Abstract
Objective
The present study investigated the capacity of the adipokine leptin to promote angiogenesis by modulating the function of circulating angiogenic cells (CAC).
Methods and Results
In vitro, leptin specifically promoted CAC adhesion to tubular endothelial structures and migration along outgrowing sprouts of endothelial cells. In vivo, stimulation of CAC with leptin increased their capacity to promote new vessel formation in the chorioallantoic membrane of chicken embryos and to improve neovascularization of ischemic murine hindlimbs. These effects required the phosphorylation of αvβ5 integrins which depended on the interaction of leptin with its receptor ObR, and on JAK2- and PLCγ-mediated activation of Src kinase. Protein tyrosine phosphatase (PTP1)B, a negative regulator of leptin signaling, was overexpressed in CAC from obese, hyperleptinemic individuals, and this was associated with insensitivity of CAC to the angiogenic effects of leptin. Weight loss (by 30±15 kg) normalized PTP1B expression in CAC and restored their responsiveness to leptin. A similar, dose-dependent response was found after incubation of CAC from the obese with a PTP1B inhibitor ex vivo.
Conclusions
Our results point to the ObR-Src kinase-αvβ5 cross-talk as a distinct novel component of the network of specific interactions between integrins and cytokine receptors in angiogenesis.
Keywords: angiogenesis, circulating angiogenic cells, leptin, obesity, PTP1B, Src kinase
Introduction
Angiogenesis, the formation of new blood vessels from the existing vasculature, is a complex, multi-step process involving the proliferation, migration, and remodeling of endothelial cells in response to growth factors and cytokines. The adipose tissue-derived cytokine leptin has been shown to exert proangiogenic effects on endothelial cells,1, 2 and leptin stimulated corneal neovascularization in rats and in leptin-deficient ob/ob mice.3 On the other hand, prevention of leptin binding to its receptor inhibited angiogenesis,4 and leptin receptor-deficient mice exhibited reduced basal capillary density and defective revascularization of ischemic muscles.5
Angiogenesis requires the interplay of various cell types, among which circulating angiogenic cells (CAC) appear to be of particular interest. These cells, formerly considered to represent a subpopulation of endothelial progenitor cells (EPC)6 and now regarded as a distinct (progenitor) cell type,7 are derived from the peripheral blood after expansion in culture. They have been shown to augment neovascularization after tissue ischemia8 and promoted endothelial repair after vascular injury.9 The vasculoprotective properties of CAC and other progenitor cells are defined by their ability to adhere to molecules released from injured tissues, allowing them to home and transmigrate at sites of injury or ischemia.10 These steps are regulated by integrins, the transmembrane receptor heterodimers connecting the extracellular matrix to cytoskeletal and signaling molecules. Importantly, the activation status of integrins appears to be modulated by soluble growth factors and cytokines, and reciprocal communication between signaling pathways has been proposed to underlie the synergism of specific growth factors with integrins involved in angiogenesis.11
In the present study, we began to analyze the effects of leptin on the angiogenic properties of CAC in vitro and in vivo, particularly seeking to dissect the intracellular mechanisms mediating the cross-talk between the leptin receptor and angiogenic integrins. Our findings suggest an important role of the cytoplasmic protein kinase Src in linking leptin receptor activation to β5 integrin phosphorylation, followed by downstream signal transduction and angiogenesis. Constantly elevated leptin levels, such as found in obesity, are accompanied by elevated levels of protein tyrosine phosphatase 1B, a negative regulator of leptin signaling, and an attenuated angiogenic response of CAC to leptin. This latter defect can be fully restored by PTP1B inhibition or, preferably, successful weight loss.
Methods
Cells were isolated from peripheral human blood, cultivated and characterized according to an established protocol.6, 9 They were CD31+, CD34−, CD45+, displayed limited proliferation ability, and did not form de novo blood vessels. Therefore, in accordance with a recently proposed definition,7 the cells used in the present study were termed circulating angiogenic cells. A detailed description of the methods is provided in the online supplemental file.
Results
Leptin Enhances the Angiogenic Properties of CAC in vitro
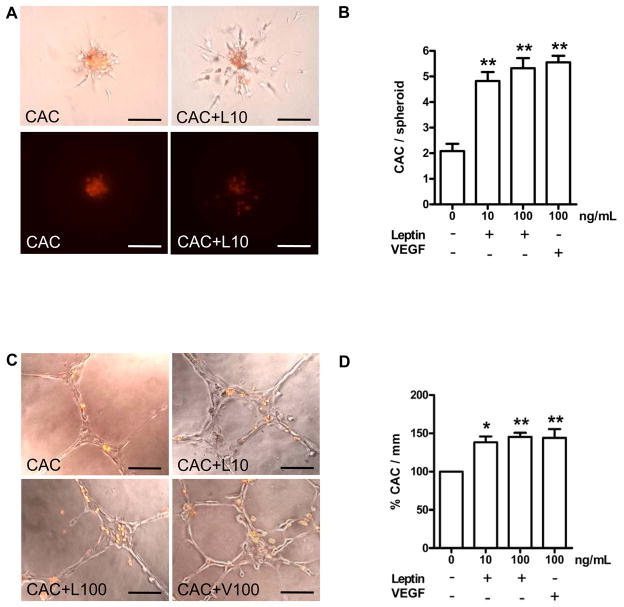
The effects of leptin on the capacity of CAC to interact with co-cultured human endothelial cells (HUVEC) were examined using two in vitro angiogenesis assays. In the three-dimensional spheroid angiogenesis assay, pretreatment of CAC with leptin dose-dependently enhanced the number of CAC adherent to outgrowing HUVEC sprouts (Fig. 1A; summarized findings in Fig. 1B), and it increased the cumulative length of sprouts outgrowing from co-incubated HUVEC (not shown). The effects of leptin were comparable to those of vascular endothelial growth factor (VEGF, 100 ng/mL). In the matrigel assay, preincubation of CAC with leptin (10 and 100 ng/mL) increased the number of CAC adherent to endothelial cell networks provided by HUVEC (Fig. 1C; summarized results, Fig. 1D). Again, the effects of leptin were comparable to those induced by VEGF. The effects of leptin on the CAC-HUVEC interactions could be completely reversed i) by co-incubation with leptin-neutralizing antibodies; ii) by function-blocking antibodies against the leptin receptor; and iii) by siRNA-mediated downregulation of ObR expression (supplemental Fig. IA and B; please see www.ahajournals.org). Of note, stimulation of CAC with leptin did not affect their low proliferation potential or VEGF expression (not shown).
Figure 1.
CAC were control-treated with PBS (CAC), or stimulated with 10 or 100 ng/mL recombinant human leptin (CAC+L10, CAC+L100), or 100 ng/mL VEGF (CAC+V100) for 24 hours, followed by assessment of their angiogenic properties in the spheroid (A, B) or matrigel assay (C, D). Displayed are the number of CAC (red fluorescence signal) migrating along sprouts of co-incubated HUVEC (B) and the number of CAC per mm of endothelial cell networks (D). *P<0.05, and **P<0.01 vs. control-treated CAC defined as 100%. Size bars represent 100 μm.
Leptin Promotes the Angiogenic Properties of CAC in vivo
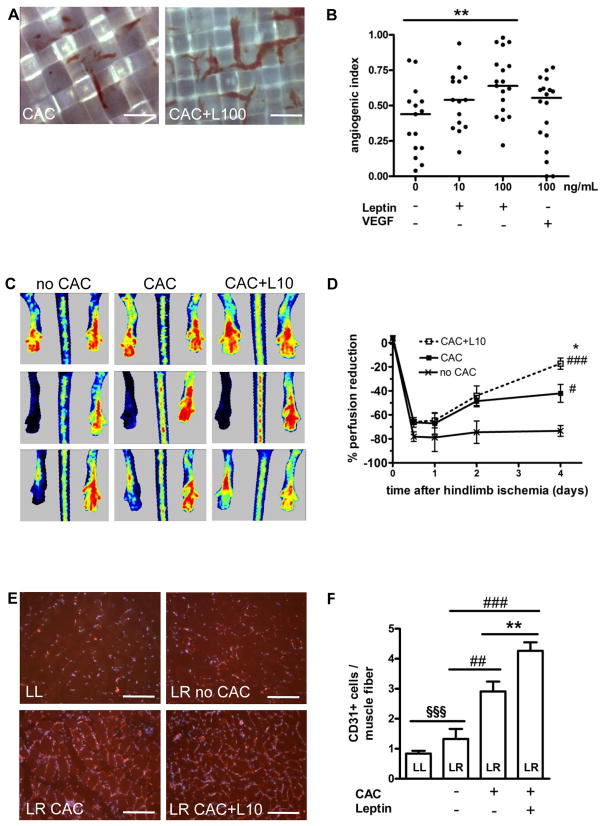
CAC-collagen onplants were placed on top of the chorioallantoic membrane (CAM) of chick embryos. Our analysis revealed that pretreatment of CAC with leptin increased the angiogenic index, defined as the number of grids containing newly formed blood vessels as a proportion of the total number of grids (Fig. 2A, quantitative analysis, Fig. 2B), and similar results were obtained after stimulation of CAC with 100 ng/mL VEGF. The angiogenic effects of leptin could be completely inhibited by preincubation with ObR-neutralizing antibodies (not shown).
Figure 2.
(A) Leptin increased the potential of CAC to promote the ingrowth of blood vessels from the chick CAM into collagen-coated grids. Representative findings after treatment of CAC with 100 ng/mL leptin (CAC+L100) vs. control-treated CAC. Size bars, 50 μm. (B) Summarized results from one out of 5 independent experiments. **P<0.01 for comparison of the first vs. third set of data. (C and D) Laser doppler blood flow measurements in hindlimbs of mice treated with leptin-stimulated vs. control-treated CAC or PBS alone before (upper row), immediately after (middle row) and four days following induction of ischemia (lower panels). *P<0.05 for CAC+L10 vs. CAC; #P<0.05 and ###P<0.001 for CAC+L10 vs. no CAC. (E and F) Quantification of CD31-positive endothelial cells in the non-ischemic lower left (LL) or the ischemic lower right (LR) hindlimb of athymic nude mice treated with CAC compared to those receiving PBS (no CAC), and to CAC pretreated with leptin (CAC+L10). **P<0.01 for CAC+L10 vs. CAC; ##P<0.01 and ###P<0.001 vs. no CAC, §§§P<0.001 for LL vs. LR. Bars, 100 μm.
The effects of leptin on CAC-mediated neovascularization were also tested employing a mouse model of unilateral hindlimb ischemia. Laser doppler measurements revealed a significantly improved tissue perfusion in lower hindlimbs of mice treated with leptin-stimulated CAC (1×106 cells per mouse) compared to those treated with control-stimulated CAC, or medium (no CAC) alone (Fig. 2C and 2D). Immunohistological analysis demonstrated higher numbers of CD31-positive capillary endothelial cells per muscle fiber in the lower hindlimb of mice that received leptin-pretreated CAC (Fig. 2E and 2F).
The Angiogenic Effects of Leptin on CAC are Mediated by Integrin αvβ5
We have previously shown that the leptin receptor (ObR) and αvβ5 integrin are co-expressed on CAC, and that incubation of CAC with leptin enhances αvβ5 integrin surface expression and ligand binding affinity.9 In the present study, pretreatment of CAC with RGD peptides or neutralizing anti-αvβ5 integrin antibodies completely inhibited the angiogenic effects of leptin, both in vitro (matrigel assay; suppl. Fig. IIA; please see www.ahajournals.org) and in vivo (CAM assay; suppl. Fig. IIB), suggesting that integrin αvβ5 plays an important role in mediating the effects of leptin on CAC function.
Leptin Enhances Integrin β5 Phosphorylation in CAC
Incubation of CAC with leptin time-dependently enhanced the phosphorylation of β5 integrin (suppl.Fig. IIIA; please see www.ahajournals.org), without significantly affecting total β5 integrin expression; a significant increase was seen after stimulation for 1 and 10 hours (suppl. Fig. IIIB). The effects of leptin on integrin tyrosine phosphorylation did not extend to β3 integrins. Dose-curve experiments (0.1 to 1000 ng/mL; not shown) revealed maximal β5 phosphorylation after stimulation with the ‘physiological’ leptin concentration of 10 ng/mL. The β5 phosphorylation in response to leptin could be prevented by preincubation with ObR-neutralizing antibodies (suppl. Fig. IIIC). Further experiments revealed co-immunoprecipitation of ObR with β5 integrin after leptin stimulation (suppl. Fig. IIID and E).
Leptin-Induced Src Kinase Activation Mediates the Interaction Between ObR and αvβ5 Integrins
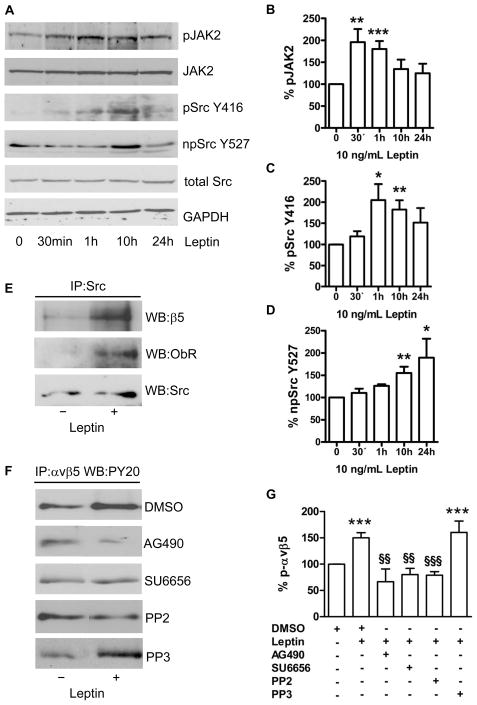
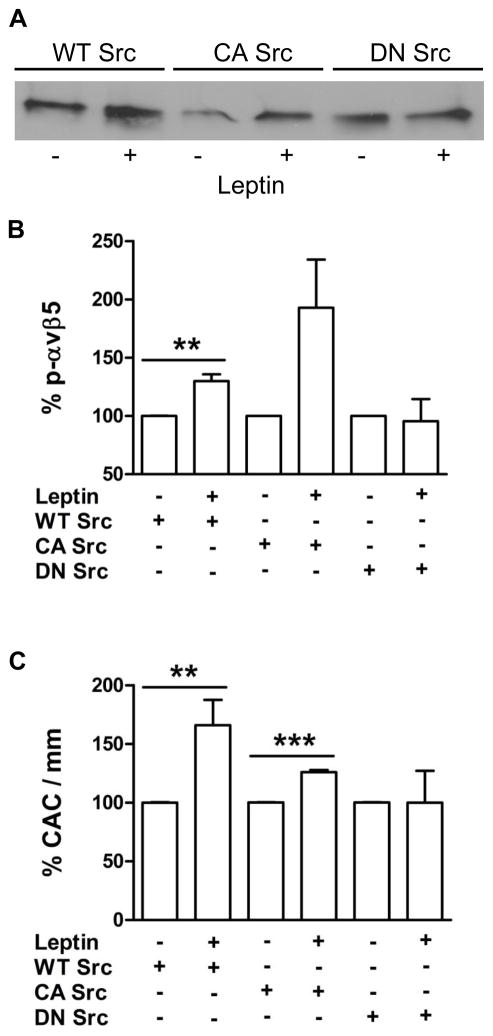
The leptin receptor ObR lacks a cytoplasmic kinase domain and transmits signals through cellular tyrosine kinases, particularly Janus kinase (JAK)2. As shown in Fig. 3A to D, stimulation of CAC with leptin (10 ng/mL) increased JAK2 tyrosine phosphorylation with a maximum effect after 30 to 60 minutes. In addition, leptin time-dependently modulated phosphorylation of Src kinase: Src phosphorylation was enhanced at tyrosine residue (Y)416, indicating Src activation, while dephosphorylation was detected at Y527, the residue located within the autoinhibitory loop of the kinase.12 Stimulation of CAC with leptin increased the amount of ObR andαvβ5 that could be co-immunoprecipitated with Src kinase (Fig. 3E). In further experiments, the effects of leptin on αvβ5 phosphorylation could be inhibited by preincubation of CAC with the JAK inhibitor AG490, or the Src kinase inhibitors SU6656 and pyrazolopyrimidine (PP)2, whereas a nonspecific analogue of the latter (PP3) or DMSO alone had no effect (Fig. 3F and G). Importantly, treatment with SU6656 or PP2, but not with DMSO or PP3, also completely abolished the effects of leptin on the angiogenic properties of CAC, both in vitro (spheroid assay; suppl. Fig. IVA; please see www.ahajournals.org) and in vivo (hindlimb ischemia model; suppl. Fig. IVB and C). Additional studies showed that the leptin-induced Src kinase activation was downstream of ObR and JAK2, since Src phosphorylation (at Y416) could be blocked by transfection of CAC with ObR siRNA (suppl. Fig. VA and B; please see www.ahajournals.org), or preincubation with AG490 or U73122 (an inhibitor of phospholipase C; suppl. Fig. VC and D). Analysis of CAC transfected with either wild-type (WT), constitutive-active (CA), or dominant-negative (DN) mutant forms of Src kinase confirmed that the effects of leptin on αvβ5 integrin phosphorylation (Fig. 4A and B), and on CAC-mediated angiogenesis in the matrigel assay (Fig. 4C) require functional Src kinase.
Figure 3.
(A) Stimulation of CAC with leptin (10 ng/mL) resulted in a time-dependent increase in the phosphorylation of JAK2 and Src kinase at tyrosine (Y) 416, along with dephosphorylation of Src at Y527. (B, C, D) Summarized results from 3–7 experiments. Results were normalized to total JAK2 (for phospho-JAK2) or total Src kinase protein (for phospho-Y416- and non-phospho-Y527-Src) and are expressed as percent of control-treated cells defined as 100%. GAPDH is shown as loading control. *P<0.05; **P<0.01; ***P<0.001. (E) Immunoprecipitation followed by Western blot analysis showed that leptin promoted the association of Src kinase with ObR and integrin β5. (F) The leptin-induced increase of αvβ5 phosphorylation could be reversed by inhibition of JAK2 (AG490) and Src kinase (PP2 or SU6656), whereas PP3 or vehicle (DMSO) had no effect. (G) Summary of 3–5 experiments.***P<0.001 vs. control-treated cells defined as 100%; §§P<0.01, and §§§P<0.001 for leptin-stimulated CAC in the presence vs. absence of the JAK2 or Src kinase inhibitor, respectively.
Figure 4.
Leptin enhanced αvβ5 phosphorylation (A; immunoprecipitation of αvβ5 followed by Western blot for phosphotyrosines; quantitative analysis of 4 experiments shown in B), and the angiogenic properties (C; matrigel assay) of CAC transfected with wild-type (WT) or constitutively active (CA) Src kinase. Leptin had no effect on cells transfected with dominant-negative (DN) Src kinase. **P<0.01, and ***P<0.001 vs. unstimulated CAC defined as 100%.
Src Kinase Activation and Integrin αvβ5 Phosphorylation Also Mediate the Angiogenic Effects of Leptin on Endothelial Cells
To examine whether the effects of leptin on Src-mediated αvβ5 integrin phosphorylation operate in endothelial cells, HUVEC were stimulated with leptin (suppl. Fig. VI; please see www.ahajournals.org). In these studies, αvβ5 integrin phosphorylation was enhanced after incubation with leptin (suppl. Fig. VIA). Similar to the findings in CAC, leptin induced activation of Src kinase, reflected by enhanced phosphorylation at Y416 and dephosphorylation on Y527 (suppl. Fig. VIB). Leptin also induced a time-dependent phosphorylation of FAK at Y397, the autophosphorylation site accompanying integrin activation.13 In addition, FAK was phosphorylated at Y861 and Y925 upon stimulation with leptin (suppl. Fig. VIC). These tyrosine residues promote the binding of Src kinase and the coupling of FAK to integrin αvβ5,14 and also create binding sites for SH2 domain-containing adaptor proteins, including Grb2; the latter step results in activation of the Ras-Raf-ERK cascade15 which regulates proliferation, differentiation, and migration during angiogenesis.
Leptin Is Incapable of Stimulating Angiogenesis in CAC from Obese Individuals: Effects of Weight Loss
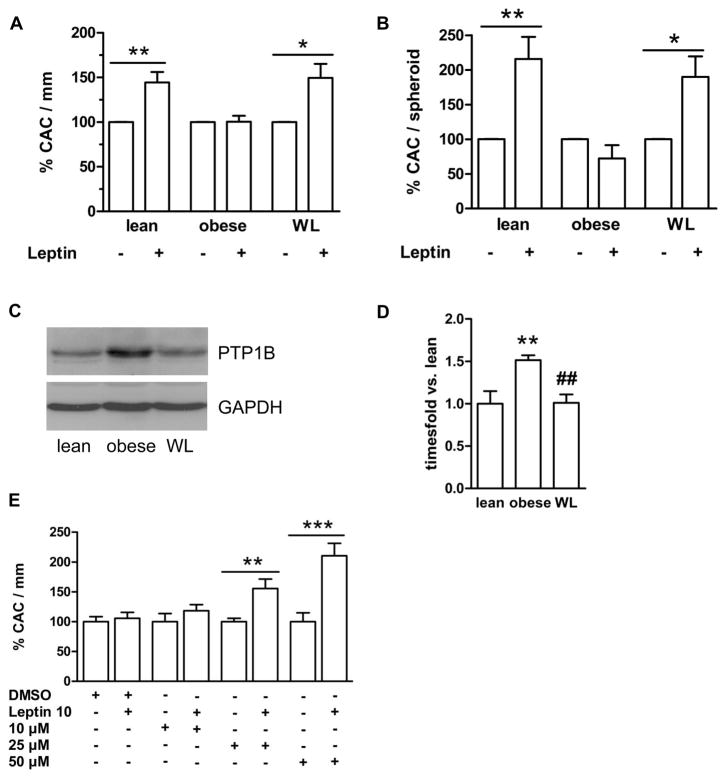
Obesity is associated with chronically elevated circulating leptin levels and resistance to the weight-reducing effects of the adipokine. We examined the angiogenic response to leptin in CAC derived from severely obese individuals (n=21; body weight, 135±6.0 kg, BMI, 43±1.4 kg/m2) at the time of enrollment in a professional weight reduction program, and compared it to age- and sex-matched normal-weight controls (n=21; 68±2.1 kg, 22±0.5 kg/m2). As expected, plasma leptin levels were elevated in the obese (59±10 vs. 4.4±0.8 ng/mL in lean controls; P<0.001). As summarized in Fig. 5A, stimulation of CAC from obese individuals with leptin (10 ng/mL) failed to enhance their adherence to endothelial cell networks provided by HUVEC in the matrigel assay; this was in contrast to the leptin responsiveness of ‘lean’ CAC. Leptin also failed to increase the number of CAC adherent to outgrowing HUVEC sprouts (Fig. 5B), or the cumulative length of sprouts outgrowing from co-incubated HUVEC (not shown) in the spheroid assay, suggesting that CAC are resistant to the angiogenic effects of leptin in obesity. Similar findings were observed with higher concentrations (100 ng/mL) of leptin (P<0.05 in the matrigel assay; and P<0.001 in the spheroid assay; n=4; not shown).
Figure 5.
CAC, isolated from lean controls, or obese individuals, before and after weight loss (WL), were stimulated with leptin (10 ng/mL) followed by analysis of angiogenesis in the matrigel (A) and the spheroid (B) assay. Leptin responsiveness was abolished in CAC from obese individuals but restored after weight loss. *P<0.05 and **P<0.01 vs. control-treated cells. (C) Representative Western blot analysis of unstimulated CAC cell lysates revealed increased basal PTP1B protein levels in obese individuals, while weight loss normalized PTP1B expression. (D) Quantitative analysis of n=9 subjects per group. Results were normalized to PTP1B expression in lean controls. **P<0.01 vs. lean, ##P<0.01 vs. formerly obese. (E) Ex vivo inhibition of PTP1B restored the responsiveness of CAC from obese subjects to leptin in the matrigel assay. **P<0.01 and ***P<0.001.
Western blot analysis of known negative regulators of leptin signaling revealed significantly elevated levels of the non-receptor protein tyrosine phosphatase (PTP)1B in CAC from obese persons (Fig. 5C and D). This finding generated the question whether the expression levels of negative regulators of leptin signaling can return to normal and the CAC responsiveness to leptin be restored following therapeutic weight loss. Indeed, follow-up studies of obese individuals after significant weight loss (n=18; mean weight reduction, 30±15 kg, P<0.001 vs. baseline; mean circulating leptin levels, 21±4.2 ng/mL; P=0.001 vs. baseline) revealed normalization of the responsiveness of CAC to the angiogenic effects of leptin, both in the matrigel (Fig. 5A) and in the spheroid angiogenesis assay (Fig. 5B). The functional improvement of CAC was accompanied by a significant reduction of PTP1B expression levels in these cells (Fig. 5C and D). The importance of PTP1B in (reversibly) attenuating the response of CAC to leptin in obesity as opposed to the lean state was supported by further experiments in which we found that the effects of leptin on the angiogenic properties of CAC from obese individuals could be dose-dependently restored by inhibition of PTP1B (Fig. 5E).
Discussion
It has been reported that leptin may promote angiogenesis.1, 2 The present study set out to address the exact mechanisms mediating the angiogenic effects of the adipokine, particularly focussing on how leptin may modulate the functional properties of circulating angiogenic cells. Our findings can be summarized as follows: 1) leptin exerts angiogenic effects in vitro and in vivo which are mediated, at least in part, by CAC; 2) these effects appear to depend on a specific interaction between the leptin receptor ObR and αvβ5 integrins; 3) phosphorylation of the β5 integrin chain by leptin requires the JAK2- and, possibly, PLCγ-mediated activation of Src kinase; 4) in the chronic hyperleptinemia which characterizes human obesity, negative regulators of leptin signaling, particularly PTP1B, are overexpressed in CAC, preventing the angiogenic response of these cells to leptin; and 5) therapeutic weight loss, or PTP1B inhibition, may restore the responsiveness of CAC to leptin.
The leptin receptor is expressed on numerous cell types including endothelial cells,1, 2 CD34-positive hematopoietic cells,16 and peripheral blood-derived early and late outgrowth endothelial progenitor cells.9, 17 We recently observed that leptin increased the adhesion, transmigration, and incorporation of early outgrowth progenitor cells into experimental arterial lesions.9 Therefore, we hypothesized that the angiogenic effects of leptin may be mediated by an enhancement of the vasculoprotective function of these cells. Of note, it was recently proposed to change the nomenclature of early outgrowth endothelial progenitor cells to ‘circulating angiogenic cells’ in order to better differentiate them from endothelial colony forming cells. Regardless of the nomenclature however, the importance of CAC (isolated according to the protocol used in the present study) for vascular homeostasis and particularly for the angiogenic process remains undisputed.7 Using two in vitro and two in vivo angiogenesis assays, we could demonstrate that leptin specifically promoted the adhesion and incorporation of CAC to structures provided by endothelial cells. The physiological relevance of these observations was strongly supported by our findings that leptin increased new vessel formation in the chorioallantoic membrane of chick embryos and also improved perfusion and neovascularization in ischemic murine himdlimbs.
The results of the present study indicate that β5 integrin activation is essential for mediating the effects of leptin on CAC function and angiogenesis. It has previously been reported that αvβ5 mediates at least some of the angiogenic endothelial cell responses to VEGF during new vessel formation.18, 19 We could now show that leptin specifically inducesβ5 (but not β3) phosphorylation, and that leptin binding to ObR induces the formation of immunocomplexes of this cytokine receptor with αvβ5 integrins. Consistently, β5 integrin phosphorylation in response to leptin was prevented by antibodies blocking the interaction of leptin with its receptor. Thus, our results point to the ObR-αvβ5 cross-talk as a novel paradigm of specific interactions between integrins and growth factor/cytokine receptors in angiogenesis.20
As integrins do not possess intrinsic enzymatic activity, cytoplasmic tyrosine kinases, notably of the Src family, are important regulators of outside-in integrin signaling.21 While most studies have thus far focused on the association between Src kinases and β3 integrins, it could also be shown that αvβ5 integrin-mediated activation of c-Raf depends on Src kinase22 and triggers the Ras-Raf-ERK pathway. We now show that treatment with leptin time-dependently enhanced activation of Src kinase, both in CAC and in endothelial cells, as indicated by phosphorylation at Y416 and a parallel increase of dephosphorylation at Y527 within its autoinhibitory element.21 The physiological relevance of the leptin-induced, ObR-specific Src activation is further supported by our observation that inhibition of Src kinase completely abolished the effects of leptin on the angiogenic properties of the CAC, and that the leptin-induced phosphorylation of integrin β5 and angiogenesis was absent in CAC transfected with inactive, i.e. dominant-negative forms of Src kinase.
How can Src family kinases function as a link between ObR and integrin activation? In our studies, Src co-immunoprecipitated together with ObR following leptin stimulation of CAC, and incubation with the JAK2 inhibitor AG490 prevented the leptin-induced Src activation. Tyrosine phosphorylation of ObR by JAK2 may create docking sites for the SH2 domain of Src kinase.23 Previous studies have indeed suggested that leptin signaling may involve activation of Src family tyrosine kinases,24 and that JAK kinase is required for optimal activation of Src.23 In this regard, leptin might promote the direct association of Src with activated JAK2 via N-terminal phosphotyrosines such as occur after angiotensin II stimulation.25 In addition, PLCγ, which becomes activated downstream of JAK2 in response to leptin,26 may facilitate the localization of Src within integrin signaling complexes.27 Consistently, our experiments revealed that Src kinase activation following leptin stimulation of CAC could be reduced by inhibition of PLCγ.
Protein tyrosine phosphatases (PTP) contribute to the activity regulation of receptor tyrosine kinases. In the setting of chronic hyperleptinemia associated with obesity, dephosphorylation of JAK2 by PTP1B is thought to provide a negative feedback control of leptin signaling in the central nervous system,28 and studies in mice have demonstrated that lack of PTP1B prevents ‘central’ leptin resistance and diet-induced obesity.29, 30 Our findings now support the existence of ‘peripheral’ CAC resistance to leptin in human obesity: PTP1B expression was elevated in CAC from obese, hyperleptinemic individuals compared to age-and sex-matched lean controls, along with a reduced responsiveness of CAC from the obese to the angiogenic effects of leptin. Moreover, and importantly, weight loss was associated with normalized PTP1B protein levels and a restored responsiveness of CAC to the angiogenic effects of leptin. Inhibition of PTP1B ‘reproduced’ the effects of weight loss, i.e. restored the impaired angiogenic response of CAC from obese individuals to leptin.
Interestingly, PTP1B may also directly activate Src kinase by dephosphorylation at Y527 and thus reversal of its constitutive autoinhibition.21 In fact, our own studies confirmed basal (leptin-independent) dephosphorylation of Src at Y527 in CAC from obese individuals (not shown). However, is unlikely that these findings might translate into an increased angiogenic capacity of CAC in human obesity. On the contrary, we have previously observed that CAC from obese individuals exhibited impaired angiogenic properties compared to lean controls.31 Thus, despite the complexity of the proangiogenic and antiangiogenic mechanisms involved, the predominating effects of PTP1B in obesity appear to involve the dephosphorylation and thus negative regulation both of angiogenic growth factor receptors32 and of regulators of cell motility and proliferation.33
In conclusion, the findings of the present study demonstrate that the angiogenic effects of the adipocytokine leptin are mediated, at least in part, by circulating angiogenic cells and involve Src kinase-dependent activation of the angiogenic integrin αvβ5. Our results point to the leptin receptor-αvβ5 synergy as a novel, distinct, and physiologically relevant component of a network of specific interactions between integrins and growth factor or cytokine receptors during angiogenic processes. Moreover, they suggest that elevated PTP1B expression levels may be responsible for the resistance of CAC to the angiogenic and potentially vasculoprotective effects of leptin in obese individuals. Future studies will need to explore inhibition of PTP1B as a possible therapeutic target, besides weight loss, in obesity.
Supplementary Material
Acknowledgments
We wish to thank Drs. Elena Deryugina (The Scripps Research Institute, La Jolla, CA, USA), Thomas Korff (University of Heidelberg, Germany), and Wulf Ito (University of Luebeck, Germany) for helpful advice regarding the CAM assay, the spheroid angiogenesis assay, and the murine hindlimb ischemia model. We also thank Prof. Tatiana V. Byzova (The Cleveland Clinic, Cleveland, OH, USA) for providing Src kinase plasmids.
Source of Funding
Parts of the study were awarded the Young Investigator Award 2007 of the American Heart Association, Council on Arteriosclerosis, Thrombosis and Vascular Biology (to N.M.H.). The study was in part supported by a grant from the National Institutes of Health (NIH HL-75736; to Z.M.R.).
Footnotes
Disclosures
none.
References
- 1.Bouloumie A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res. 1998;83:1059–1066. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 2.Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, Flores-Riveros JR. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 3.Suganami E, Takagi H, Ohashi H, Suzuma K, Suzuma I, Oh H, Watanabe D, Ojima T, Suganami T, Fujio Y, Nakao K, Ogawa Y, Yoshimura N. Leptin stimulates ischemia-induced retinal neovascularization: possible role of vascular endothelial growth factor expressed in retinal endothelial cells. Diabetes. 2004;53:2443–2448. doi: 10.2337/diabetes.53.9.2443. [DOI] [PubMed] [Google Scholar]
- 4.Iversen PO, Drevon CA, Reseland JE. Prevention of leptin binding to its receptor suppresses rat leukemic cell growth by inhibiting angiogenesis. Blood. 2002;100:4123–4128. doi: 10.1182/blood-2001-11-0134. [DOI] [PubMed] [Google Scholar]
- 5.Schiekofer S, Galasso G, Sato K, Kraus BJ, Walsh K. Impaired revascularization in a mouse model of type 2 diabetes is associated with dysregulation of a complex angiogenic-regulatory network. Arterioscler Thromb Vasc Biol. 2005;25:1603–1609. doi: 10.1161/01.ATV.0000171994.89106.ca. [DOI] [PubMed] [Google Scholar]
- 6.Asahara T, Murohara T, Sullivan A, Silver M, van der ZR, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 7.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroeter MR, Leifheit M, Sudholt P, Heida NM, Dellas C, Rohm I, Alves F, Zientkowska M, Rafail S, Puls M, Hasenfuss G, Konstantinides S, Schäfer K. Leptin enhances the recruitment of endothelial progenitor cells into neointimal lesions after vascular injury by promoting integrin-mediated adhesion. Circ Res. 2008;103:536–544. doi: 10.1161/CIRCRESAHA.107.169375. [DOI] [PubMed] [Google Scholar]
- 10.Chavakis E, Urbich C, Dimmeler S. Homing and engraftment of progenitor cells: A prerequisite for cell therapy. J Mol Cell Cardiol. 2008;45:514–522. doi: 10.1016/j.yjmcc.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Mahabeleshwar GH, Feng W, Reddy K, Plow EF, Byzova TV. Mechanisms of integrin-vascular endothelial growth factor receptor cross-activation in angiogenesis. Circ Res. 2007;101:570–580. doi: 10.1161/CIRCRESAHA.107.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roskoski R., Jr Src protein-tyrosine kinase structure and regulation. Biochem Biophys Res Commun. 2004;324:1155–1164. doi: 10.1016/j.bbrc.2004.09.171. [DOI] [PubMed] [Google Scholar]
- 13.Salazar EP, Rozengurt E. Src family kinases are required for integrin-mediated but not for G protein-coupled receptor stimulation of focal adhesion kinase autophosphorylation at Tyr-397. J Biol Chem. 2001;276:17788–17795. doi: 10.1074/jbc.M100984200. [DOI] [PubMed] [Google Scholar]
- 14.Eliceiri BP, Puente XS, Hood JD, Stupack DG, Schlaepfer DD, Huang XZ, Sheppard D, Cheresh DA. Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J Cell Biol. 2002;157:149–160. doi: 10.1083/jcb.200109079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlaepfer DD, Jones KC, Hunter T. Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: summation of both c-Src- and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol Cell Biol. 1998;18:2571–2585. doi: 10.1128/mcb.18.5.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gainsford T, Willson TA, Metcalf D, Handman E, McFarlane C, Ng A, Nicola NA, Alexander WS, Hilton DJ. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc Natl Acad Sci U S A. 1996;93:14564–14568. doi: 10.1073/pnas.93.25.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolk R, Deb A, Caplice NM, Somers VK. Leptin receptor and functional effects of leptin in human endothelial progenitor cells. Atherosclerosis. 2005;183:131–139. doi: 10.1016/j.atherosclerosis.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 18.Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct alpha v integrins. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- 19.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 20.Ross RS. Molecular and mechanical synergy: cross-talk between integrins and growth factor receptors. Cardiovasc Res. 2004;63:381–390. doi: 10.1016/j.cardiores.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 21.Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23:7928–7946. doi: 10.1038/sj.onc.1208080. [DOI] [PubMed] [Google Scholar]
- 22.Hood JD, Frausto R, Kiosses WB, Schwartz MA, Cheresh DA. Differential alphav integrin-mediated Ras-ERK signaling during two pathways of angiogenesis. J Cell Biol. 2003;162:933–943. doi: 10.1083/jcb.200304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene. 2000;19:5662–5679. doi: 10.1038/sj.onc.1203925. [DOI] [PubMed] [Google Scholar]
- 24.Jiang L, Li Z, Rui L. Leptin stimulates both JAK2-dependent and JAK2-independent signaling pathways. J Biol Chem. 2008;283:28066–28073. doi: 10.1074/jbc.M805545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sayeski PP, Ali MS, Hawks K, Frank SJ, Bernstein KE. The angiotensin II-dependent association of Jak2 and c-Src requires the N-terminus of Jak2 and the SH2 domain of c-Src. Circ Res. 1999;84:1332–1338. doi: 10.1161/01.res.84.11.1332. [DOI] [PubMed] [Google Scholar]
- 26.Dellas C, Schäfer K, Rohm IK, Lankeit M, Leifheit M, Loskutoff DJ, Hasenfuss G, Konstantinides S. Leptin signalling and leptin-mediated activation of human platelets: importance of JAK2 and the phospholipases Cgamma2 and A2. Thromb Haemost. 2007;98:1063–1071. [PubMed] [Google Scholar]
- 27.Epple H, Cremasco V, Zhang K, Mao D, Longmore GD, Faccio R. Phospholipase Cgamma2 modulates integrin signaling in the osteoclast by affecting the localization and activation of Src kinase. Mol Cell Biol. 2008;28:3610–3622. doi: 10.1128/MCB.00259-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng A, Uetani N, Simoncic PD, Chaubey VP, Lee-Loy A, McGlade CJ, Kennedy BP, Tremblay ML. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev Cell. 2002;2:497–503. doi: 10.1016/s1534-5807(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 29.Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med. 2006;12:917–924. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- 30.Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 31.Heida NM, Müller JP, Cheng I, Leifheit M, Faustin V, Riggert J, Hasenfuss G, Konstantinides S, Schäfer K. Effects of obesity and weight loss on the functional properties of early outgrowth endothelial progenitor cells. J Am Coll Cardiol. doi: 10.1016/j.jacc.2009.09.031. in press. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura Y, Patrushev N, Inomata H, Mehta D, Urao N, Kim HW, Razvi M, Kini V, Mahadev K, Goldstein BJ, McKinney R, Fukai T, Ushio-Fukai M. Role of protein tyrosine phosphatase 1B in vascular endothelial growth factor signaling and cell-cell adhesions in endothelial cells. Circ Res. 2008;102:1182–1191. doi: 10.1161/CIRCRESAHA.107.167080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mertins P, Eberl HC, Renkawitz J, Olsen JV, Tremblay ML, Mann M, Ullrich A, Daub H. Investigation of protein-tyrosine phosphatase 1B function by quantitative proteomics. Mol Cell Proteomics. 2008;7:1763–1777. doi: 10.1074/mcp.M800196-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.