusion at arterial pressure. However, at venous pressure myocardin expression fell by 70% within 48 h (Fig. 2D).
Fig. 2.
Expression of genes associated with smooth muscle phenotype. Quantitative real-time PCR analysis for (A) SM-MHC, (B) SMα-actin, (C) SM22α and (D) myocardin obtained as described in Materials and methods. Data for each test RNA were normalised to the expression of 18S RNA from the same sample. Data are presented as average fold change ± SEM from control normalised to 18S RNA. (Arterial pressure, n = 7, venous pressure n = 4). Open bars: arterial pressure, solid bars: venous pressure. (∗p < 0.05, ∗∗p < 0.01 compared to t = 0.)
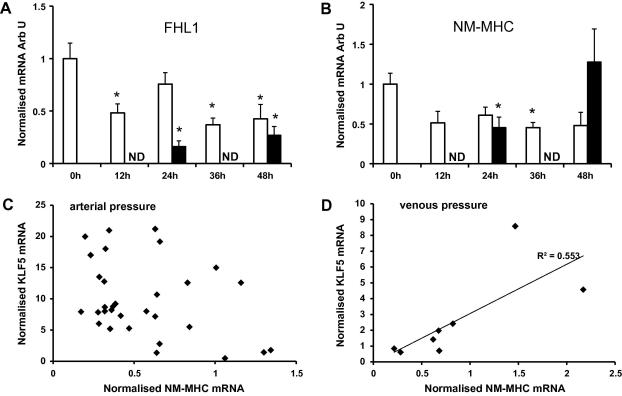
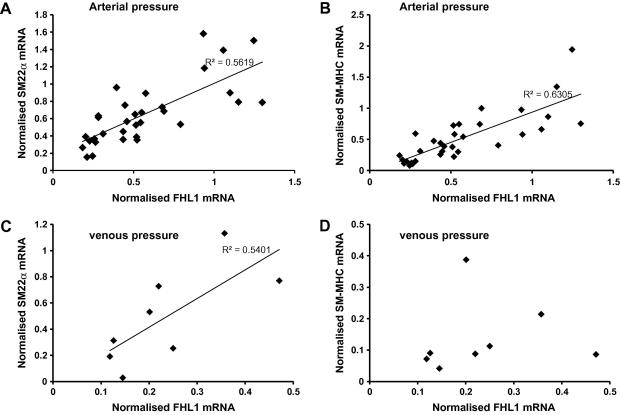
The four and half LIM domain protein FHL1, is also a potential regulator of VSMC phenotype [16]. FHL1 expression fell in response to perfusion but the reduction in expression was greater in vessels exposed to venous compared to arterial pressure (Fig. 3A). FHL1 expression was positively correlated with SM-MHC, SM22α and SMα-actin in vessels perfused at arterial pressure (ρ = 0.82, p < 0.0001, ρ = 0.86, p < 0.0001, ρ = 0.61, p < 0.01, respectively, Fig. 4). The correlation was maintained in the samples perfused at venous pressure for FHL1 with SM22α (ρ = 0.78, p = 0.05) and SMα -actin (ρ = 0.81, p = 0.03) but not with SM-MHC.
Fig. 3.
Expression of FHL1 and NM-MHC in veins. Quantitative real-time PCR analysis for (A) FHL1, and (B) NM-MHC obtained as described in Methods. Data are presented as average fold change ± SEM from control normalised to 18S RNA. (Arterial pressure, n = 7, venous pressure n = 4.) Open bars: arterial pressure, solid bars: venous pressure. (∗p < 0.05, ∗∗p < 0.01 compared to t = 0.) Correlation of NM-MHC and KLF5 expression at (C) arterial pressure, and (D) venous pressure.
Fig. 4.
Correlation of FHL1 expression with SM-MHC and SM22α at arterial and venous pressure. The expression of FHL1 was compared to the expression of (A) and (C) SM22α and (B) and (D) SM-MHC in the same sample. All samples perfused at either arterial (A and B) or venous pressure (C and D) were included in the analysis irrespective of the length of perfusion. ρ is quoted statistically significant correlations (p < 0.01).
In veins exposed to arterial pressure the expression of NM-MHC mimicked the expression of SM-MHC, reducing by approximately 50% within 12 h then remained stable. In contrast in veins perfused at venous pressure NM-MHC expression initially fell but returned to the control level by the end of the experiment (Fig. 3B). The expression of NM-MHC was correlated with KLF5 expression (ρ = 0.79, p = 0.037) at venous but not at arterial pressure (Fig. 3C and D).
Discussion
Our data show that perfusion of veins at arterial pressure increases the expression of KLF5 that is followed by increased expression of c-myc, cyclin-D and cyclin-E. KLF5 expression has previously been shown to be associated with the proliferative phenotype of VSMCs. For example, KLF5 is expressed in proliferating VSMCs in vivo in both transplant arteriosclerosis [7] and in models of restenosis [5] (e.g. in response to stenting). Indeed, KLF5 is likely to contribute to the initiation or progression of the cell cycle in arterial VSMCs as in KLF5+/− mice, the expression of platelet derived growth factor-A (PDGF-A) is reduced and the mice have a reduced proliferative response to injury [17]. Similarly inhibition of KLF5 expression and activity inhibits arterial VSMC proliferation in vivo [5].
A role for KLF5 in VSMC proliferation and our demonstration of increased expression of cell cycle genes are consistent with the known effects of KLF5 in other cells. KLF5 is known to activate the expression of cyclin-D1 and cyclin-B1 [6] and reduce the expression of the CDK inhibitors [18]. In the case of cyclin-D1 this increase results from binding of KLF5 to the cyclin-D1 promoter [6].
These data suggests that the exposure of venous SMCs to arterial pressure elevates KLF5 expression thereby activating expression of cell cycle genes. We propose that these changes initiate VSMC proliferation which can lead to occlusion of the transplanted vein and graft failure.
The increase in KLF5 might have been expected to be associated with an increase in NM-MHC [9]. However, exposure to arterial pressure reduced the expression of NM-MHC. Furthermore, whilst KLF5 expression was correlated with NM-MHC expression at venous pressure there was no association at arterial pressure. These data indicate that increased pressure suppresses NM-MHC expression and the increase in KLF5 is not sufficient to overcome this inhibition.
The expression of both KLF5 and cyclin-E showed significant increases in samples perfused at venous pressure compared to controls after 48 h. In the perfusion system the veins become slightly distended even at venous pressure and it may be that this distension is sufficient to cause the increase in KLF5 and cyclin-E after a prolonged period of time. Alternatively it is possible that these changes are the result of perfusion for an extended period of time in the absence of plasma proteins.
The reduction in the expression of SM-MHC, SM22α and SM α-actin in response to perfusion is consistent with studies of cultured arterial SMCs [8]. However, the smaller reduction and the stabilisation of SM-MHC and SM22α expression at arterial compared with venous pressure suggest that tension in the vessel wall contributes to the expression these genes. This observation is consistent with previous observations that the expression of smooth muscle-specific genes is regulated by pressure in the developing pulmonary arterial system [19] and that intraluminal pressure maintains the expression of caldesmon and filamin in organ culture [20]. Given the importance of myocardin to VSMC differentiation it is perhaps surprising that the reduction in the expression of the smooth muscle contractile genes was not accompanied by a reduction in myocardin expression. This observation implies that myocardin does not contribute to the regulation of gene expression by wall tension.
Over expression of FHL1 has been shown to increase SM-MHC expression in SMCs [21]. FHL1 interacts with components of the cytoskeleton [22] and with transcription factors and shuttles between the cytoplasm and the nucleus [23] making it an ideal regulator of gene expression in response to cytoskeletal changes. Our data showing a strong positive correlation of FHL1 and SM-MHC expression when veins were perfused under arterial pressure but not venous pressure suggests either that FHL1 and SM-MHC are regulated by similar mechanisms under these conditions or that FHL1 contributes to the control of SM-MHC expression in response to pressure. Consistent with this suggestion we have also found a similar correlation between FHL1 expression and SM-MHC in carotid arteries and transplanted jugular veins but not in normal jugular veins of pigs (EA and PRK manuscript in preparation).
In conclusion our data show that cells respond to the increase in pressure by increasing the expression of KLF5 and inducing the expression of genes associated with the cell cycle. This increase in KLF5 is likely to be important in the initiation of the cell cycle and the development of intimal hyperplasia. Higher pressure also maintains higher levels of SM-MHC expression and alters the association between FHL1 and SM-MHC implicating FHL1 in the expression of SM-MHC under high pressure.
Acknowledgments
This work was supported by the British Heart Foundation, and the Hammersmith Hospitals Trustees’ Research Committee. EA received a BHF Scholarship.
References
- 1.Georghiou G.P., Vidne B.A., Dunning J. Does the radial artery provide better long-term patency than the saphenous vein? Interact. Cardiovasc. Thorac. Surg. 2005;4:304–310. doi: 10.1510/icvts.2005.107490. [DOI] [PubMed] [Google Scholar]
- 2.Peykar S., Angiolillo D.J., Bass T.A., Costa M.A. Saphenous vein graft disease. Minerva Cardioangiol. 2004;52:379–390. [PubMed] [Google Scholar]
- 3.Dethlefsen S.M., Shepro D., D’Amore P.A. Comparison of the effects of mechanical stimulation on venous and arterial smooth muscle cells in vitro. J. Vasc. Res. 1996;33:405–413. doi: 10.1159/000159169. [DOI] [PubMed] [Google Scholar]
- 4.Thyberg J. Differentiated properties and proliferation of arterial smooth muscle cells in culture. Int. Rev. Cytol. 1996;169:183–265. doi: 10.1016/s0074-7696(08)61987-7. [DOI] [PubMed] [Google Scholar]
- 5.Fujiu K., Manabe I., Ishihara A., Oishi Y., Iwata H., Nishimura G., Shindo T., Maemura K., Kagechika H., Shudo K., Nagai R. Synthetic retinoid Am80 suppresses smooth muscle phenotypic modulation and in-stent neointima formation by inhibiting KLF5. Circ. Res. 2005;97:1132–1141. doi: 10.1161/01.RES.0000190613.22565.13. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki T., Sawaki D., Aizawa K., Munemasa Y., Matsumura T., Ishida J., Nagai R. Kruppel-like factor 5 shows proliferation-specific roles in vascular remodeling, direct stimulation of cell growth, and inhibition of apoptosis. J. Biol. Chem. 2009;284:9549–9557. doi: 10.1074/jbc.M806230200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bafford R., Sui X.X., Wang G., Conte M. Angiotensin II and tumor necrosis factor-alpha upregulate surviving and Kruppel-like factor 5 in smooth muscle cells: potential relevance to vein graft hyperplasia. Surgery. 2006;140:289–296. doi: 10.1016/j.surg.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Owens G.K. Regulation of differentiation of vascular smooth muscle cells. Physiol. Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe N., Kurabayashi M., Shimomura Y., Kawai-Kowase K., Hoshino Y., Manabe I., Watanabe M., Aikawa M., Kuro-o M., Suzuki T., Yazaki Y., Nagai R. BTEB2, a Kruppel-like transcription factor, regulates expression of the SMemb/Nonmuscle myosin heavy chain B (SMemb/NMHC-B) gene. Circ. Res. 1999;85:182–191. doi: 10.1161/01.res.85.2.182. [DOI] [PubMed] [Google Scholar]
- 10.Muluk S.C., Vorp D.A., Severyn D.A., Gleixner S., Johnson P.C., Webster M.W. Enhancement of tissue factor expression by vein segments exposed to coronary arterial hemodynamics. J. Vasc. Surg. 1998;27:521–527. doi: 10.1016/s0741-5214(98)70327-1. [DOI] [PubMed] [Google Scholar]
- 11.Mavromatis K., Fukai T., Tate M., Chesler N., Ku D.N., Galis Z.S. Early effects of arterial hemodynamic conditions on human saphenous veins perfused ex vivo. Arterioscler. Thromb. Vasc. Biol. 2000;20:1889–1895. doi: 10.1161/01.atv.20.8.1889. [DOI] [PubMed] [Google Scholar]
- 12.Porter K.E., Nydahl S., Dunlop P., Varty K., Thrush A.J., London N.J. The development of an in vitro flow model of human saphenous vein graft intimal hyperplasia. Cardiovasc. Res. 1996;31:607–614. [PubMed] [Google Scholar]
- 13.Ellis P.D., Smith C.W., Kemp P. Regulated tissue-specific alternative splicing of enhanced green fluorescent protein transgenes conferred by alpha-tropomyosin regulatory elements in transgenic mice. J. Biol. Chem. 2004;279:36660–36669. doi: 10.1074/jbc.M405380200. [DOI] [PubMed] [Google Scholar]
- 14.Miano J.M., Vlasic N., Tota R.R., Stemerman M.B. Localization of Fos and Jun proteins in rat aortic smooth muscle cells after vascular injury. Am. J. Pathol. 1993;142:715–724. [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J., Kitchen C.M., Streb J.W., Miano J.M. Myocardin: a component of a molecular switch for smooth muscle differentiation. J. Mol. Cell Cardiol. 2002;34:1345–1356. doi: 10.1006/jmcc.2002.2086. [DOI] [PubMed] [Google Scholar]
- 16.Davis C.A., Haberland M., Arnold M.A., Sutherland L.B., McDonald O.G., Richardson J.A., Childs G., Harris S., Owens G.K., Olson E.N. PRISM/PRDM6, a transcriptional repressor that promotes the proliferative gene program in smooth muscle cells. Mol. Cell Biol. 2006;26:2626–2636. doi: 10.1128/MCB.26.7.2626-2636.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shindo T., Manabe I., Fukushima Y., Tobe K., Aizawa K., Miyamoto S., Kawai-Kowase K., Moriyama N., Imai Y., Kawakami H., Nishimatsu H., Ishikawa T., Suzuki T., Morita H., Maemura K., Sata M., Hirata Y., Komukai M., Kagechika H., Kadowaki T., Kurabayashi M., Nagai R. Kruppel-like zinc-finger transcription factor KLF5/BTEB2 is a target for angiotensin II signaling and an essential regulator of cardiovascular remodeling. Nat. Med. 2002;8:856–863. doi: 10.1038/nm738. [DOI] [PubMed] [Google Scholar]
- 18.Chen C., Benjamin M.S., Sun X., Otto K.B., Guo P., Dong X.Y., Bao Y., Zhou Z., Cheng X., Simons J.W., Dong J.T. KLF5 promotes cell proliferation and tumorigenesis through gene regulation and the TSU-Pr1 human bladder cancer cell line. Int. J. Cancer. 2006;118:1346–1355. doi: 10.1002/ijc.21533. [DOI] [PubMed] [Google Scholar]
- 19.Favot L., Hall S.M., Haworth S.G., Kemp P.R. Cytoplasmic YY1 is associated with increased smooth muscle-specific gene expression: implications for neonatal pulmonary hypertension. Am. J. Pathol. 2005;167:1497–1509. doi: 10.1016/S0002-9440(10)61236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birukov K.G., Bardy N., Lehoux S., Merval R., Shirinsky V.P., Tedgui A. Intraluminal pressure is essential for the maintenance of smooth muscle caldesmon and filamin content in aortic organ culture. Arterioscler. Thromb. Vasc. Biol. 1998;18:922–927. doi: 10.1161/01.atv.18.6.922. [DOI] [PubMed] [Google Scholar]
- 21.Kang M.A., Jeoung N.H., Kim J.Y., Lee J.E., Jung U.J., Choi M.S., Lee W.H., Kwon O.S., Lee H., Park Y.B. Up-regulation of skeletal muscle LIM protein 1 gene by 25-hydroxycholesterol may mediate morphological changes of rat aortic smooth muscle cells. Life Sci. 2007;80:460–467. doi: 10.1016/j.lfs.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 22.McGrath M.J., Cottle D.L., Nguyen M.A., Dyson J.M., Coghill I.D., Robinson P.A., Holdsworth M., Cowling B.S., Hardeman E.C., Mitchell C.A., Brown S. Four and a half LIM protein 1 binds myosin-binding protein C and regulates myosin filament formation and sarcomere assembly. J. Biol. Chem. 2006;281:7666–7683. doi: 10.1074/jbc.M512552200. [DOI] [PubMed] [Google Scholar]
- 23.Liang L., Zhang H.W., Liang J., Niu X.L., Zhang S.Z., Feng L., Liang Y.M., Han H. KyoT3, an isoform of murine FHL1, associates with the transcription factor RBP-J and represses the RBP-J-mediated transactivation. Biochim. Biophys. Acta. 2008;1779:805–810. doi: 10.1016/j.bbagrm.2008.08.001. [DOI] [PubMed] [Google Scholar]