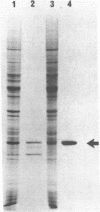
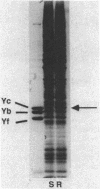
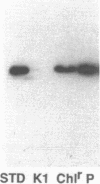
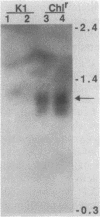
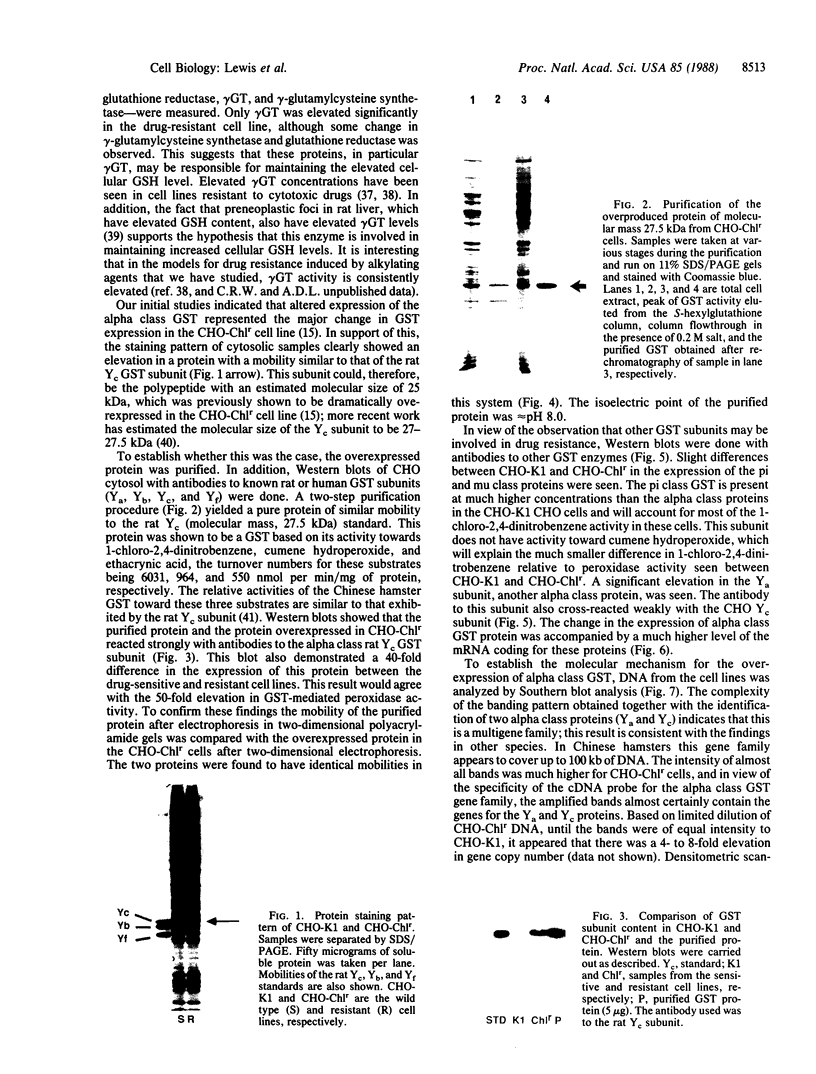
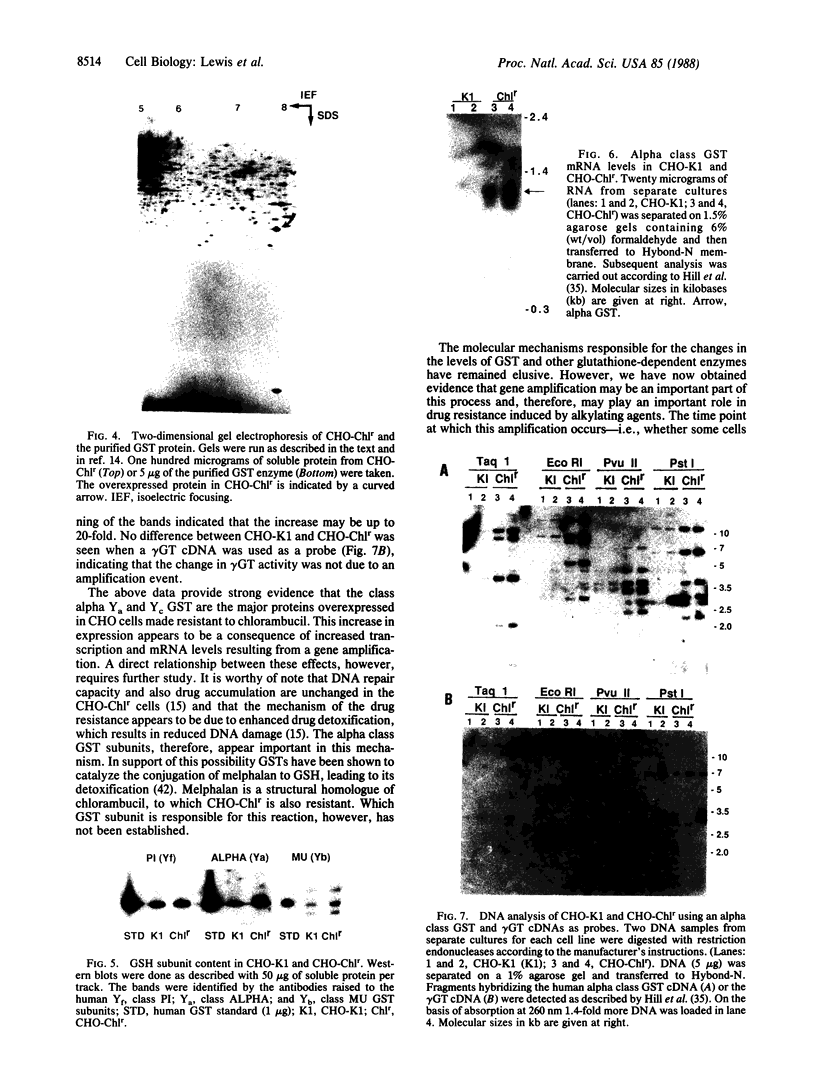
Abstract
Glutathione-dependent enzymes play a central role in the protection of cells from cytotoxic chemicals and have been implicated in the intrinsic and acquired resistance of tumors to cytotoxic drugs. We have generated a Chinese hamster ovary line resistant to bifunctional nitrogen mustards and in this report have characterized and isolated the protein that represents the major observable phenotypic difference between the drug-sensitive and drug-resistant cell lines. This purified protein is shown to be an alpha class glutathione S-transferase comprising YcYc subunits and possessing a pI value of approximately 8.0. The intracellular level of the Yc subunit is elevated greater than 40-fold in the drug-resistant cell line, which could account for the increase in glutathione S-transferase (RX:glutathione R-transferase; EC 2.5.1.18) activity toward both 1-chloro-2,4-dinitrobenzene and cumene hydroperoxide. Other glutathione S-transferase subunits within this gene family are also elevated. These changes are accompanied by a significant elevation in alpha class mRNA levels. Southern analysis indicates that the genes coding for these proteins are amplified 4- to 8-fold in the drug-resistant cell line. In addition, gamma-glutamyl transpeptidase [(5-glutamyl)-peptide:amino acid 5-glutamyltransferase; EC 2.3.2.2] activity is increased 3.6-fold in the drug-resistant Chinese hamster ovary cell line, which may explain the increase in cellular glutathione level. In this case no gene amplification was seen. These data indicate that gene amplification may be important in drug resistance toward alkylating agents and also that other enzymes in glutathione homeostasis are involved.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Seilman S., Amelizad Z., Oesch F., Wolf C. R. Identification of human cytochromes P-450 analogous to forms induced by phenobarbital and 3-methylcholanthrene in the rat. Biochem J. 1985 Dec 15;232(3):869–876. doi: 10.1042/bj2320869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams K. J., Carmichael J., Wolf C. R. Altered mouse bone marrow glutathione and glutathione transferase levels in response to cytotoxins. Cancer Res. 1985 Apr;45(4):1669–1673. [PubMed] [Google Scholar]
- Ahmad S., Okine L., Wood R., Aljian J., Vistica D. T. gamma-Glutamyl transpeptidase (gamma-GT) and maintenance of thiol pools in tumor cells resistant to alkylating agents. J Cell Physiol. 1987 May;131(2):240–246. doi: 10.1002/jcp.1041310214. [DOI] [PubMed] [Google Scholar]
- Arrick B. A., Nathan C. F. Glutathione metabolism as a determinant of therapeutic efficacy: a review. Cancer Res. 1984 Oct;44(10):4224–4232. [PubMed] [Google Scholar]
- Batist G., Tulpule A., Sinha B. K., Katki A. G., Myers C. E., Cowan K. H. Overexpression of a novel anionic glutathione transferase in multidrug-resistant human breast cancer cells. J Biol Chem. 1986 Nov 25;261(33):15544–15549. [PubMed] [Google Scholar]
- Birnboim H. C. Rapid extraction of high molecular weight RNA from cultured cells and granulocytes for Northern analysis. Nucleic Acids Res. 1988 Feb 25;16(4):1487–1497. doi: 10.1093/nar/16.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Board P. G., Webb G. C. Isolation of a cDNA clone and localization of human glutathione S-transferase 2 genes to chromosome band 6p12. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2377–2381. doi: 10.1073/pnas.84.8.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann A., Kuhlmann W., Schwarz M., Kunz W., Wolf C. R., Moll E., Friedberg T., Oesch F. Regulation and expression of four cytochrome P-450 isoenzymes, NADPH-cytochrome P-450 reductase, the glutathione transferases B and C and microsomal epoxide hydrolase in preneoplastic and neoplastic lesions in rat liver. Carcinogenesis. 1985 Apr;6(4):513–521. doi: 10.1093/carcin/6.4.513. [DOI] [PubMed] [Google Scholar]
- Carmichael J., Adams D. J., Ansell J., Wolf C. R. Glutathione and glutathione transferase levels in mouse granulocytes following cyclophosphamide administration. Cancer Res. 1986 Feb;46(2):735–739. [PubMed] [Google Scholar]
- Christman M. F., Morgan R. W., Jacobson F. S., Ames B. N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985 Jul;41(3):753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- Deml E., Oesterle D. Histochemical demonstration of enhanced glutathione content in enzyme-altered islands induced by carcinogens in rat liver. Cancer Res. 1980 Feb;40(2):490–491. [PubMed] [Google Scholar]
- Dulik D. M., Fenselau C., Hilton J. Characterization of melphalan-glutathione adducts whose formation is catalyzed by glutathione transferases. Biochem Pharmacol. 1986 Oct 1;35(19):3405–3409. doi: 10.1016/0006-2952(86)90444-2. [DOI] [PubMed] [Google Scholar]
- Farber E. The biochemistry of preneoplastic liver: a common metabolic pattern in hepatocyte nodules. Can J Biochem Cell Biol. 1984 Jun;62(6):486–494. doi: 10.1139/o84-066. [DOI] [PubMed] [Google Scholar]
- HIRONO I. Non-protein sulphydryl group in the original strain and sub-line of the ascites tumour resistant to alkylating reagents. Nature. 1960 Jun 25;186:1059–1060. doi: 10.1038/1861059a0. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hayes J. D., Gilligan D., Chapman B. J., Beckett G. J. Purification of human hepatic glutathione S-transferases and the development of a radioimmunoassay for their measurement in plasma. Clin Chim Acta. 1983 Oct 31;134(1-2):107–121. doi: 10.1016/0009-8981(83)90189-4. [DOI] [PubMed] [Google Scholar]
- Hayes J. D., Mantle T. J. Anomalous electrophoretic behaviour of the glutathione S-transferase Ya and Yk subunits isolated from man and rodents. A potential pitfall for nomenclature. Biochem J. 1986 Aug 1;237(3):731–740. doi: 10.1042/bj2370731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. E., Shaw P. H., Barth R. K., Hastie N. D. A genetic locus closely linked to a protease inhibitor gene complex controls the level of multiple RNA transcripts. Mol Cell Biol. 1985 Aug;5(8):2114–2122. doi: 10.1128/mcb.5.8.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran P., Lindahl T. Cellular defence mechanisms against alkylating agents. Cancer Surv. 1985;4(3):583–599. [PubMed] [Google Scholar]
- Kartner N., Shales M., Riordan J. R., Ling V. Daunorubicin-resistant Chinese hamster ovary cells expressing multidrug resistance and a cell-surface P-glycoprotein. Cancer Res. 1983 Sep;43(9):4413–4419. [PubMed] [Google Scholar]
- Ketterer B. Detoxication reactions of glutathione and glutathione transferases. Xenobiotica. 1986 Oct-Nov;16(10-11):957–973. doi: 10.3109/00498258609038976. [DOI] [PubMed] [Google Scholar]
- Kimball R. E., Reddy K., Peirce T. H., Schwartz L. W., Mustafa M. G., Cross C. E. Oxygen toxicity: augmentation of antioxidant defense mechanisms in rat lung. Am J Physiol. 1976 May;230(5):1425–1431. doi: 10.1152/ajplegacy.1976.230.5.1425. [DOI] [PubMed] [Google Scholar]
- Kitahara A., Satoh K., Nishimura K., Ishikawa T., Ruike K., Sato K., Tsuda H., Ito N. Changes in molecular forms of rat hepatic glutathione S-transferase during chemical hepatocarcinogenesis. Cancer Res. 1984 Jun;44(6):2698–2703. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laperche Y., Bulle F., Aissani T., Chobert M. N., Aggerbeck M., Hanoune J., Guellaën G. Molecular cloning and nucleotide sequence of rat kidney gamma-glutamyl transpeptidase cDNA. Proc Natl Acad Sci U S A. 1986 Feb;83(4):937–941. doi: 10.1073/pnas.83.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A. D., Hayes J. D., Wolf C. R. Glutathione and glutathione-dependent enzymes in ovarian adenocarcinoma cell lines derived from a patient before and after the onset of drug resistance: intrinsic differences and cell cycle effects. Carcinogenesis. 1988 Jul;9(7):1283–1287. doi: 10.1093/carcin/9.7.1283. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Alin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Meyer D. J., Beale D., Tan K. H., Coles B., Ketterer B. Glutathione transferases in primary rat hepatomas: the isolation of a form with GSH peroxidase activity. FEBS Lett. 1985 May 6;184(1):139–143. doi: 10.1016/0014-5793(85)80670-0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967 Jul;70(1):158–169. [PubMed] [Google Scholar]
- Robson C. N., Alexander J., Harris A. L., Hickson I. D. Isolation and characterization of a Chinese hamster ovary cell line resistant to bifunctional nitrogen mustards. Cancer Res. 1986 Dec;46(12 Pt 1):6290–6294. [PubMed] [Google Scholar]
- Robson C. N., Lewis A. D., Wolf C. R., Hayes J. D., Hall A., Proctor S. J., Harris A. L., Hickson I. D. Reduced levels of drug-induced DNA cross-linking in nitrogen mustard-resistant Chinese hamster ovary cells expressing elevated glutathione S-transferase activity. Cancer Res. 1987 Nov 15;47(22):6022–6027. [PubMed] [Google Scholar]
- Russo A., Carmichael J., Friedman N., DeGraff W., Tochner Z., Glatstein E., Mitchell J. B. The roles of intracellular glutathione in antineoplastic chemotherapy. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1347–1354. doi: 10.1016/0360-3016(86)90169-0. [DOI] [PubMed] [Google Scholar]
- Seelig G. F., Meister A. Gamma-glutamylcysteine synthetase. Interactions of an essential sulfhydryl group. J Biol Chem. 1984 Mar 25;259(6):3534–3538. [PubMed] [Google Scholar]
- Smith G. D., Ding J. L., Peters T. J. A sensitive fluorimetric assay for gamma-glutamyl transferase. Anal Biochem. 1979 Nov 15;100(1):136–139. doi: 10.1016/0003-2697(79)90122-2. [DOI] [PubMed] [Google Scholar]
- Soma Y., Satoh K., Sato K. Purification and subunit-structural and immunological characterization of five glutathione S-transferases in human liver, and the acidic form as a hepatic tumor marker. Biochim Biophys Acta. 1986 Feb 14;869(3):247–258. doi: 10.1016/0167-4838(86)90064-6. [DOI] [PubMed] [Google Scholar]
- Stark G. R. DNA amplification in drug resistant cells and in tumours. Cancer Surv. 1986;5(1):1–23. [PubMed] [Google Scholar]
- Stockman P. K., Beckett G. J., Hayes J. D. Identification of a basic hybrid glutathione S-transferase from human liver. Glutathione S-transferase delta is composed of two distinct subunits (B1 and B2). Biochem J. 1985 Apr 15;227(2):457–465. doi: 10.1042/bj2270457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman P. K., McLellan L. I., Hayes J. D. Characterization of the basic glutathione S-transferase B1 and B2 subunits from human liver. Biochem J. 1987 May 15;244(1):55–61. doi: 10.1042/bj2440055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson S. S., Huber B. E., Sorrell S., Fojo A., Pastan I., Gottesman M. M. Expression of the multidrug-resistant gene in hepatocarcinogenesis and regenerating rat liver. Science. 1987 May 29;236(4805):1120–1122. doi: 10.1126/science.3576227. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C., Maker H. S., Lehrer G. M. Sensitive fluorometric assays for glutathione peroxidase and reductase. Anal Biochem. 1980 Aug;106(2):512–516. doi: 10.1016/0003-2697(80)90556-4. [DOI] [PubMed] [Google Scholar]
- Wolf C. R., Lewis A. D., Carmichael J., Adams D. J., Allan S. G., Ansell D. J. The role of glutathione in determining the response of normal and tumor cells to anticancer drugs. Biochem Soc Trans. 1987 Aug;15(4):728–730. doi: 10.1042/bst0150728. [DOI] [PubMed] [Google Scholar]