Abstract
Disruption of dopamine homeostasis may lead to dopaminergic neuron degeneration, a proposed explanation for the specific vulnerability of dopaminergic neurons in Parkinson's disease. While expression of human α-synuclein in C. elegans results in dopaminergic neuron degeneration, the effects of α-synuclein on dopamine homeostasis and its contribution to dopaminergic neuron degeneration in C. elegans have not been reported. Here, we examined the effects of α-synuclein overexpression on worm dopamine homeostasis. We found that α-synuclein expression results in upregulation of dopamine synthesis and content, and redistribution of dopaminergic synaptic vesicles, which significantly contribute to dopaminergic neuron degeneration. These results provide in vivo evidence supporting a critical role for dopamine homeostasis in supporting dopaminergic neuron integrity.
Introduction
Abnormal dopamine (DA) metabolism, which produces reactive oxygen species (ROS), may lead to dopaminergic (DAergic) neuron degeneration and has been proposed to be related to the pathogenesis of Parkinson's Disease (PD) [1]–[6]. For example, overexpression of tyrosine hydroxylase (TH) in primary neuronal cultures of Drosophila embryos induces cellular degeneration [1] and vesicular monoamine transporter (VMAT) loss-of-function mice show nigrostriatal neurogdegeneration [2].
Some in vitro or ex vivo evidence also suggests a connection between dopamine homeostasis and α-synuclein, the central player of PD pathology [3]–[11]. Thus, expression of pathogenic α-synuclein mutants enhances cytosolic catecholamine levels in human mesencephalic cells, PC12 cells and mouse chromaffin cells [12], [13]. Moreover, genetic disruption of vesicular dopamine storage induces age-dependent alterations in the nigrostriatal dopamine system and progressive nigral cell loss in α-synuclein positive, but not in α-synuclein negative mice [2]. Reduction of cytosolic dopamine content either genetically or pharmacologically prevents hαSyn-mediated neuronal degeneration in vitro [1]. It also has been suggested that α-synuclein overexpression disrupts vesicular pH, leading to the increased cytosolic catechol species [13].
Genetic model organisms such as yeast, Drosophila and C. elegans are valuable surrogates for the study of certain aspects of neurodegenerative diseases, including investigations of α-synuclein toxicity [5], [14]–[22]. For example, genes involved in protein trafficking have recently been identified to be involved in α-synuclein toxicity, leading to the hypothesis that α-synuclein mediated altered intracellular trafficking regulates dopamine homeostasis [5].
Expression of human α-synuclein (hαSyn) in DAergic neurons of C. elegans results in their degeneration [21], [22]. Yet, the effects of hαSyn expression on dopamine homeostasis have not been addressed in this useful organism. Here, we used hαSyn-expressing C. elegans lines to examine the toxic effects of hαSyn on dopamine homeostasis and its contribution to hαSyn-mediated DAergic neuron degeneration.
Results
hαSyn Expression Induces DAergic Neuron Degeneration
We first characterized the expression of dat-1 promoter-driven hαSyn by using immunohistochemistry and confocal microscopy. Positive hαSyn immunostaining was found exclusively in DAergic neurons, marked with dat-1 promoter-driven DsRed, demonstrating the specificity of hαSyn expression in our transgenic lines (Figure S1).
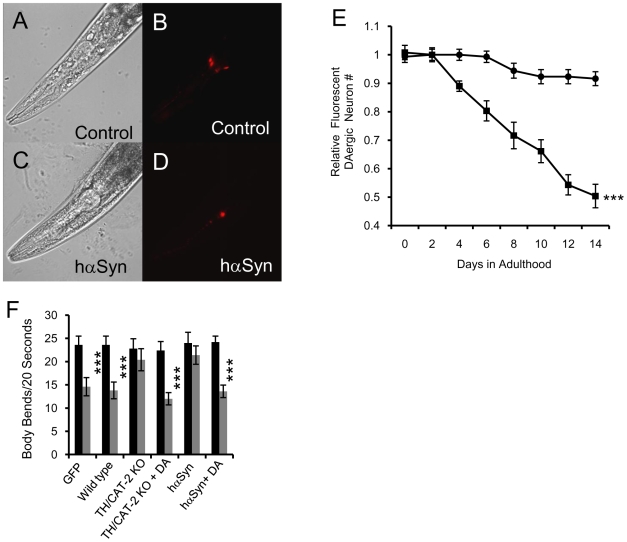
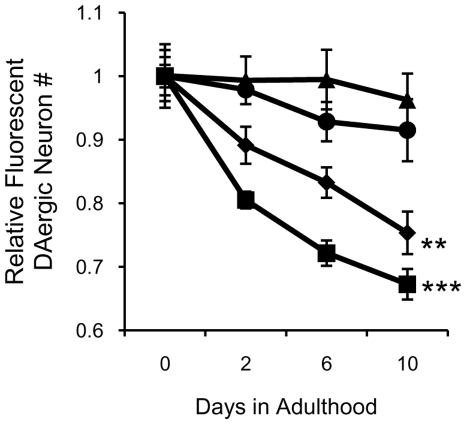
Previous efforts to express wild type or pathogenic hαSyn in worms led to loss of the fluorescent DAergic neuron marker due to degeneration of DAergic neurons [5], [20]. Consistent with these reports, our hαSyn-expressing line, but not the control line, displayed an age-related progressive decline in the number of fluorescent DAergic neurons (Figure 1A–E). Another hαSyn-expressing line also exhibited a similar decline in the number of fluorescent DAergic neurons (data not shown). This conclusion was further confirmed by TH immunostaining experiments (Figure S2) and similar experiments where both a non-functional CAT-2/TH::GFP fusion protein [23], [24] and DsRed were used as DAergic neuron markers (Figure S3).
Figure 1. hαSyn expression leads to DAergic neuron degeneration.
A–D, Confocal images of living day 10 adult control (AB) or hαSyn-expressing (CD) worms with DAergic neuron specific expression of DsRed. A and C, Bright field; B and D, DsRed. E, Number of fluorescent DAergic neurons in hαSyn-expressing (squares) and control (circles) lines. ***, p<0.005 (two-way ANOVA). Error bars represent the SEM (standard error of the mean) for three independent experiments. In each experiment, the n of each sample varied from 20 to 50. F, Basal slowing response of day 2 adult worms. TH/CAT-2 KO or cat-2 is a TH/CAT-2 knockout (KO) worm line. GFP indicates a wild type line expressing GFP in DAergic neurons. Food response experiments were conducted with (grey bars) or without (black bars) food. ***, p<0.0001 (t-test); n varied from 6 to 20. Error bars indicate SEM.
We next investigated the effect of hαSyn expression on the function of worm DAergic neurons by measuring the basal slowing response, a food-sensing behavior regulated by dopamine neurotransmission [25]. The worm basal slowing response was used to assess the effect of hαSyn expression on the function of DAergic neurons [21]. As found in cat-2, a knockout mutant of worm TH, hαSyn-expressing worms had an impaired basal slowing response, which returned to control levels in the presence of 0.5 mM exogenous dopamine (Figure 1F). Thus, animals of the hαSyn expressing line were functionally deficient in dopamine.
Consistent with our hαSyn expression pattern, the enhanced slowing response, a food response behavior regulated by serotonin neurotransmission [25], was not affected in hαSyn expressing animals (Figure S4).
Taken together, these results lead us to conclude that hαSyn expression induces degeneration of DAergic neurons in our hαSyn expressing lines, similar to previous reports.
hαSyn Expression Induces a Motor Capacity Deficit
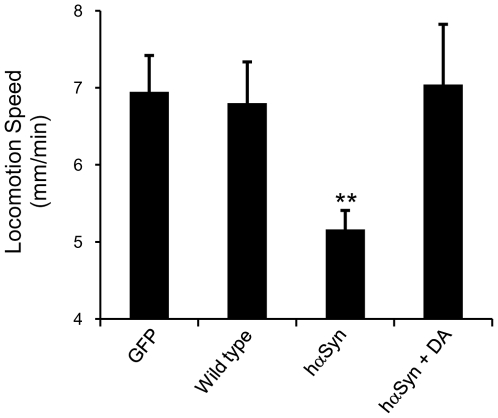
We next quantified the effect of hαSyn expression on worm motor capacity, which had not been assessed previously in worms specifically expressing hαSyn in DAergic neurons [5], [21]. In general, there are two methods to access motor capacity in worms: body bending frequency and centroid velocity [25]–[28]. Body bending frequency is the number of sinusoidal waves made by a worm during a given time period, while centroid velocity quantifies the physical displacement of a worm's centroid. Body bending frequency can be uncoupled from centroid displacement by genetic mutations and ageing [26], [29]. We observed that L4 and day 1 adult worms exhibit similar body bending frequencies, although adult worms move much faster than L4 worms, as quantified by their centroid velocity (Cao and Feng, unpublished data). Because the centroid velocity of worm locomotion has been utilized to quantify age-related changes in motor capacity and provides more sensitive and reliable quantification of worm motor activity [26], [27], this parameter was selected to address the effect of hαSyn expression on the worm motor system. Indeed, hαSyn expressing worms exhibited a deficit in motor activity that was restored by adding 1 mM dopamine (Figure 2), a finding consistent with observations in a Drosophila PD model [17].
Figure 2. hαSyn expression leads to a motor deficit.
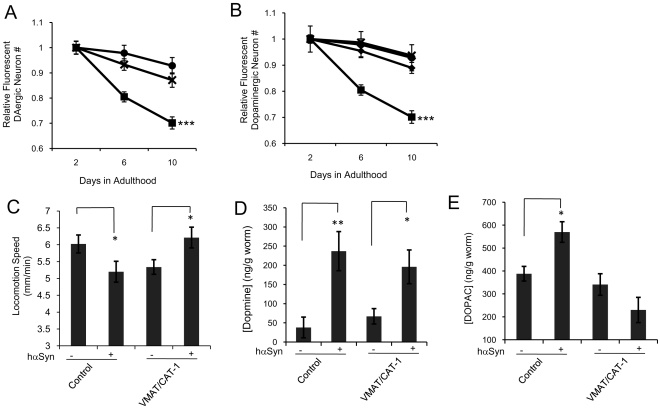
Locomotion speed was quantified in day 2 adult worms. **: p<0.01 (one way ANOVA with Dunnet's post-hoc test). n varies 10 to 15. Error bars indicate SEM. This deficit was not observed after addition of 1 mM DA.
hαSyn Expression Results in Altered Dopamine Metabolism
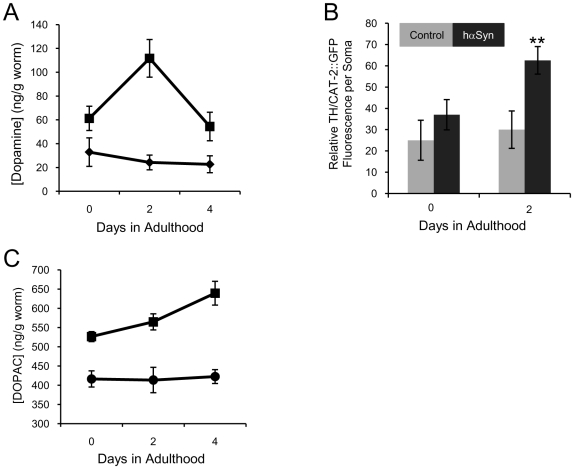
Despite their functional deficiency in dopamine neurotransmission, hαSyn expressing worms surprisingly exhibited a remarkable upregulation of dopamine content from L4 to day 4 in adulthood (Figure 3A), as measured by liquid chromatography-mass spectrometry (LC-MS). We obtained similar results and reached the same conclusion (data not shown) by using conventional high performance liquid chromatography (HPLC) as well. Consistently, the fluorescence intensity of a non-functional TH/CAT-2::GFP fusion protein [23], [24] in day 2 adult hαSyn expressing worms was significantly elevated (Figure 3B).
Figure 3. hαSyn expression leads to altered dopamine metabolism.
A, Quantification of dopamine content in worms with (squares) or without (diamonds) hαSyn expression is shown as a function of age. Error bars represent the SEM of 3 independent experiments. Each experiment was done with ∼200 worms per sample. B, Quantification of CAT-2::GFP florescence in DAergic neurons of EM641 worms (a worm line expressing a non-functional CAT-2::GFP) either with (black bars) or without (gray bars) hαSyn expression. ** p<0.01 (t-test). n varied from 9 to 12. Error bar, SEM. C, Quantification of DOPAC content in hαSyn expressing (squares) or control (circles) worms. Error bars represent the SEM of 3 independent experiments. Each experiment was done with ∼400 worms per sample.
Abnormal dopamine metabolism may produce cytotoxic molecules such as hydrogen peroxide, superoxide radicals and dopamine-quinone through two pathways, namely auto-oxidation and deamination by monoamine oxidase (MO). Dopamine deamination also yields 3,4-dihydroxyphenylacetic acid (DOPAC), a non-toxic metabolite that can be used to monitor dopamine deamination-specific oxidative stress [12], [30].
We found that hαSyn-expressing worms displayed an age-related accumulation of DOPAC leading to a significantly higher DOPAC content than control worms (Figure 3C), thereby providing evidence for an hαSyn-mediated disruption of dopamine metabolism. Dopamine-quinone was not detected in any worms (data not shown), possibly because dopamine auto-oxidation is negligible in vivo. This quinone can be oxidized to several other species [30] or become adducted to glutathione and/or thiol groups of native proteins [31]. Nevertheless, we conclude that hαSyn expression alters dopamine metabolism in worms.
hαSyn Expression Redistributes Dopamine Synaptic Vesicles
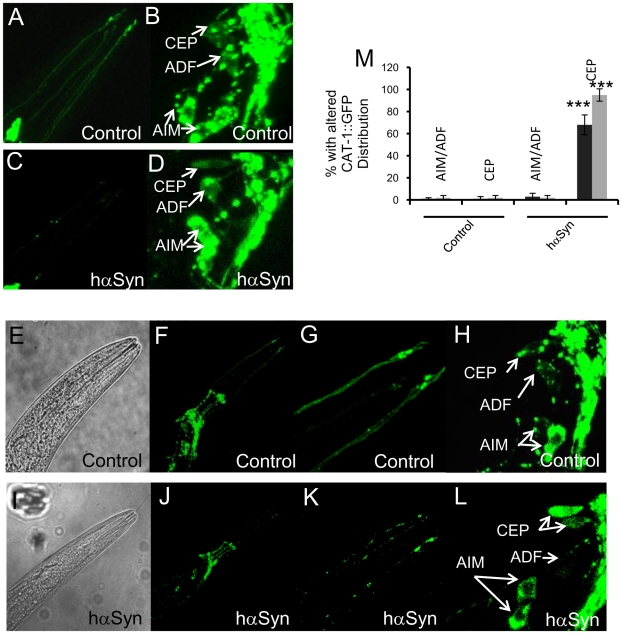
Dopamine is loaded into synaptic vesicles by a VMAT and pathogenic α-synuclein impairs dopamine storage in mammalian cell lines [32], [33]. To further investigate whether hαSyn expression affects dopamine homeostasis in worms, we crossed our hαSyn expressing line with a worm line expressing CAT-1::GFP [34]. CAT-1 is the sole worm homolog of VMAT. In worms expressing only VMAT/CAT-1::GFP but not hαSyn, the observed VMAT/CAT-1::GFP expression pattern of DAergic neurites was continuously linear with a few bright spots at both L2 (Figure 4A) and L4 (Figure 4E–G) stages, a finding consistent with previous reports [34]–[36]. In contrast, many bright VMAT/CAT-1::GFP spots appeared in the remarkably weakened linear fluorescent DAergic neurites of hαSyn expressing L2 worms (Figure 4C). Such an hαSyn mediated alteration of VMAT/CAT-1::GFP distribution further developed, and VMAT/CAT-1::GFP fluorescence of DAergic neurites was only located in discrete punctate spots without visible lines in L4 worms (Figure 4I–M), which was prior to the obvious start of DAergic neuron degeneration in this worm variant.
Figure 4. hαSyn expression disrupts dopamine synaptic vesicle distribution.
A–L, Typical confocal laser scanning VMAT/CAT-1::GFP (A–D, F–H, J–L) or bright field (E, I) images of living L2 (A–D) or L4 (E–L) nuls26 (a worm line expressing VMAT-CAT-1::GFP) worms expressing (C–D, I–L) or not expressing (A–B, E–H) hαSyn. A–D, are GFP images that show DAergic (CEP, specifically) dendrites (A, C) or DAergic/serotonergic somas (B, D) of L2 worms. G and K are magnified areas of F and J, respectively, that show DAergic dendrites (CEP) of L4 worms. H and L, are magnified areas of F and J, respectively, that show DAergic and serotonergic somas of L4 worms. M, Quantification of CAT-1::GFP redistribution in DAergic neurons (CEP) and serotonergic neurons (AIM and ADF) of L2 (black bars, n = 5) and L4 worms (gray bars, n = 8). ***: p<0.0001 (t-test). Error bars indicate SEM.
Also consistent with previous reports [34]–[36], VMAT/CAT-1::GFP in DAergic somas of control worms was excluded from the nucleus and formed a punctate pattern in both DAergic and serotonergic neuron somas (Figure 4B and H). hαSyn expression disrupted this pattern of VMAT/CAT-1::GFP expression exclusively in DAergic but not serotonergic neurons as early as L2 (Figure 4D, L–M). From this evidence, we conclude that hαSyn expression causes dopamine synaptic vesicle maldistribution.
Disruption of hαSyn-Mediated Dopamine Homeostasis Contributes to DAergic Neuron Degeneration
The next step was to determine whether hαSyn-mediated disruption of dopamine homeostasis contributes to DAergic neuron degeneration in worms. In rodents, exogenous expression of DAT-1, a dopamine transporter, leads to neuronal degeneration. In worms, overexpression of TH/CAT-2 produces DAergic neuron (CEP) abnormalities [22]. Here, we found that hαSyn induced DAergic neuron degeneration more slowly in worms with a cat-2 mutant background (Figure 5), indicating that hαSyn-mediated DAergic neuron degeneration is related to dopamine homeostasis.
Figure 5. Knockout of TH protects DAergic neurons from hαSyn expression toxicity.
A. Quantification of fluorescent DAergic neurons in hαSyn expressing wild type (squares) or hαSyn expressing TH/CAT-2 KO (diamonds) worms and in control wild type (triangles) or TH/CAT-2 KO (circles) worms. Error bars represent the SEM of three independent experiments. **, p<0.01; ***, p<0.005 (Two-way ANOVA). In each experiment, n of each sample varied from 10 to 30.
Dopamine is toxic in the cytosol but not in synaptic vesicles [1], [2], [37]. Consistently, we found that VMAT/CAT-1 knockout worms displayed slightly faster rates of DAergic neuron degeneration than controls (Figure 6A). If hαSyn-mediated altered dopamine metabolism contributes to hαSyn-mediated dopamine neuron degeneration, one would expect that in vivo overexpression of VMAT/CAT-1 would ameliorate hαSyn mediated DAergic neuron degeneration. Indeed, we found that VMAT/CAT-1 overexpression [34], [38] did prevent the hαSyn-mediated DAergic neuron degeneration (Figure 6B) and motor activity deficit (Figure 6C).
Figure 6. Overexpression of VMAT protects DAergic neurons from hαSyn toxicity.
A, Quantification of fluorescent DAergic neurons of wild type (circles), VMAT/CAT-1 KO (crosses), and hαSyn expressing wild type (squares) worms. Error bars represent the SEM of three independent experiments. In each experiment, n of each sample varied from 10 to 25. ***, p<0.005 (Two-way ANOVA). B, Quantification of fluorescent DAergic neurons in wild type (circles), hαSyn expressing wild type(squares), VMAT/CAT-1 overexpressing (diamonds), and hαSyn expressing VMAT/CAT-1 overexpressing (crosses) worms. Error bars represent the SEM of three independent experiments. In each experiment, n of each sample varied from 10 to 30. ***, p<0.005 (Two-way ANOVA). C, Locomotion speed of day 2 adult worms of control and VMAT/CAT-1 overexpressing lines with or without hαSyn expression. *: p<0.01 (t-test). n≥10. Error bars:SEM. D. Quantification of [dopamine] in day 2 adult worms of control and VMAT/CAT-1 overexpressing lines with or without hαSyn expression. Error bars represent the SEM of three independent experiments. In each experiment, the number of worms per sample was about 200. *: p<0.01 (t-test), **: p<0.005 (t-test). E. Quantification of [DOPAC] in day 2 adult worms of control and VMAT/CAT-1 overexpressing lines with or without hαSyn expression. Error bars represent the SEM of three independent experiments. In each experiment, the number of worms per sample was about 200. *: p<0.01 (t-test).
Critically, VMAT/CAT-1 overexpression prohibited hαSyn-mediated [DOPAC] upregulation (Figure 6E), but not [dopamine] upregulation (Figure 6D), providing evidence that enhanced sequestration of dopamine protects DAergic neurons from the toxicity of hαSyn expression by affecting dopamine turnover. Thus, hαSyn-mediated disruption of dopamine homeostasis significantly contributes to the observed DAergic neuron degeneration and loss of motor activity. Consistent with this conclusion, hαSyn expression disturbed the VMAT/CAT-1::GFP expression pattern in L2 organisms before significant DAergic neuron degeneration starts (Figures 4 and 6B), and this disruption persisted in the cat-2 mutant background (Figure S5), wherein DAergic neuron degeneration was prevented.
Discussion
Using in vitro and ex vivo mammalian or drosophila cell cultures, α-synuclein was found to disrupt dopamine homeostasis. Here, we provide in vivo evidence to support a critical relationship between α-synuclein and dopamine homeostasis. α-Synuclein may regulate dopamine homeostasis through multiple mechanisms [13], such as dopamine synthesis/breakdown [39], [40], compartmentalization [41] and recycling [42]. Consistently, we found that α-synuclein expression altered the expression of CAT-2/TH and distribution of dopamine synaptic vesicles.
Why did we observe an hαSyn mediated dopamine functional deficit along with upregulated dopamine synthesis and content? One possibility to explain this paradox is that hαSyn alters dopamine synaptic vesicle trafficking or packing, which may reduce the availability of dopamine synaptic vesicles at synapses and stimulate dopamine synthesis through feedback control mechanisms [43], [44]. Insufficient loading of unregulated dopamine into vesicles, therefore, could result in the observed altered dopamine metabolism. Indeed, α-synuclein was proposed to intervene directly in dopamine synaptic loading in mammals [12], [32], [33]. But this possibility should be further explored and validated with mammalian models.
In a previous study, investigators observed that heterological hαSyn expression in worm DAergic neurons induced dopamine deficiency rather than upregulation [21]. Interestingly, hαSyn expression did not cause degeneration of DAergic somas in their worm lines either. The less severe cytotoxicity of hαSyn in their worm line, compared with our hαSyn expressing lines and a line reported by Caldwell's group [22], may be due to different levels of protein expression.
It is worthy to point out that knockout of TH/CAT-2 or overexpression of VMAT/CAT-1 did not completely protect DAergic neurons from hαSyn-mediated degeneration. Consistently, the effect of knocking out VMAT/CAT-1 on DAergic degeneration was not as pronounced as that resulting from hαSyn expression, indicating that hαSyn-mediated cytotoxicity is not solely caused by the disruption of dopamine homeostasis. Indeed, α-synuclein mediated modification of chaperone-mediated autophagy (CMA) also plays a critical role in DAergic neuron loss in mammals [45].
Materials and Methods
C. elegans Strains
The promoter of dat-1 was cloned and linked to a full-length cDNA encoding hαSyn, DsRed or GFP according to a previous description [21]. Transgenic lines expressing hαSyn were generated by injecting constructs of hαSyn (10 ng/µl per injection), DsRed or GFP under the control of the dat-1 (encoding dopamine transporter) promoter sequence [46]. Two transgenic lines expressing hαSyn were obtained and both exhibited similar hαSyn toxicity. After the transgenic line expressing hαSyn was integrated, this integrated line was backcrossed 4× with wild type worms. To produce lines expressing both hαSyn and CAT-1::GFP or CAT-2::GFP, the transgenic hαSyn expressing worm line was crossed into nuls26 or EM641, respectively [24], [34]. All other worm protocols involved standard methods [47]. cat-1 and cat-2 mutants used were e1111 and e1112, respectively. N2 was used as the wild type.
Immunochemistry
Worms were fixed with formaldehyde and stained with goat anti-hαSyn antibody according to published protocols with slight modifications [48]. All antibodies were purchased from Millipore.
Microscopy
All confocal experiments were conducted with a Leica TCS SP2 confocal microscope. The spectra used were: DsRed(λex = 543nm and λem = 580–630nm) and GFP(λex = 488nm and λem = 510–530nm). To count fluorescent DAergic neuron numbers, living worms were immobilized with 30 mM sodium azide on 5% agarose pads and examined with a Leica DMI3000 microscope or a Leica TCS SP2 confocal microscope according to a published method with modifications [21]. Specifically, fluorescent DAergic neurons numbers were counted manually. The existence of a fluorescent DAergic soma was evaluated by its fluorescence intensity, its position in animals and the position of its dendrites of a candidate neuron. The position of a neuron in worms and the position of its dendrites are relatively unchanged in worms throughout their life [49]. To obtain consistent data, an observer was warmed up with 10–20 day 1 animals from an integrated wild type line expressing DsRed in DAergic neurons, every day when such an experiment was conducted. These animals have eight fluorescent DAergic somas. In these experiments, representative images were captured with an Andor iXonEM 885 EMCCD camera and SimImaging (Feng, Z. unpublished software) (when Lecica DMI3000 microscope was used) or a Leica TCS SP2 confocal microscope. All images were processed and analyzed with National Instruments Vision Assistant 7.1.
Behavioral Analyses
Worm basal/enhanced slowing responses with and without dopamine pretreatment were obtained as previously described [21], [25]. Locomotion speed was collected by using Automated and Quantitative Analysis of Behavior of Nematode (AQUABN) with a protocol described previously [26], [27]. After a 10-minute video was collected, the average speed from minutes 7–10 was computed to eliminate the locomotion acclimation phase. For dopamine rescue experiments, dopamine was added to the liquid medium before pouring Nematode Growth Medium (NGM) plates. Animals were then raised and experiments were conducted on dopamine containing NGM plates. These dopamine-exposed, hαSyn-expressing animals exhibited DAergic neurite and soma degeneration phenotypes similar to hαSyn expressing animals raised on regular NGM plates (data not shown).
Dopamine and DOPAC Measurements
Samples were prepared as described [21] and filtered with a 0.45 µm Millipore filter before being injected into tandem LC-MS that employed an ESI probe in the positive ion mode. The column used was a C18 Discovery HS (5µm narrow bore), 15 cm long with a 2.1 mm diameter. The mobile phase used for elution was composed of solvent A (10 mM ammonium formate, pH 3.0) and solvent B (acetonitrile) with ratios ranging from 97% – 80% of solvent A. The detector was set up for single ion monitoring m/z 150–210.
Statistical Analysis
Statistical significance was analyzed by using Statistica (StatSoft, Inc.). T-tests, ANOVA with Bonferroni corrections or Dunnet's post-hoc analyses were used for their appropriate applications.
Supporting Information
Immunohistochemical analysis of hαSyn expression in transgenic C. elegans. A–D, Confocal images of a formaldehyde-fixed day 2 adult worm with DAergic neuron specific expression of hαSyn and DsRed. A, Bright field (BF). B, DsRed. C, hαSyn immunostaining (green). D. Merged image of B and C.
(0.80 MB TIF)
Immunohistochemical analysis of TH expression in transgenic C. elegans. A–B, Confocal images of formaldehyde-fixed day 0 (A) or day 10 (B) worm with DAergic neuron specific expression of hαSyn and DsRed. Left, TH immuostaining; Middle, DsRed; Right, Merged image of TH staining and DsRed; C, Quantification of DAergic neuron degeneration by using TH staining (green) or DsRed (Red). Data represents mean ± S.E.M., n = 10. 1 represents 6.8 and 6.3 DAergic neurons in DsRed and TH staining experiments, representatively.
(0.69 MB TIF)
Correlation of DAergic neuron degeneration with CAT-2::GFP and DsRed. A–B, Confocal images of living day 0 (A) or day 10 worms (B) with DAergic neuron specific expression of CAT-2::GFP, DsRed and hαSyn. Left, CAT-2::GFP; Middle, DsRed; Right, Merged image of CAT-2::GFP and DsRed. (C) Quantification of DAergic neuron degeneration by using CAT-2::GFP (green) or DsRed (red) in hαSyn-expressing (diamonds) and control (circles) lines. Data represent mean ± S.E.M., n = 30. Error bars may hide in symbols. ***, p<0.005 (Two-way ANOVA to compare hαSyn expressing and control line) (green: CAT-2::GFP; Red DsRed). 1 represents 7.9 and 7.7 in DsRed and CAT-2/TH::GFP experiments, respectively.
(1.44 MB TIF)
hαSyn expression does not affect serotonin neurotransmission. Enhanced slowing responses of day 2 adult worms. GFP indicates a wild type worm line expressing GFP in DAergic neurons. Food response experiments were conducted with (grey bars) or without (black bars) food.
(0.23 MB TIF)
hαSyn expression disrupts dopamine synaptic vesicle distribution in TH/CAT-2 knockout background. A–D, Typical bright field (A) or confocal laser scanning VMAT/CAT-1::GFP (B–D) images of living L2 worms expressing both VMAT/CAT-1::GFP and hαSyn in a TH/CAT-2 knockout background. C and D are magnified areas of B that show DAergic and serotonergic somas (C) or DAergic dendrites of CEPs (D), respectively. E, Quantification of CAT-1::GFP redistribution in CEPs of L2 worms expressing both VMAT/CAT-1::GFP and hαSyn in wild type (n = 5) or a TH/CAT-2 knockout background (n = 5). Error bar:SEM.
(0.98 MB TIF)
Acknowledgments
We thank Kris Kramp, Alex Ward and Beverly Piggott for technical support; Drs. Leslie Webster, Daewoo Lee, Martin Snider, Stewart Loh, Ruth Siegel, Mark Smith, Vernon Anderson and John Mieyal for discussions; Drs. Scott Emmons, Joshua Kaplan and the Caenorhabditis Genetics Center for providing worm strains; and Dr. Yoshikazu Imanishi for assistance with confocal microscopy. Z. F. is a scholar of the Mt. Sinai Health Care Foundation Scholars Program in the Basic Sciences.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work is partially supported by Mt. Sinai Healthcare Scholarship from Mt. Sinai Healthcare Foundation (www.mtsinaifoundation.org/) to ZF and a MERIT Review grant of the Department of Veterans Affairs (www.research.va.gov/) to EAP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Park SS, Schulz EM, Lee D. Disruption of dopamine homeostasis underlies selective neurodegeneration mediated by alpha-synuclein. Eur J Neurosci. 2007;26:3104–3112. doi: 10.1111/j.1460-9568.2007.05929.x. [DOI] [PubMed] [Google Scholar]
- 2.Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, et al. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 2007;27:8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auluck PK, Meulener MC, Bonini NM. Mechanisms of Suppression of {alpha}-Synuclein Neurotoxicity by Geldanamycin in Drosophila. J Biol Chem. 2005;280:2873–2878. doi: 10.1074/jbc.M412106200. [DOI] [PubMed] [Google Scholar]
- 4.Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science. 2002;295:865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- 5.Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson TM, Dawson VL. Neuroprotective and neurorestorative strategies for Parkinson's disease. Nat Neurosci. 2002;5(Suppl):1058–1061. doi: 10.1038/nn941. [DOI] [PubMed] [Google Scholar]
- 7.Lee VM, Trojanowski JQ. Mechanisms of Parkinson's disease linked to pathological alpha-synuclein: new targets for drug discovery. Neuron. 2006;52:33–38. doi: 10.1016/j.neuron.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 8.Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 9.Nussbaum RL, Polymeropoulos MH. Genetics of Parkinson's disease. Hum Mol Genet. 1997;6:1687–1691. doi: 10.1093/hmg/6.10.1687. [DOI] [PubMed] [Google Scholar]
- 10.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 11.Vila M, Przedborski S. Genetic clues to the pathogenesis of Parkinson's disease. Nat Med. 2004;10(Suppl):S58–62. doi: 10.1038/nm1068. [DOI] [PubMed] [Google Scholar]
- 12.Lotharius J, Barg S, Wiekop P, Lundberg C, Raymon HK, et al. Effect of mutant alpha-synuclein on dopamine homeostasis in a new human mesencephalic cell line. J Biol Chem. 2002;277:38884–38894. doi: 10.1074/jbc.M205518200. [DOI] [PubMed] [Google Scholar]
- 13.Mosharov EV, Staal RG, Bove J, Prou D, Hananiya A, et al. Alpha-synuclein overexpression increases cytosolic catecholamine concentration. J Neurosci. 2006;26:9304–9311. doi: 10.1523/JNEUROSCI.0519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, et al. The Parkinson's disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci U S A. 2008;105:145–150. doi: 10.1073/pnas.0710685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gitler AD, Chesi A, Geddie ML, Strathearn KE, Hamamichi S, et al. Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet. 2009;41:308–315. doi: 10.1038/ng.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auluck PK, Bonini NM. Pharmacological prevention of Parkinson disease in Drosophila. Nat Med. 2002;8:1185–1186. doi: 10.1038/nm1102-1185. [DOI] [PubMed] [Google Scholar]
- 17.Feany MB, Bender WW. A Drosophila model of Parkinson's disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Feany MB. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat Neurosci. 2005;8:657–663. doi: 10.1038/nn1443. [DOI] [PubMed] [Google Scholar]
- 19.Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, et al. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson's disease. Science. 2007;317:516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- 20.Lakso M, Vartiainen S, Moilanen AM, Sirvio J, Thomas JH, et al. Dopaminergic neuronal loss and motor deficits in Caenorhabditis elegans overexpressing human alpha-synuclein. J Neurochem. 2003;86:165–172. doi: 10.1046/j.1471-4159.2003.01809.x. [DOI] [PubMed] [Google Scholar]
- 21.Kuwahara T, Koyama A, Gengyo-Ando K, Masuda M, Kowa H, et al. Familial Parkinson mutant alpha-synuclein causes dopamine neuron dysfunction in transgenic Caenorhabditis elegans. J Biol Chem. 2006;281:334–340. doi: 10.1074/jbc.M504860200. [DOI] [PubMed] [Google Scholar]
- 22.Cao S, Gelwix CC, Caldwell KA, Caldwell GA. Torsin-mediated protection from cellular stress in the dopaminergic neurons of Caenorhabditis elegans. J Neurosci. 2005;25:3801–3812. doi: 10.1523/JNEUROSCI.5157-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lints R, Emmons SW. Regulation of sex-specific differentiation and mating behavior in C. elegans by a new member of the DM domain transcription factor family. Genes Dev. 2002;16:2390–2402. doi: 10.1101/gad.1012602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lints R, Emmons SW. Patterning of dopaminergic neurotransmitter identity among Caenorhabditis elegans ray sensory neurons by a TGFbeta family signaling pathway and a Hox gene. Development. 1999;126:5819–5831. doi: 10.1242/dev.126.24.5819. [DOI] [PubMed] [Google Scholar]
- 25.Sawin ER, Ranganathan R, Horvitz HR. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–631. doi: 10.1016/s0896-6273(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 26.Hsu AL, Feng Z, Hsieh MY, Xu XZ. Identification by machine vision of the rate of motor activity decline as a lifespan predictor in C. elegans. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng Z, Li W, Ward A, Piggott BJ, Larkspur ER, et al. A C. elegans model of nicotine-dependent behavior: regulation by TRP-family channels. Cell. 2006;127:621–633. doi: 10.1016/j.cell.2006.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W, Feng Z, Sternberg PW, Xu XZ. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature. 2006;440:684–687. doi: 10.1038/nature04538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geng W, Cosman P, Berry CC, Feng Z, Schafer WR. Automatic tracking, feature extraction and classification of C elegans phenotypes. IEEE Trans Biomed Eng. 2004;51:1811–1820. doi: 10.1109/TBME.2004.831532. [DOI] [PubMed] [Google Scholar]
- 30.Pezzella A, d'Ischia M, Napolitano A, Misuraca G, Prota G. Iron-mediated generation of the neurotoxin 6-hydroxydopamine quinone by reaction of fatty acid hydroperoxides with dopamine: a possible contributory mechanism for neuronal degeneration in Parkinson's disease. J Med Chem. 1997;40:2211–2216. doi: 10.1021/jm970099t. [DOI] [PubMed] [Google Scholar]
- 31.Spencer JP, Jenner P, Daniel SE, Lees AJ, Marsden DC, et al. Conjugates of catecholamines with cysteine and GSH in Parkinson's disease: possible mechanisms of formation involving reactive oxygen species. J Neurochem. 1998;71:2112–2122. doi: 10.1046/j.1471-4159.1998.71052112.x. [DOI] [PubMed] [Google Scholar]
- 32.Lotharius J, Brundin P. Impaired dopamine storage resulting from alpha-synuclein mutations may contribute to the pathogenesis of Parkinson's disease. Hum Mol Genet. 2002;11:2395–2407. doi: 10.1093/hmg/11.20.2395. [DOI] [PubMed] [Google Scholar]
- 33.Lotharius J, Brundin P. Pathogenesis of Parkinson's disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci. 2002;3:932–942. doi: 10.1038/nrn983. [DOI] [PubMed] [Google Scholar]
- 34.Duerr JS, Frisby DL, Gaskin J, Duke A, Asermely K, et al. The cat-1 gene of Caenorhabditis elegans encodes a vesicular monoamine transporter required for specific monoamine-dependent behaviors. J Neurosci. 1999;19:72–84. doi: 10.1523/JNEUROSCI.19-01-00072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shim J, Umemura T, Nothstein E, Rongo C. The unfolded protein response regulates glutamate receptor export from the endoplasmic reticulum. Mol Biol Cell. 2004;15:4818–4828. doi: 10.1091/mbc.E04-02-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sze JY, Zhang S, Li J, Ruvkun G. The C. elegans POU-domain transcription factor UNC-86 regulates the tph-1 tryptophan hydroxylase gene and neurite outgrowth in specific serotonergic neurons. Development. 2002;129:3901–3911. doi: 10.1242/dev.129.16.3901. [DOI] [PubMed] [Google Scholar]
- 37.Caudle WM, Colebrooke RE, Emson PC, Miller GW. Altered vesicular dopamine storage in Parkinson's disease: a premature demise. Trends Neurosci. 2008;31:303–308. doi: 10.1016/j.tins.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Hobert O, Tessmar K, Ruvkun G. The Caenorhabditis elegans lim-6 LIM homeobox gene regulates neurite outgrowth and function of particular GABAergic neurons. Development. 1999;126:1547–1562. doi: 10.1242/dev.126.7.1547. [DOI] [PubMed] [Google Scholar]
- 39.Alerte TN, Akinfolarin AA, Friedrich EE, Mader SA, Hong CS, et al. Alpha-synuclein aggregation alters tyrosine hydroxylase phosphorylation and immunoreactivity: lessons from viral transduction of knockout mice. Neurosci Lett. 2008;435:24–29. doi: 10.1016/j.neulet.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tehranian R, Montoya SE, Van Laar AD, Hastings TG, Perez RG. Alpha-synuclein inhibits aromatic amino acid decarboxylase activity in dopaminergic cells. J Neurochem. 2006;99:1188–1196. doi: 10.1111/j.1471-4159.2006.04146.x. [DOI] [PubMed] [Google Scholar]
- 41.Park SS, Lee D. Selective loss of dopaminergic neurons and formation of Lewy body-like aggregations in alpha-synuclein transgenic fly neuronal cultures. Eur J Neurosci. 2006;23:2908–2914. doi: 10.1111/j.1460-9568.2006.04844.x. [DOI] [PubMed] [Google Scholar]
- 42.Wersinger C, Sidhu A. Attenuation of dopamine transporter activity by alpha-synuclein. Neurosci Lett. 2003;340:189–192. doi: 10.1016/s0304-3940(03)00097-1. [DOI] [PubMed] [Google Scholar]
- 43.Kehr W, Carlsson A, Lindqvist M, Magnusson T, Atack C. Evidence for a receptor-mediated feedback control of striatal tyrosine hydroxylase activity. J Pharm Pharmacol. 1972;24:744–747. doi: 10.1111/j.2042-7158.1972.tb09104.x. [DOI] [PubMed] [Google Scholar]
- 44.Bozzi Y, Borrelli E. Dopamine in neurotoxicity and neuroprotection: what do D2 receptors have to do with it? Trends Neurosci. 2006;29:167–174. doi: 10.1016/j.tins.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Yang Q, She H, Gearing M, Colla E, Lee M, et al. Regulation of neuronal survival factor MEF2D by chaperone-mediated autophagy. Science. 2009;323:124–127. doi: 10.1126/science.1166088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wragg RT, Hapiak V, Miller SB, Harris GP, Gray J, et al. Tyramine and octopamine independently inhibit serotonin-stimulated aversive behaviors in Caenorhabditis elegans through two novel amine receptors. J Neurosci. 2007;27:13402–13412. doi: 10.1523/JNEUROSCI.3495-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stiernagle T. Maintenance of C. elegans. WormBook. 2006:1–11. doi: 10.1895/wormbook.1.101.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duerr JS. Immunohistochemistry. WormBook. 2006:1–61. doi: 10.1895/wormbook.1.105.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunohistochemical analysis of hαSyn expression in transgenic C. elegans. A–D, Confocal images of a formaldehyde-fixed day 2 adult worm with DAergic neuron specific expression of hαSyn and DsRed. A, Bright field (BF). B, DsRed. C, hαSyn immunostaining (green). D. Merged image of B and C.
(0.80 MB TIF)
Immunohistochemical analysis of TH expression in transgenic C. elegans. A–B, Confocal images of formaldehyde-fixed day 0 (A) or day 10 (B) worm with DAergic neuron specific expression of hαSyn and DsRed. Left, TH immuostaining; Middle, DsRed; Right, Merged image of TH staining and DsRed; C, Quantification of DAergic neuron degeneration by using TH staining (green) or DsRed (Red). Data represents mean ± S.E.M., n = 10. 1 represents 6.8 and 6.3 DAergic neurons in DsRed and TH staining experiments, representatively.
(0.69 MB TIF)
Correlation of DAergic neuron degeneration with CAT-2::GFP and DsRed. A–B, Confocal images of living day 0 (A) or day 10 worms (B) with DAergic neuron specific expression of CAT-2::GFP, DsRed and hαSyn. Left, CAT-2::GFP; Middle, DsRed; Right, Merged image of CAT-2::GFP and DsRed. (C) Quantification of DAergic neuron degeneration by using CAT-2::GFP (green) or DsRed (red) in hαSyn-expressing (diamonds) and control (circles) lines. Data represent mean ± S.E.M., n = 30. Error bars may hide in symbols. ***, p<0.005 (Two-way ANOVA to compare hαSyn expressing and control line) (green: CAT-2::GFP; Red DsRed). 1 represents 7.9 and 7.7 in DsRed and CAT-2/TH::GFP experiments, respectively.
(1.44 MB TIF)
hαSyn expression does not affect serotonin neurotransmission. Enhanced slowing responses of day 2 adult worms. GFP indicates a wild type worm line expressing GFP in DAergic neurons. Food response experiments were conducted with (grey bars) or without (black bars) food.
(0.23 MB TIF)
hαSyn expression disrupts dopamine synaptic vesicle distribution in TH/CAT-2 knockout background. A–D, Typical bright field (A) or confocal laser scanning VMAT/CAT-1::GFP (B–D) images of living L2 worms expressing both VMAT/CAT-1::GFP and hαSyn in a TH/CAT-2 knockout background. C and D are magnified areas of B that show DAergic and serotonergic somas (C) or DAergic dendrites of CEPs (D), respectively. E, Quantification of CAT-1::GFP redistribution in CEPs of L2 worms expressing both VMAT/CAT-1::GFP and hαSyn in wild type (n = 5) or a TH/CAT-2 knockout background (n = 5). Error bar:SEM.
(0.98 MB TIF)