Abstract
The interleukin (IL)-1 family cytokines are regulated on transcriptional and posttranscriptional levels. Pattern recognition and cytokine receptors control pro-IL-1β transcription while inflammasomes regulate the proteolytic processing of pro-IL-1β. The NLRP3 inflammasome, however, assembles in response to extracellular ATP, poreforming toxins or crystals only in the presence of proinflammatory stimuli. How activation of gene transcription by signaling receptors enables the NLRP3 activation remains elusive and controversial. Here, we show that cell priming through multiple signaling receptors induce NLRP3 expression, which we identified to be a critical checkpoint for NLRP3 activation. Signals provided by NF-κB activators are necessary but not sufficient for NLRP3 activation and a second stimulus, such as ATP or crystal-induced damage is required for NLRP3 activation.
Introduction
Members of the Toll-like receptor (TLR) and C-type lectin receptor families signal in response to microbial or altered endogenous molecules when presented extracellularly or in endo-lysosomal compartments (1, 2, 3). In the cytoplasm, Nod-like receptors (NLRs) and Rig-I-like helicases respond to defined microbial components gaining access to the cytosol (3).
Most innate signaling receptors respond to a relatively restricted ligand spectrum (4). In contrast, diverse molecular entities including bacteria, viruses, purified microbial products, components of dying cells, small molecule immune activators and crystalline or aggregated materials can activate the NLR protein NLRP3 (5). The molecular mechanisms of how NLRP3 can recognize such a diverse array of activators and the role of transcriptionally active signaling receptors for the activation of the NLRP3 inflammasome are controversial and mechanistically poorly understood (2, 5–8). Upon activation, NLRP3 forms a so-called inflammasome complex with the adaptor molecule ASC, which controls the activation of caspase-1. Activated caspase-1, in turn, cleaves pro-IL-1β and pro-IL-18 into the biologically active, secreted forms (9).
Here, we demonstrate expression of NLRP3 itself is tightly controlled by the activity of multiple signaling receptors. We reveal that enhanced expression of NLRP3 in response to NF-κB is sufficient for NLRP3 inflammasome activation by ATP or pore-forming toxins or crystals. Thus, macrophages need to acquire a licensing signal provided by a transcriptionally active signaling receptor, which enables them to respond to NLRP3 activators.
Material and methods
Mice
Mice were kindly provided as indicated: NLRP3-KO and ASC-KO (Millenium Pharmaceuticals); TLR2-KO, TLR4-KO, TLR7-KO, IRAK4-KO, MAL-KO, TRIF-KO, MyD88-KO and TRAM-KO (S. Akira, Osaka University, Japan); TLR3-KO (R. Flavell, Yale University, New Haven); MD2-KO (K. Miyake, Tokyo University, Japan). C57BL/6 were purchased from Jackson Laboratories. All animal experiments were approved by the UMass Animal Care and Use Committee.
Reagents
Adenosin triphosphate (ATP), poly(dA-dT), muramyl dipeptide (MDP), nigericin, cycloheximide and Bay11-7082 were from Sigma-Aldrich. Pam2CysK4, poly(I:C), ultra-pure LPS, R848, iE-DAP were from Invivogen. Anti-TLR4 Abs (UT18 and MTS510) were from eBioscience. The anti-NLRP3 pAB was raised against the NLRP3 pyrin domain that was expressed in E. coli. mAb against mouse pro-IL-1β was from the National Cancer Institute and the mouse α-actin mAb was from Sigma.
Cell stimulation and analysis
Human PBMCs were isolated by density-gradient centrifugation, stimulated in complete RPMI and the caspase-1 FMK-YVAD-FMK FLICA kit (Immunochemistry Technologies) was used to stain active caspase-1. CD14-positive cells were analyzed for FLICA positivity by flow cytometry. The respective local ethics committees approved experiments involving PBMCs. Immortalized macrophage cell lines were generated as described (10). Caspase-1 was detected in serum-free cell supernatants or cell lysates by SDS PAGE using caspase-1 pAb (sc-514; Santa Cruz). Human ASC and NLRP3 were cloned from cDNA into the lentiviral plasmid FugW and immortalized macrophages were transduced as described (12). Quantitative real-time PCR was performed as described (12). Primer sequences are available upon request. The mouse NLRP3 promoter (-3000 bp to 0 bp upstream of the transcription start site 1) was cloned from cDNA into pGL3-basic. HEK293T cells were transfected with luciferase reporter plasmid and expression plasmids (100ng each) using Lipofectamine.
Microscopy
A Leica SP2 AOBS confocal LSM was used. ASC pyroptosomes were quantified by epifluorescence microscopy and ImageJ software.
Results and discussion
Assembly of the NLRP3 inflammasome requires priming signals functioning upstream of ASC
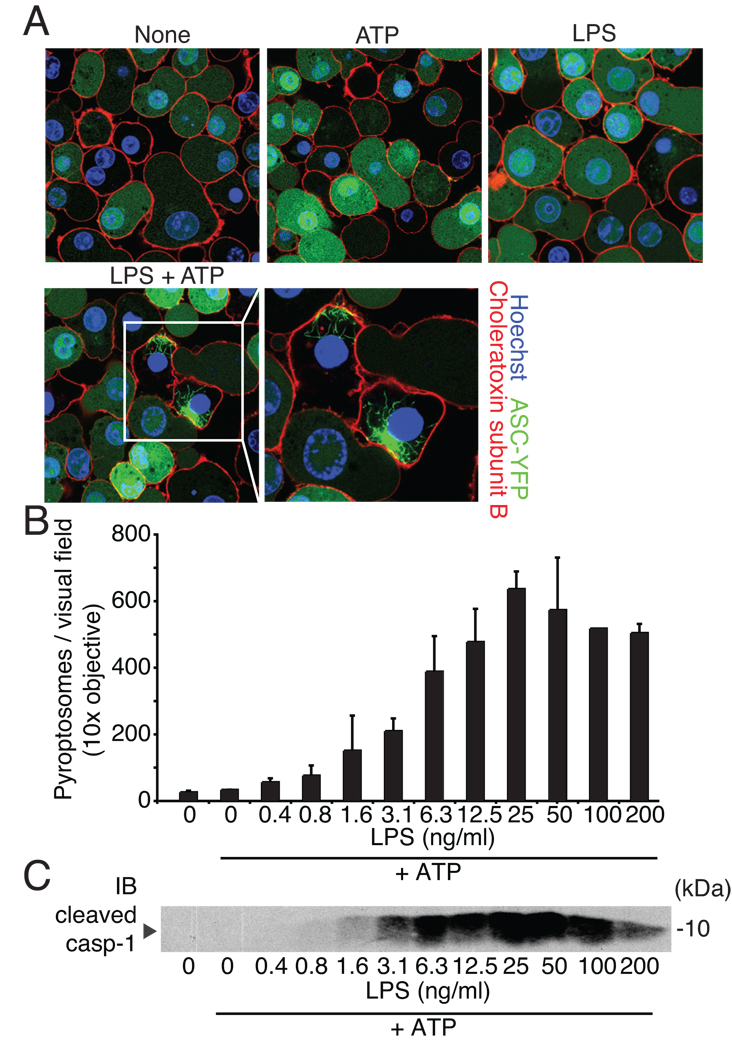
Fluorescent ASC forms speck-like structures, termed pyroptosomes, upon activation (13). We generated immortalized mouse macrophages expressing ASC-YFP to test whether the NLRP3 inflammasome can assemble upstream of caspase-1 in the absence of priming. Resting cells or cells treated with ATP or LPS alone uniformly expressed ASC-YFP throughout the cells. However, when LPS-primed cells were treated with ATP, ASC-YFP formed large, irregularly shaped pyroptosomes indicating that LPS signaling was also required for ASC pyroptosome formation upon NLRP3 activation (Fig. 1A). Thus, mouse macrophages require two stimuli for NLRP3 inflammasome activation even at the level of ASC similar to what is observed at the level of caspase-1 (Supplementary Fig. 1A and (6)). Furthermore, pyroptosomes dose-responsively formed in response to LPS and ATP and caspase-1 cleavage closely correlated with the number of pyroptosomes (Fig. 1C, D and Supplementary Fig. 1B). Synthetic LPS or TLR2 and 7 activators also induced pyroptosomes together with ATP (Supplementary Fig. 1C, D) suggesting that TLR activators and not an undefined contaminant were responsible for pyroptosome formation in response to ATP. In line with previous observations, pyroptosome formation in response to the AIM2 inflammasome activator - transfected double-stranded DNA (poly(dA-dT)) - did not require LPS priming (Supplementary Fig. 1B and (12, 14)).
Figure 1. Caspase-1 activation and the formation of ASC pyroptosomes require priming.
(A) C57BL/6 expressing ASC-YFP left untreated or stimulated as indicated and counterstained for membranes (choleratoxin subunit B (red)) and nuclei (Hoechst dye (blue)) were analyzed by confocal microscopy. (B) ASC-CFP pyroptosome formation after LPS priming and subsequent ATP stimulation was analyzed by epifluorescence microscopy. (C) Immunoblot for cleaved caspase-1 in supernatants from cells stimulated as in (B). Data are representative of three independent experiments (error bars, s.d. in B).
Different signaling receptor family members are able to license NLRP3 inflammasome activation
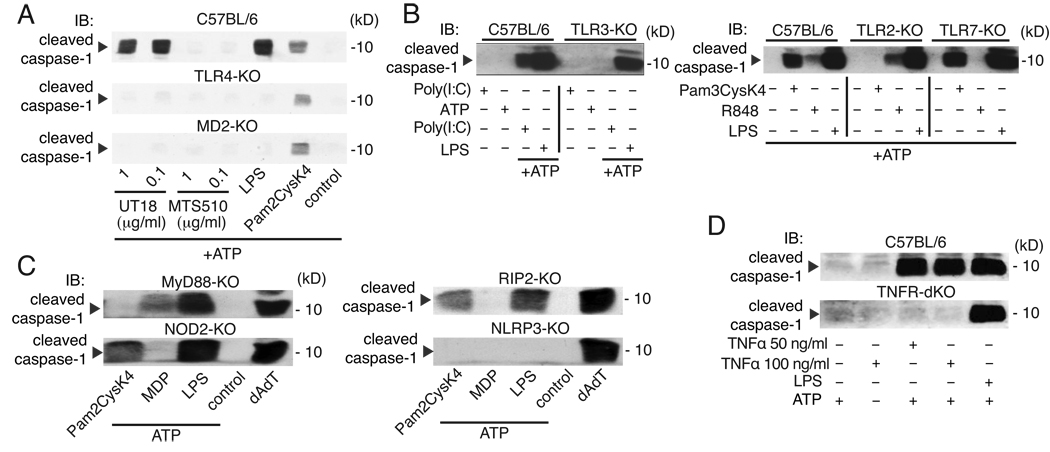
We next aimed at dissecting the influence of TLR4 signaling on NLRP3 inflammasome activation from TLR-independent mechanisms. We made use of an activating anti-TLR4/MD-2 antibody (clone UT18) and tested whether activation of TLR4 in the absence of LPS was sufficient for priming of the NLRP3 inflammasome. Pre-incubation with an activating antibody, but not with a blocking TLR4/MD-2 antibody (clone MTS510), led to the cleavage of caspase-1 in wild-type macrophages when stimulated with ATP. Furthermore, activation by the stimulating Ab was dependent on TLR4 and MD-2 consistent with the fact that it fails to bind and activate TLR4 or MD-2 alone (15) (Fig. 2A). Furthermore, activation of TLRs 2, 3 and 7 also induced priming of the NLRP3 inflammasome and macrophages lacking TLRs for the respective stimuli failed to activate caspase-1 after activation via ATP (Fig. 2B).
Figure 2. PRR activation is required for NLRP3 inflammasome activation.
(A) Immunoblot of caspase-1 in wild type or knock-out macrophages primed for 4h with LPS (200 ng/ml), Pam2CysK4 (50 ng/ml), TLR4 activating (UT18), or TLR4 blocking (MTS510) antibodies and subsequently stimulated with ATP (1h). Cells from wild-type or knock-out macrophages were left untreated or stimulated for 4h with (B) poly(I:C) (1 µg/ml), Pam2CysK4 (50 ng/ml), or R848 (0.5 µg/ml) or with (C) Pam2CysK4 (50 ng/ml), MDP (10 µg/ml), LPS (200 ng/ml), or transfected with poly(dA-dT). ATP was added for 1h as indicated. (D) Wild type or TNFR1/2 dKO macrophages were stimulated with TNFα as indicated or with LPS as in (A) and analyzed for caspase-1 activation. Immunoblot analyses of cleaved caspase-1 from supernatants are show. Data are from one representative experiment out of three (A, B) or two (C–D).
We next analyzed the requirement for TLR signaling for NLRP3 priming. Macrophages deficient in MyD88 or TRIF responded normally to LPS and ATP with cleavage of caspase-1, while macrophages doubly deficient in both MyD88 and TRIF or TLR4 failed to respond (Supplementary Fig. 2A). These results show that both TRIF-and MyD88-dependent signaling pathways can compensate for each other in their ability to induce priming for NLRP3 activation. Of note, the priming of macrophages was also a necessary step for NLRP3 inflammasome activation by other established NLRP3 activators such as the pore-forming toxin nigericin or crystalline activators (Supplementary Fig. 2B, C) suggesting that priming is generally required for NLRP3 inflammasome activation.
MDP, which engages NOD2, also mediated priming for ATP responsiveness and the activity was dependent on RIP2, the downstream signaling transducer of this signaling pathway (Fig. 2C). We further observed that priming with a cytokine stimulus (TNF-α) was sufficient to induce caspase-1 activation by ATP. Notably, TNF-α failed to prime cells obtained from mice doubly deficient in TNFR1 and 2, while LPS was able to prime these cells (Fig. 2D). Collectively, these results suggest that multiple transcriptionally active signaling receptors can prime macrophages for subsequent NLRP3 inflammasome activation.
Priming is required for NLRP3 activation in human monocytes
Interleukin-1 receptor-associated kinase 4 (IRAK4) is essentially required for signal transduction to NF-κB downstream of MyD88 (24). Indeed, ligands for TLRs that exclusively signal via the adapter MyD88 also failed to prime IRAK4-deficient macrophages for NLRP3-mediated ATP reactivity while signaling cascades that operate independently of MyD88 induced priming IRAK4-independently (Supplementary Fig. 3A). This differential requirement for priming allowed us to address the role of priming for NLRP3 inflammasome activation in the human system. We obtained monocytes from healthy volunteers and from a patient with a loss-of-function mutation (Q293X) in IRAK4(25) and tested their ability to activate caspase-1 upon ATP stimulation after priming with IRAK-4-dependent and -independent stimuli. Only primed monocytes displayed a robust caspase-1 activation upon ATP stimulation while the AIM2 inflammasome activation was independent this type of priming (Supplementary Fig. 3B). When primed with IRAK-4 independent ligands monocytes from the IRAK4-mutant patient responded robustly with caspase-1 activation after ATP stimulation. In contrast and consistent with IRAK4 deficiency in the murine system, we failed to observe priming activity towards ATP after TLR2 or TLR7/8 stimulation (Supplementary Fig. 3B). Thus, these data indicate that NLRP3 activation is also critically dependent on priming activity by signaling receptors in human cells.
NF-κB-dependent signals regulate NLRP3 expression
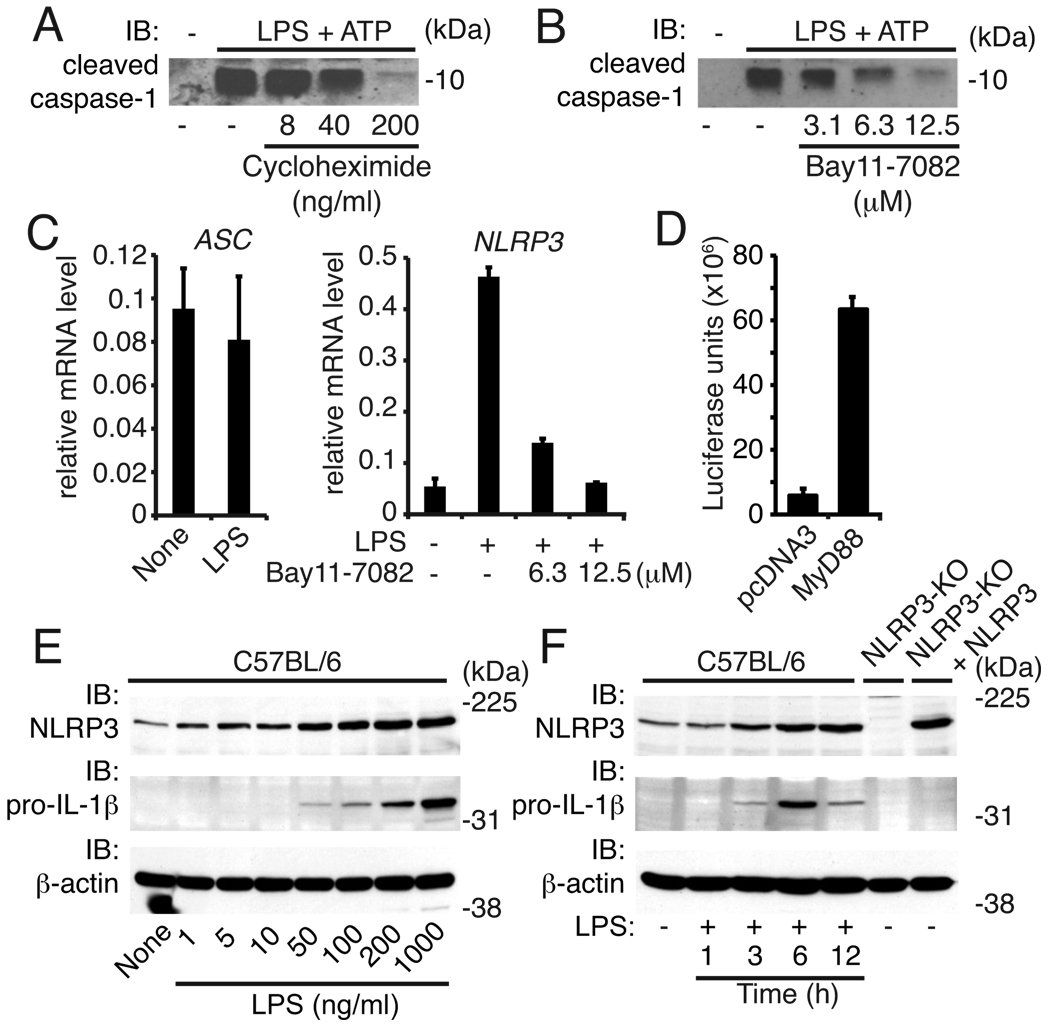
Treatment of macrophages with the protein synthesis inhibitor cycloheximide dose-responsively led to reduced caspase-1 activation obtained by the combination of LPS and ATP indicating that protein de novo synthesis was functionally limiting in mouse macrophages (Fig. 3A). In addition, priming of the NLRP3 inflammasome was dose-dependently reduced by a specific inhibitor of NF-κB (Bay11-7082) suggesting a key role for NF-κB for priming (Fig. 3B).
Figure 3. NLRP3 induction involves NF-κB activity.
Caspase-1 immunoblot from supernatants of wild type macrophages pretreated with (A) cycloheximide or (B) Bay11-7082 as indicated for 1h followed by LPS (200 ng/ml, 4h) and stimulated with ATP (1h). (C) Messenger RNA expression of ASC or NLRP3 in LPS primed or untreated macrophages. Cells were pretreated with Bay11-7082 for 1h where indicated. (D) HEK293T were transfected with pcDNA3-MyD88 or control (pcDNA3) together with a NLRP3 promoter reporter and assessed for luciferase activity after 20h. (E) Immunoblots for NLRP3, pro-IL-1β and β-actin in lysates from C57BL/6 macrophages treated with LPS for 6h as indicated, or, (F) treated with LPS (200 ng/ml) for the indicated periods of time. Controls are lysates from NLRP3-KO macrophages with and without heterologous NLRP3 expression. Data are from one representative experiment of three (A-D) or of two (E, F) experiments (error bars, s.d.).
Overexpression of ASC is not sufficient to overcome the priming requirement for NLRP3 activation (Fig. 1) suggesting that the NF-κB-induced activity was acting upstream of ASC. Consistent with this idea, we found that LPS stimulation did not change Asc mRNA levels but led to strong, NF-κB-dependent, increases in Nlrp3 mRNA in mouse macrophages (Fig. 3C). These studies are in line with a report demonstrating NLRP3 induction by TNF and TLR ligands in human cells (16). To analyze the putative Nlrp3 promoter activity, we cloned the promoter region entailing −3000 to 0 bp upstream of the Nlrp3 transcription start site and constructed a luciferase reporter gene construct. We made use of the fact that heterologous overexpression of MyD88 activates downstream signaling and therefore expressed the Nlrp3 promoter-luciferase construct alone or together with MyD88. Indeed, co-expression of the Nlrp3 promoter-luciferase construct with MyD88 led to a ~10-fold induction of luciferase activity indicating that MyD88-mediated signaling can activate the promoter of Nlrp3. Notably, around 40% of patients with autoinflammatory diseases present with classical clinical symptoms without carrying any mutations in the coding region of NLRP3 (17). Recently, unique NLRP3 promoter sequence variants leading to enhanced NLRP3 promoter activity were identified in patients with autoinflammatory diseases that lack NLRP3 coding sequence mutations. This suggest that dysregulated NLRP3 expression could evoke autoinflammatory symptoms (18).
We further found that LPS stimulation led to both NLRP3 and pro-IL-1β protein induction in a dose- and time-dependent manner (Fig. 3E, F). In addition, LPS failed to induce NLRP3 or pro-IL-1β in cells lacking TLR4 or doubly-deficient in MyD88 and TRIF and NF-κB inhibition led to a dose-dependent reduction of NLRP3 protein induction by L P S (Supplementary Fig. 4A, B). Collectively, these data indicate that NLRP3 expression is tightly controlled by signals culminating in the activation of NF-κB.
NLRP3 expression is a limiting factor for NLRP3 inflammasome activation
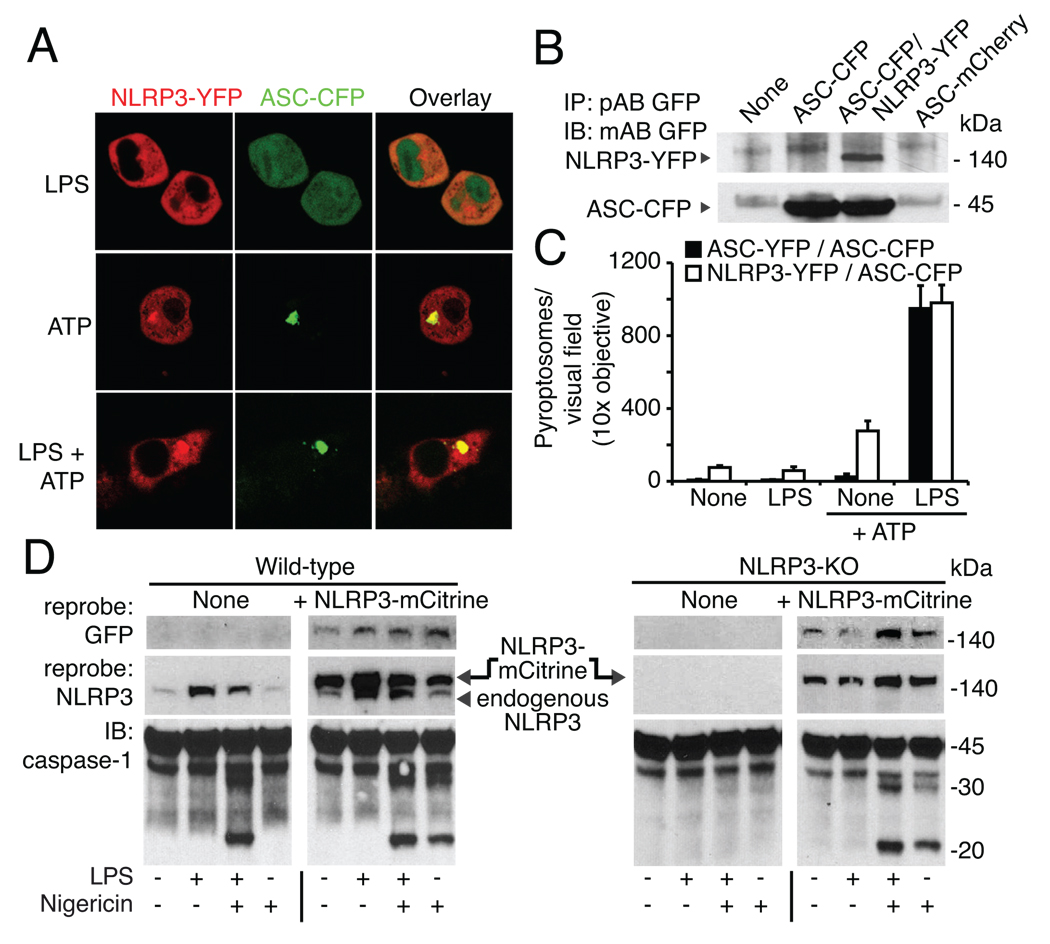
To determine if enhanced expression of NLRP3 was not only required, but also sufficient for NLRP3 inflammasome activation by a ‘bona fide’ NLRP3 stimulus, we next generated macrophage cell lines expressing NLRP3 controlled by a constitutively active viral promoter (Fig. 4A–D). In contrast to cell lines overexpressing ASC alone, cells that overexpressed fluorescent NLRP3 together with fluorescent ASC responded to NLRP3 stimuli in the absence of a prior priming step with the formation of NLRP3/ASC pyroptosomes (Fig. 4A, C). We next assessed caspase-1 activity in wild-type and NLRP3-deficient macrophages with and without overexpressed NLRP3. Consistent with earlier results, LPS priming led to significant induction of NLRP3 protein in wild-type cells and LPS was required for caspase-1 cleavage in combination with an NLRP3 activator. NLRP3-mCitrine overexpressing wild-type cells, however, responded to nigericin (or ATP, data not shown) in the absence of prior priming (Fig. 4D; left). Moreover, heterologous expression of NLRP3-mCitrine functionally reconstituted NLRP3-KO macrophages, which also did not require priming to induce a functionally active NLRP3 inflammasome (Fig. 4D, right). Notably, overexpression of NLRP3 per se had no influence on caspase-1 cleavage in the absence of nigericin stimulation demonstrating the need for an NLRP3 activator at this level of expression. Altogether, these results establish that the requirement for priming of the NLRP3 inflammasome can be explained by the restricted expression of NLRP3 itself, and that the NLRP3 expression level is a limiting step for the NLRP3 inflammasome activation in macrophages (2, 5). We revealed that the NLRP3 inflammasome is, in fact, rather restricted in its ability to directly recognize microbial derived substances. We found that the activation of the NLRP3 inflammasome requires two steps, which are controlled by different mechanisms. First, NLRP3 expression itself needs to be transcriptionally induced, and a second, posttranscriptional step leads to the activation of NLRP3 allowing for NLRP3 inflammasome assembly. The key mediator of immunity, NF-κB, plays a critical role for the priming of the NLRP3 inflammasome. We thus speculate that other NF-κB activators such as UV light or reactive oxygen species can also induce NLRP3 priming.
Figure 4. Stable NLRP3 expression is sufficient for priming-independent NLRP3 activation.
Macrophages were stimulated for 4h and ATP or nigericin were added for an additional 1h as indicated. (A) Confocal microscopy of caspase-1-KO macrophages expressing ASC-CFP and NLRP3-YFP. (B) Expression of ASC-CFP and NLRP3-YFP assessed by GFP immunoblot after GFP immunoprecipitation. (C) ASC pyroptosome quantification in caspase-1-KO macrophages expressing ASC-CFP and NLRP3-YFP or ASC-CFP and ASC-YFP (D) Immortalized C57BL/6 (left) or NLRP3-KO (right) macrophages with or without stable expression of NLRP3-mCitrine were left untreated or primed with LPS (200 ng/ml) for (4h), and stimulated with nigericin for 1h as indicated. Caspase-1 immunoblots of combined supernatant and cell lysates are shown. NLRP3 expression was verified by immunoblotting for GFP and NLRP3. One representative experiment out of three (A, C-D) or two (B) is shown.
The fact that priming is a necessary step for NLRP3 inflammsome assembly suggests that macrophages need to acquire a signal that indicates either the presence of infection (via activation of PRRs by microbial products) or the activation of other cells (via the presence of pro-inflammatory cytokines) in order to commit to sense danger signals in their immediate environment via the activation of the NLRP3 inflammasome. This dual stimulation requirement may operate to prevent accidental or uncontrolled NLRP3 activation, which can have devastating consequences for the host as exemplified by the clinical presentation of patients with auto-inflammatory diseases (5, 17).
Supplementary Material
Acknowledgements
We would like to acknowledge P. Vandenabeele (University of Ghent, Ghent, Belgium) for supplying a caspase-1 p20 antibody.
This work was supported by grants from the NIH AI-065483 (to E.L.), AI-067497 (to K.A.F.) and and AG14357 and AR055398 (to E. S. A.), by a grant of the Dana Foundation (to E.L.), by the German Research Foundation (to V.H., Ho2783/2-1) and a grant by the Canadian Institutes for Health Research (to D. P. S.).
References
- 1.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annual review of immunology. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 2.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi O, Akira S. MDA5/RIG-I and virus recognition. Curr Opin Immunol. 2008;20:17–22. doi: 10.1016/j.coi.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annual review of immunology. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 6.Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-kappaB-driven protein synthesis. J Immunol. 2005;175:7611–7622. doi: 10.4049/jimmunol.175.11.7611. [DOI] [PubMed] [Google Scholar]
- 7.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annual review of immunology. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 10.Roberson SM, Walker WS. Immortalization of cloned mouse splenic macrophages with a retrovirus containing the v-raf/mil and v-myc oncogenes. Cell Immunol. 1988;116:341–351. doi: 10.1016/0008-8749(88)90236-5. [DOI] [PubMed] [Google Scholar]
- 11.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009 doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohta S, Bahrun U, Shimazu R, Matsushita H, Fukudome K, Kimoto M. Induction of long-term lipopolysaccharide tolerance by an agonistic monoclonal antibody to the toll-like receptor 4/MD-2 complex. Clin Vaccine Immunol. 2006;13:1131–1136. doi: 10.1128/CVI.00173-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Connor W, Jr, Harton JA, Zhu X, Linhoff MW, Ting JP. Cutting edge: CIAS1/cryopyrin/PYPAF1/NALP3/CATERPILLER 1.1 is an inducible inflammatory mediator with NF-kappa B suppressive properties. J Immunol. 2003;171:6329–6333. doi: 10.4049/jimmunol.171.12.6329. [DOI] [PubMed] [Google Scholar]
- 17.Masters SL, Simon A, Aksentijevich I, Kastner DL. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease (*) Annual review of immunology. 2009;27:621–668. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson JP, Mueller JL, Misaghi A, Anderson S, Sivagnanam M, Kolodner RD, Hoffman HM. Initial description of the human NLRP3 promoter. Genes Immun. 2008;9:721–726. doi: 10.1038/gene.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.