SUMMARY
Olfactory signals are transduced by a large family of odorant receptor proteins, each of which corresponds to a unique glomerulus in the first olfactory relay of the brain. Cross-talk between glomeruli has been proposed to be important in olfactory processing, but it is not clear how these interactions shape the odor responses of second-order neurons. In the Drosophila antennal lobe (a region analogous to the vertebrate olfactory bulb), we selectively remove most inter-glomerular input to identified second-order olfactory neurons. We find this broadens the odor tuning of these neurons, implying that inter-glomerular inhibition dominates over inter-glomerular excitation. The strength of this inhibitory signal scales with total feedforward input to the entire antennal lobe, and has similar tuning in different glomeruli. A substantial portion of this inter-glomerular inhibition acts at a presynaptic locus, and our results imply this is mediated by both GABAA and GABAB receptors on the same nerve terminal.
A sensory stimulus generally triggers activity in multiple neural processing channels, each of which carries information about some feature of that stimulus. The concept of a processing channel has a particularly clear anatomical basis in the first relay of the olfactory system, which is typically divided into glomerular compartments. Each glomerulus receives input from many first-order olfactory receptor neurons (ORNs), all of which express the same odorant receptor. Each second-order neuron receives direct ORN input from a single glomerulus, and thus all the first- and second-order neurons corresponding to a glomerulus constitute a discrete processing channel. An odorant typically triggers activity in multiple glomeruli, and local interneurons that interconnect glomeruli provide a substrate for cross-talk between channels.
The Drosophila antennal lobe is a favored model for investigating olfactory processing because it contains only ~50 glomeruli1, each of which corresponds to an identified type of ORN and an identified type of postsynaptic projection neuron (PN)2–5. Several recent studies of the Drosophila antennal lobe have produced divergent views of the relative importance of inter-glomerular connections. One model proposes that PN odor responses are almost completely determined by feedforward excitation6, 7. This model ascribes little importance to cross-talk between glomerular processing channels. An alternative model proposes that inter-glomerular connections make an important contribution to shaping PN odor responses8–12. However, this has not been demonstrated by showing a change in PN odor responses when lateral inputs to that PN are removed. (We use the word “lateral” as a synonym for “inter-glomerular”.)
In principle, several features of olfactory processing in the Drosophila antennal lobe could reflect either intra- or inter-glomerular events. For example, most PNs are more broadly tuned to odors than their presynaptic ORNs8, 10. This could reflect a purely intra-glomerular nonlinear process, such as short-term synaptic depression at ORN-PN connections. Alternatively, it could be due to the fact that lateral excitatory connections exist between glomeruli7, 11, 12. It is also unclear whether inhibitory epochs in PN odor responses8–10 reflect inter- or intra-glomerular events. Many GABAergic interneurons form connections between glomeruli9, 13, 14, but several recent studies have failed to observe any inter-glomerular inhibition7, 11, 12. These studies removed all the direct ORN inputs to an identified PN, and asked whether lateral input could be evoked in that PN by olfactory stimulation of other ORN types. In all cases, lateral inputs to PNs were excitatory. This raises the possibility that inter-glomerular inhibition might not exist, and inhibitory PN odor responses might merely reflect intra-glomerular feedback15–17. This is an important issue because intra- and inter-glomerular inhibition have different consequences for how olfactory representations are transformed in this circuit.
In this study, we addressed three questions: Do inter-glomerular interactions make a substantial contribution to PN odor responses? Do these interactions include lateral inhibition? If so, how does this occur, and why has it been difficult to observe?
Removing lateral input to PNs
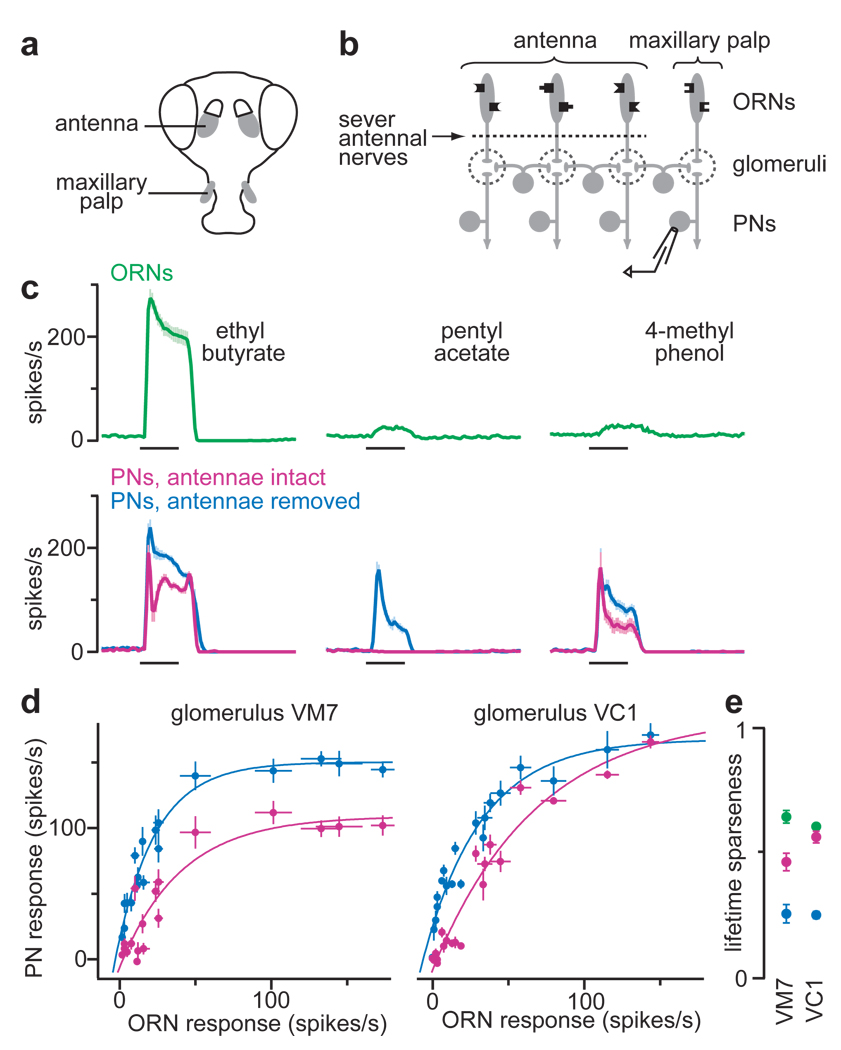
We began by investigating what happens to PN odor responses when most lateral input to that PN is removed. We took advantage of the fact that the fly has two olfactory organs. About 90% of ORNs are contained in the antennae, with 10% in the maxillary palp (Fig. 1a). Palp ORNs express odorant receptors not expressed in the antennae, and project to palp glomeruli that are distinct from glomeruli targeted by antennal ORNs3, 4. Antennal and palp glomeruli are interconnected by local interneurons. Acute removal of the antennae eliminates 90% of the input to the antennal lobe, and thus most excitatory drive to local interneurons (Fig. 1b). If lateral connections are mainly excitatory7, 11, 12, then removing the antennae should decrease the odor responses of palp PNs.
Figure 1. Removing lateral input disinhibits PNs.
a, Drosophila olfactory organs.
b, Experimental configuration.
c, Spiking responses of ORNs and PNs for the palp glomerulus VM7 (n = 4–12 for each response). PNs are shown with and without lateral input from antennal glomeruli. Throughout, black bars represent the 500-msec odor stimulus period.
d, Input-output functions for palp glomeruli VM7 and VC1. Each point represents the average PN response to an odor versus the response of the cognate ORNs. PNs are shown with antennae intact (magenta) or removed (blue). Responses to 18 of 20 odors are significantly disinhibited in VM7; 13 of 20 in VC1 (p < 0.05, t-tests).
e, Odor selectivity of ORNs (green) and cognate PNs with antennae intact (magenta) or removed (blue). Lifetime sparseness = 0 for an unselective cell, 1 for a maximally selective cell. All within-glomerulus comparisons are significant (p < 0.002, Mann-Whitney U-test), except ORNs versus antennae-intact-PNs for VC1.
We performed this experiment in two different palp glomeruli (VM7 and VC1). Surprisingly, removing the antennae increased most of the odor responses of these PNs (Fig. 1c,d; Supplementary Figs. 2,3). No odor responses were decreased. This implies that most of our odors normally evoke lateral inhibitory input to these glomeruli, and this outweighs the effect of lateral excitatory input.
We examined the input-output function of each glomerulus by plotting the strength of each PN odor response versus the strength of the cognate ORN response to the same odor. These input-output functions were nonlinear (Fig. 1d), which is typical for most glomeruli10. When we removed most lateral input to PNs, the nonlinearity persisted (Fig. 1d), and PNs became even more broadly tuned (Fig. 1e). This argues that broad PN tuning8, 10 results mainly from purely intra-glomerular mechanisms. These could include short-term synaptic depression at ORN-PN connections, and/or an intrinsic ceiling on PN firing rates. Lateral excitation should tend to broaden PN tuning even more, but lateral inhibition evidently counteracts this.
One clue to the significance of lateral inputs is that in the intact antennal lobe circuit, PN responses cannot be predicted purely on the basis of feedforward excitatory inputs10. Two odors can elicit similar responses in an ORN, but divergent responses in a postsynaptic PN. For example, pentyl acetate and 4-methyl phenol evoke similar activity in VM7 ORNs, but not in VM7 PNs (Fig. 1c), implying that these odors recruit different lateral inputs to this glomerulus. After antennae were removed, these odors evoked similar responses in VM7 PNs (Fig. 1c). Overall, removing the antenna increased the correlation between the ranked odor preferences of ORNs and their cognate PNs (Spearman’s ρ increased from 0.82 to 0.88 for VM7, from 0.89 to 0.94 for VC1; p < 0.02 for each comparison, Mann-Whitney U-test).
Lateral inhibition suppresses ORN input
Several recent studies have failed to observe lateral inhibition in the Drosophila antennal lobe7, 11, 12. These studies silenced all direct ORN inputs to a PN, and focused on lateral input to that PN evoked by stimulating ORNs presynaptic to other glomeruli. We reasoned that some lateral inhibition might target ORN axon terminals; if so, this would only be observed when the direct ORN inputs to a PN are active. GABAergic inhibition at ORN axon terminals has been described previously in the olfactory bulb15–21, and synapses from GABAergic interneurons onto ORN axon terminals have been found in an insect antennal lobe22.
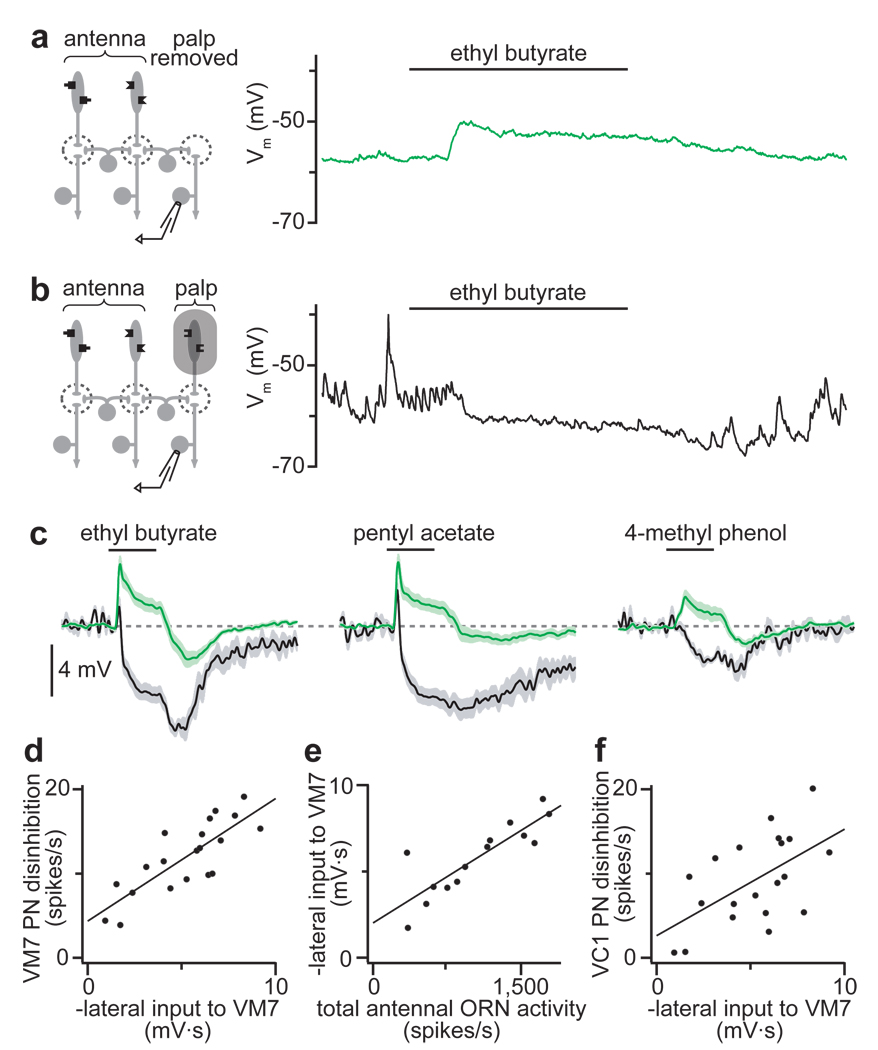
To test the idea that some lateral inhibition is presynaptic, we asked how lateral input to glomerulus VM7 depends on the activity of VM7 ORNs. Each VM7 ORN fires spontaneously at ~10 spikes/s, and consequently these PNs are bombarded by spontaneous spike-driven EPSPs (Supplementary Fig. 4a). When we silenced VM7 ORNs by removing the maxillary palps, large spontaneous EPSPs disappeared in these PNs (Fig. 2a). In this experimental configuration, stimulating the antennae with odorants depolarized VM7 PNs (Fig. 2a), which is consistent with previous reports that lateral input is excitatory when direct ORN inputs are silent7, 11, 12. Next, we asked whether preserving spontaneous activity in VM7 ORNs would allow us to observe lateral inhibition. To prevent odor-evoked activity in VM7 ORNs, we covered the maxillary palps with a plastic shield. We inferred that the shield did not prevent spontaneous activity in VM7 ORNs because we observed normal spontaneous EPSPs in VM7 PNs (Fig. 2b). The shield was clearly an effective barrier to odors because it blocked the normal strong excitatory response to ethyl butyrate in VM7 PNs (Fig. 2b, Supplementary Fig. 4). Rather, when the palps were shielded, stimulating the antennae with odorants suppressed spontaneous EPSPs in VM7 PNs (Fig. 2b,c). This was accompanied by hyperpolarization of the membrane potential, which would reflect (at least in part) the removal of ongoing depolarization produced by EPSP bombardment.
Figure 2. Lateral inhibition suppresses spontaneous EPSCs and scales with total ORN input.
a, Left: experimental configuration. Right: recording from a PN in glomerulus VM7. Olfactory stimulation evokes depolarization. Spontaneous EPSPs are absent.
b, Left: palps are shielded from odors. Right: recording from a PN in glomerulus VM7. Olfactory stimulation suppresses spontaneous EPSPs.
c, Average VM7 PN responses as in a (green) or b (black), n = 6–7 PNs for each condition. When ORNs are shielded (black), inhibition dominates. When ORNs are absent (green), excitation dominates throughout the stimulus period. (Off-inhibition likely reflects lateral postsynaptic inhibition, see Supplementary Fig. 5.)
d, Lateral input to VM7 (as in b) is correlated with the disinhibition in VM7 PNs after antennal removal (see Fig. 1b). Each point represents a different odor. Lateral input is measured as the time-integrated change in membrane potential.
e, Lateral input to VM7 is correlated with total antennal ORN spiking activity evoked by each odor.
f, Lateral input to VM7 is correlated with the disinhibition in VC1 PNs after antennal removal.
For all 20 odors in our panel, lateral input depolarized VM7 PNs when their cognate ORNs were absent (as in Fig. 2a), and hyperpolarized these PNs when their ORNs were spontaneously active (as in Fig. 2b). This argues that a substantial component of lateral inhibition acts at a presynaptic locus. This does not mean that all lateral inhibition is presynaptic; indeed there is evidence for an additional postsynaptic component (Supplementary Fig. 5).
Inhibition scales with total ORN input
When palp ORNs were shielded, different odors evoked different amounts of inhibition in glomerulus VM7 (Fig. 2c, black traces). We hypothesized that this odor tuning could explain why antennal removal disinhibits some VM7 PN odor responses more than others (Fig. 1c,d). To test this, we measured the amount of inhibition evoked by each odor in the shielded-palps experiment, and compared this to the change in PN spiking responses to that odor after antennal removal. These two measures were well-correlated (Fig. 2d, n = 20 odors, Pearson’s r2 = 0.65, p < 0.0001), which argues that these two experimental paradigms measure the same underlying phenomenon.
The odor tuning of lateral input must reflect the connectivity of the local interneurons that mediate this inhibition. Many individual GABAergic interneurons innervate all glomeruli9, 12, 13, suggesting that lateral inhibition to each glomerulus might reflect pooled input from all ORNs. If so, then the size of lateral input to a glomerulus should correlate with the total ORN activity evoked by that odor. We estimated total ORN activity by summing the spiking responses of each antennal ORN type23, and found that this measure predicted the strength of lateral inhibition evoked by each odor in the shielded-palps experiment (Fig. 2e, n = 14 odors, Pearson’s r2 = 0.73, p < 0.0005).
If lateral inhibition to all glomeruli scales with total ORN activity, then lateral inhibitory input to each glomerulus would show the same odor tuning. We have already shown that the odor tuning of lateral input to VM7 is a good predictor of which odor responses were most disinhibited in VM7 PNs after antennal removal (Fig. 2d). As expected, it also partially predicted which odor responses were most disinhibited in a different palp glomerulus, VC1 (Fig. 2f, n = 20 odors, Pearson’s r2 = 0.32, p < 0.01). However, this correlation was weaker than the correlation with disinhibition in VM7. This leaves open the possibility that there may be some differences in the odor tuning of lateral inhibitory input to different glomeruli (see Discussion).
GABA mediates presynaptic inhibition
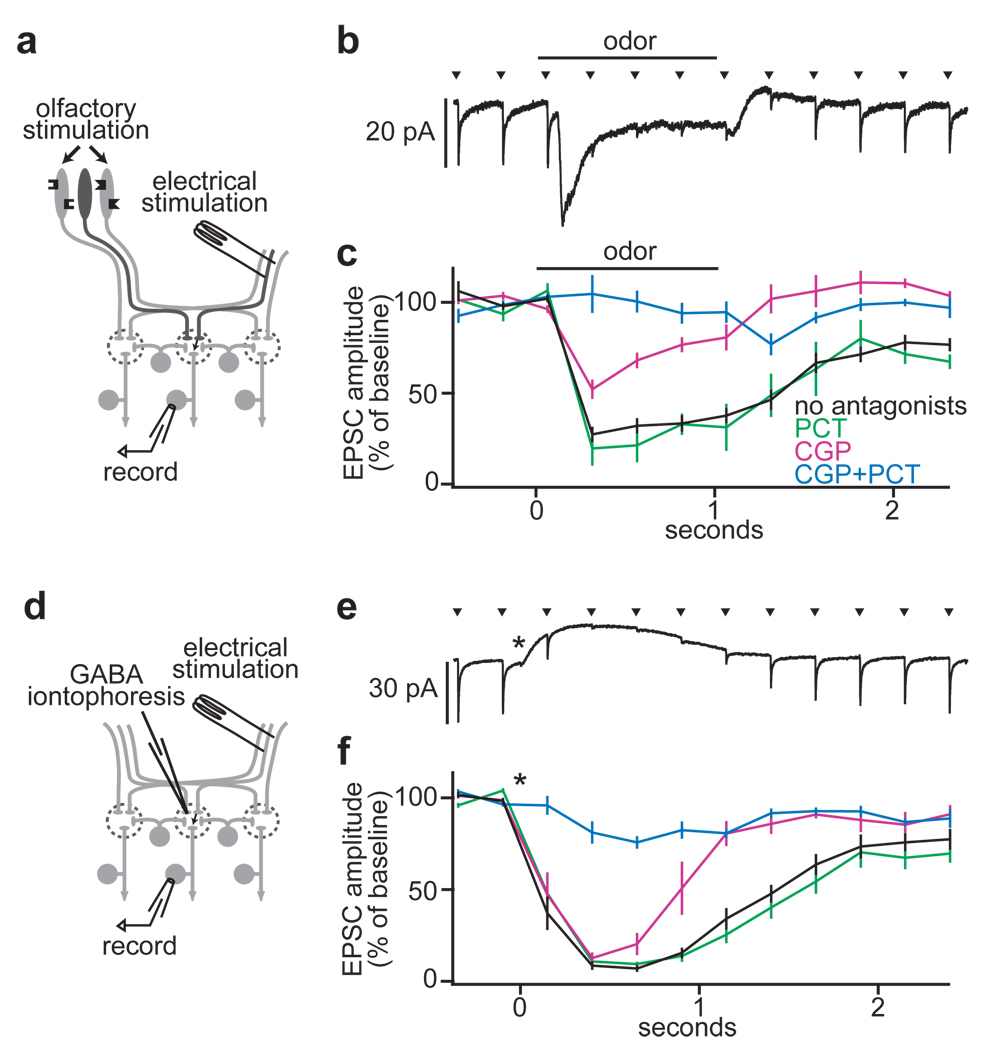
Our results suggest that much of the lateral inhibition in this circuit acts by suppressing ORN-PN synaptic transmission. To test this, we monitored ORN-PN synaptic strength in one glomerulus while recruiting lateral input to that glomerulus (Fig. 3a). We recorded from an identified PN while electrically stimulating the ipsilateral antennal nerve to evoke excitatory postsynaptic currents (EPSCs). Next, we used an odor to stimulate ORNs in the remaining intact antenna (and the maxillary palps). Because most glomeruli receive bilateral ORN input2, olfactory stimulation of the contralateral antenna drives activity in ipsilateral glomeruli. Finally, we prevented odors from recruiting direct ORN input to the recorded PN by mutating the odorant receptor gene normally expressed by its ORNs.
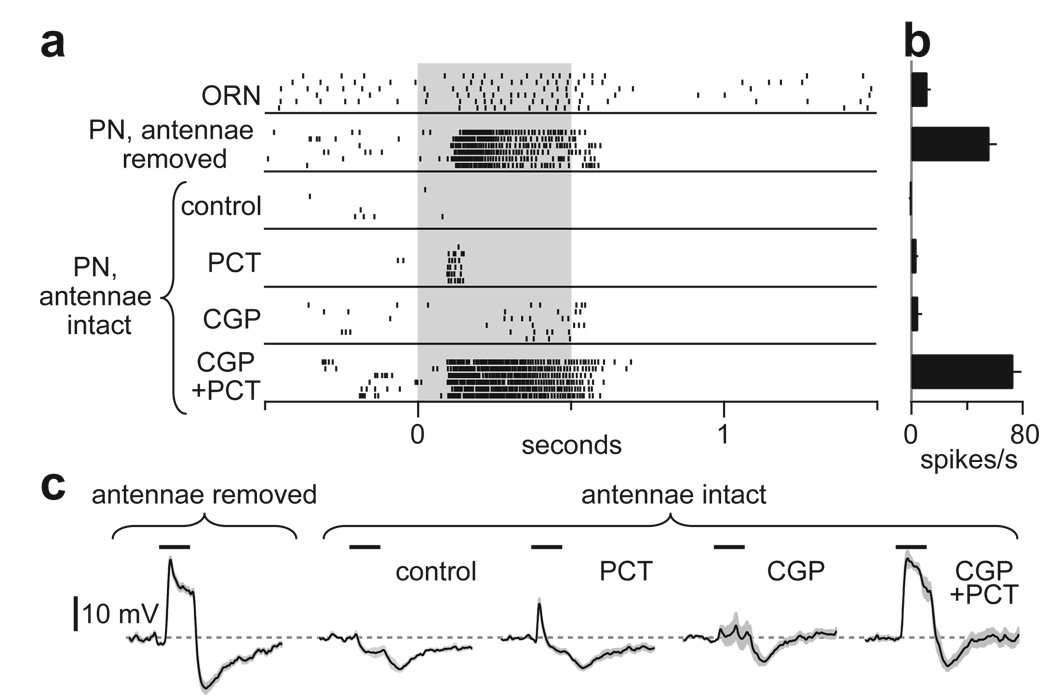
Figure 3. Lateral GABAergic suppression of ORN-PN synapses.
a, Experimental configuration.
b, Electrical stimulation of the antennal nerve (arrowheads) evokes EPSCs in a PN (average of 20 trials). Olfactory stimulation (500 ms) inhibits EPSCs. Note that odor also evokes a transient inward current reflecting lateral postsynaptic excitation; this is resistant to GABA antagonists (Supplementary Fig. 6).
c, Inhibition is blocked by the GABAB antagonist CGP54626 together with the GABAA antagonist picrotoxin (control n = 12, PCT n = 5, CGP n = 5, CGP+PCT n = 5). All pairwise comparisons are significantly different except control versus PCT (p < 0.05, t-tests).
d, Experimental configuration, substituting GABA iontophoresis for olfactory stimulation.
e, As in b for GABA iontophoresis (asterisk). Note that GABA also evokes an outward current.
f, As in c for GABA iontophoresis (control n = 13, PCT n = 5, CGP n = 6, CGP+PCT n = 7). All pairwise comparisons between conditions are significantly different except control versus PCT (p < 0.05, t-tests).
As predicted, olfactory stimulation of the contralateral antenna inhibited EPSCs evoked by ipsilateral nerve stimulation (Fig. 3b,c). We could mimic this inhibition by iontophoresing GABA into the antennal lobe neuropil (Fig. 3d–f). A GABAB receptor antagonist blocked the late phase of this inhibition, but had only a modest effect on the early phase (Fig. 3c,f). Adding a GABAA antagonist to the GABAB antagonist blocked the residual early portion of the inhibition (Fig. 3c,f). The GABAA antagonist alone had no effect (Fig. 3c,f). Taken together, these results suggest that both GABAA and GABAB receptors are present on the same ORN axon terminals, and either GABAA or GABAB receptors alone are sufficient to mediate substantial inhibition of EPSCs just after GABA release. The late phase of inhibition evidently involves only GABAB receptors.
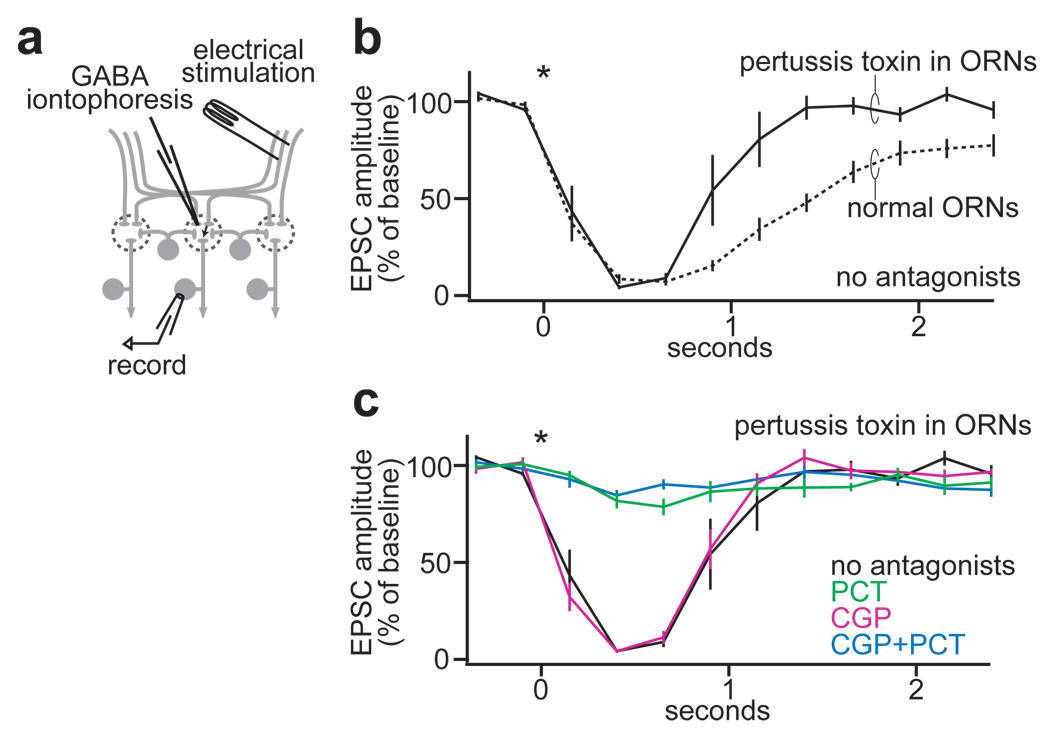
Presynaptic inhibition is generally associated with a change in the way a synapse responds to paired electrical pulses24. We found that both the GABAA and GABAB components of EPSC inhibition are associated with an increase in the paired-pulse ratio (Supplementary Fig. 7). This implies that the independent actions of both GABAA and GABAB receptors are at least partially presynaptic. As a further test of this model, we genetically abolished GABAB signaling selectively in presynaptic ORNs. We used an ORN-specific promoter25 to drive expression of pertussis toxin26, a selective inhibitor of some types of G-proteins. In these flies, GABA still inhibited ORN-PN EPSCs, but now this inhibition had a briefer duration than in wild-type flies (Fig. 4a,b). Unlike in wild-type flies, this inhibition was completely resistant to the GABAB antagonist and completely blocked by the GABAA antagonist (compare Fig. 4c to Fig. 3f). As a negative control, we confirmed that this phenotype requires both the ORN-specific promoter and the toxin transgene (Supplementary Fig. 8). This demonstrates that GABAB receptors inhibit ORN-PN synapses at a purely presynaptic locus.
Figure 4. Genetic evidence that GABAB receptors inhibit ORN-PN synapses at a presynaptic locus.
a, Experimental configuration.
b, When pertussis toxin is specifically expressed in ORNs, GABAergic inhibition of EPSCs (solid line, n = 11) is more transient than in control flies (dotted line, n = 13, reproduced from Fig. 3f).
c, Pertussis toxin expression in ORNs renders the GABAergic inhibition of EPSCs completely insensitive to CGP, and completely sensitive to PCT (black trace reproduced from a, CGP n = 5, PCT n = 6, CGP+PCT n = 5). Compare Fig. 3f.
If activation of either GABAA or GABAB receptors is sufficient to mediate strong lateral inhibition, then blockade of both receptors should be required to mimic the removal of lateral input. To test this, we again recorded from palp PNs in glomerulus VM7. Normally, the odor pentyl acetate weakly excites VM7 ORNs and inhibits VM7 PNs. When most lateral input is removed (by removing the antennae), this odor strongly excites these PNs (Fig. 1c). We could not mimic this disinhibition by applying either a GABAA or a GABAB receptor antagonist alone. However, the two antagonists together produced strong disinhibition that resembled the effect of removing the antennae (Fig. 5).
Figure 5. GABA receptor antagonists mimic removal of lateral input to a PN.
a, Rasters show spiking responses to pentyl acetate (gray) in a VM7 ORN and a VM7 PN. With antennae intact, both antagonists are required to mimic the effect of antennal removal on PNs. (Note some off-inhibition in PNs persists after antennal removal or in the presence of antagonists; this probably reflects the off-inhibition in VM7 ORNs.)
b, Average spike rates during odor stimulus period, minus baseline spike rates (n = 5–6 PNs for each condition).
c, Average membrane potential responses to pentyl acetate in VM7 PNs (n = 5–6 PNs for each).
Discussion
Many previous studies have shown that odors can inhibit spiking in olfactory bulb mitral cells and antennal lobe projection neurons (see refs. 27–32 for early examples), but in principle this inhibition could be purely intra-glomerular15–17. Experiments in vitro have revealed several types of inter-glomerular circuits33–35, but some of these circuits are evidently not recruited by olfactory stimuli15. Here we have directly demonstrated an important role for inhibitory interactions between olfactory glomeruli in vivo.
Our results argue that a substantial component of inter-glomerular inhibition occurs at a presynaptic locus. Previous studies in other species have shown that GABA can inhibit release from ORN axon terminals15–21. Our results imply that in Drosophila this is mediated by both GABAA and GABAB receptors. This arrangement is unusual but not unique; for example, there are several instances of GABAA and GABAB inhibition at the same presynaptic site in other neural circuits36–39. Ionotropic and metabotropic receptors act with different kinetics, and so this arrangement might ensure that inhibition spans a broad time window. Although both receptor types were co-active during most of the odor response, we noticed that GABAA receptors were required for the a brief early phase of inhibition after odor onset (Fig. 5), while GABAB receptors were required for the long, late phase (Fig. 3 and Fig. 5).
We have shown that lateral inhibitory input to a glomerulus roughly scales with total ORN activity. This would imply that the odor tuning of GABAergic input to each glomerulus is approximately similar. However, the effects of lateral input may nevertheless be somewhat glomerulus-specific. Even if the odor tuning of GABA release were identical in all glomeruli, the efficacy of presynaptic inhibition will vary with presynaptic membrane potential40, 41. As a result, the same inhibitory signal should be more effective in some glomeruli than in others. Also, in some glomeruli, lateral inhibition might be outweighed by lateral excitation. This would explain why other studies have found that some PNs can be excited by odors that do not excite their cognate ORNs8, 10, 42.
In sum, we propose that this form of gain control represents a flexible balance between sensitivity and efficiency. When total ORN input is weak, lateral inhibition is minimal, and ORN-PN synapses are strong. When an odorant recruits vigorous ORN input to many glomeruli, GABAergic interneurons inhibit ORN neurotransmitter release. This should prevent a stimulus from saturating the dynamic range of many PN types simultaneously. Because this mechanism suppresses responses that are strong and redundant, it may tend to decrease cross-correlations between the output of different glomeruli, and thus promote a more efficient neural code for odors.
Methods Summary
In vivo whole-cell patch clamp recordings from PNs and extracellular recordings from ORNs were performed essentially as previously described8–11, 42. Antagonists (CGP54626 50 µM and picrotoxin 5 µM) were added to the saline which perfused the brain. Pertussis toxin was expressed under control of the Or83b-Gal4 driver. All data in Results are mean values, averaged across experiments, ± s.e.m. (except for raw electrophysiological traces and rasters). The odor stimulus period was 500 ms (shown as black bar in Figures).
Supplementary Material
Acknowledgments
We are grateful to K. Ito, L. Luo, G. Roman, D.P. Smith, L.M. Stevens, and L.B. Vosshall for gifts of fly stocks. We thank G. Laurent, A.W. Liu, and members of the Wilson lab for helpful conversations. This work was funded by a grant from the NIDCD, the Pew, McKnight, Sloan, and Beckman Foundations (to R.I.W.). S.R.O. was partially supported by a NSF fellowship.
Footnotes
Full Methods are available in the online version of the paper at www.nature.com/nature.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature. A figure summarizing the main result of this paper is available in Supplementary Information.
Author Contributions. S.R.O. performed the experiments and analyzed the data. S.R.O. and R.I.W. designed the experiments and wrote the paper.
Author Information. Reprints and permissions are available at www.nature.com/reprints.
REFERENCES
- 1.Laissue PP, et al. Three-dimensional reconstruction of the antennal lobe in Drosophila melanogaster. J. Comp. Neurol. 1999;405:543–552. [PubMed] [Google Scholar]
- 2.Stocker RF, Lienhard MC, Borst A, Fischbach KF. Neuronal architecture of the antennal lobe in Drosophila melanogaster. Cell Tissue Res. 1990;262:9–34. doi: 10.1007/BF00327741. [DOI] [PubMed] [Google Scholar]
- 3.Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr. Biol. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 4.Fishilevich E, Vosshall LB. Genetic and functional subdivision of the Drosophila antennal lobe. Curr. Biol. 2005;15:1548–1553. doi: 10.1016/j.cub.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 5.Marin EC, Jefferis GS, Komiyama T, Zhu H, Luo L. Representation of the glomerular olfactory map in the Drosophila brain. Cell. 2002;109:243–255. doi: 10.1016/s0092-8674(02)00700-6. [DOI] [PubMed] [Google Scholar]
- 6.Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 7.Root CM, Semmelhack JL, Wong AM, Flores J, Wang JW. Propagation of olfactory information in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11826–11831. doi: 10.1073/pnas.0704523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson RI, Turner GC, Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science. 2004;303:366–370. doi: 10.1126/science.1090782. [DOI] [PubMed] [Google Scholar]
- 9.Wilson RI, Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J. Neurosci. 2005;25:9069–9079. doi: 10.1523/JNEUROSCI.2070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhandawat V, Olsen SR, Gouwens NW, Schlief ML, Wilson RI. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat. Neurosci. 2007;10:1474–1482. doi: 10.1038/nn1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsen SR, Bhandawat V, Wilson RI. Excitatory interactions between olfactory processing channels in the Drosophila antennal lobe. Neuron. 2007;54:89–103. doi: 10.1016/j.neuron.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shang Y, Claridge-Chang A, Sjulson L, Pypaert M, Miesenbock G. Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell. 2007;128:601–612. doi: 10.1016/j.cell.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stocker RF, Heimbeck G, Gendre N, de Belle JS. Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J. Neurobiol. 1997;32:443–456. doi: 10.1002/(sici)1097-4695(199705)32:5<443::aid-neu1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Ng M, et al. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron. 2002;36:463–474. doi: 10.1016/s0896-6273(02)00975-3. [DOI] [PubMed] [Google Scholar]
- 15.McGann JP, et al. Odorant representations are modulated by intra- but not interglomerular presynaptic inhibition of olfactory sensory neurons. Neuron. 2005;48:1039–1053. doi: 10.1016/j.neuron.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 16.Murphy GJ, Darcy DP, Isaacson JS. Intraglomerular inhibition: signaling mechanisms of an olfactory microcircuit. Nat. Neurosci. 2005;8:354–364. doi: 10.1038/nn1403. [DOI] [PubMed] [Google Scholar]
- 17.Vucinic D, Cohen LB, Kosmidis EK. Interglomerular center-surround inhibition shapes odorant-evoked input to the mouse olfactory bulb in vivo. J. Neurophysiol. 2006;95:1881–1887. doi: 10.1152/jn.00918.2005. [DOI] [PubMed] [Google Scholar]
- 18.Nickell WT, Behbehani MM, Shipley MT. Evidence for GABAB-mediated inhibition of transmission from the olfactory nerve to mitral cells in the rat olfactory bulb. Brain Res. Bull. 1994;35:119–123. doi: 10.1016/0361-9230(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 19.Wachowiak M, Cohen LB. Presynaptic inhibition of primary olfactory afferents mediated by different mechanisms in lobster and turtle. J. Neurosci. 1999;19:8808–8817. doi: 10.1523/JNEUROSCI.19-20-08808.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aroniadou-Anderjaska V, Zhou FM, Priest CA, Ennis M, Shipley MT. Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABA(B) heteroreceptors. J. Neurophysiol. 2000;84:1194–1203. doi: 10.1152/jn.2000.84.3.1194. [DOI] [PubMed] [Google Scholar]
- 21.Wachowiak M, et al. Inhibition of olfactory receptor neuron input to olfactory bulb glomeruli mediated by suppression of presynaptic calcium influx. J. Neurophysiol. 2005;94:2700–2712. doi: 10.1152/jn.00286.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Distler PG, Boeckh J. Synaptic connections between identified neuron types in the antennal lobe glomeruli of the cockroach, Periplaneta americana: II. Local multiglomerular interneurons. J. Comp. Neurol. 1997;383:529–540. doi: 10.1002/(sici)1096-9861(19970714)383:4<529::aid-cne9>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 24.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 25.Larsson MC, et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 26.Ferris J, Ge H, Liu L, Roman G. G(o) signaling is required for Drosophila associative learning. Nat. Neurosci. 2006;9:1036–1040. doi: 10.1038/nn1738. [DOI] [PubMed] [Google Scholar]
- 27.Shibuya T, Ai N, Takagi SF. Response types of single cells in the olfactory bulb. Proc. Jpn. Acad. 1962;38:231–233. [Google Scholar]
- 28.Macrides F, Chorover SL. Olfactory bulb units: activity correlated with inhalation cycles and odor quality. Science. 1972;175:84–87. doi: 10.1126/science.175.4017.84. [DOI] [PubMed] [Google Scholar]
- 29.Mathews DF. Response patterns of single units in the olfactory bulb of the rat to odor. Brain Res. 1972;47:389–400. doi: 10.1016/0006-8993(72)90647-6. [DOI] [PubMed] [Google Scholar]
- 30.Tanabe T, Iino M, Takagi SF. Discrimination of odors in olfactory bulb, pyriform-amygdaloid areas, and orbitofrontal cortex of the monkey. J. Neurophysiol. 1975;38:1284–1296. doi: 10.1152/jn.1975.38.5.1284. [DOI] [PubMed] [Google Scholar]
- 31.Meredith M, Moulton DG. Patterned response to odor in single neurones of goldfish olfactory bulb: influence of odor quality and other stimulus parameters. J. Gen. Physiol. 1978;71:615–643. doi: 10.1085/jgp.71.6.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaput M, Holley A. Single unit responses of olfactory bulb neurones to odour presentation in awake rabbits. J. Physiol. Paris. 1980;76:551–558. [PubMed] [Google Scholar]
- 33.Isaacson JS, Strowbridge BW. Olfactory reciprocal synapses: dendritic signaling in the CNS. Neuron. 1998;20:749–761. doi: 10.1016/s0896-6273(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 34.Urban NN, Sakmann B. Reciprocal intraglomerular excitation and intra- and interglomerular lateral inhibition between mouse olfactory bulb mitral cells. J Physiol. 2002;542:355–367. doi: 10.1113/jphysiol.2001.013491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aungst JL, et al. Centre-surround inhibition among olfactory bulb glomeruli. Nature. 2003;426:623–629. doi: 10.1038/nature02185. [DOI] [PubMed] [Google Scholar]
- 36.Stuart GJ, Redman SJ. The role of GABAA and GABAB receptors in presynaptic inhibition of Ia EPSPs in cat spinal motoneurones. J. Physiol. 1992;447:675–692. doi: 10.1113/jphysiol.1992.sp019023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matthews G, Ayoub GS, Heidelberger R. Presynaptic inhibition by GABA is mediated via two distinct GABA receptors with novel pharmacology. J. Neurosci. 1994;14:1079–1090. doi: 10.1523/JNEUROSCI.14-03-01079.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer Y, Parnas I. Differential activation of two distinct mechanisms for presynaptic inhibition by a single inhibitory axon. J. Neurophysiol. 1996;76:3807–3816. doi: 10.1152/jn.1996.76.6.3807. [DOI] [PubMed] [Google Scholar]
- 39.Fischer Y, Parnas I. Activation of GABAB receptors at individual release boutons of the crayfish opener neuromuscular junction produces presynaptic inhibition. J. Neurophysiol. 1996;75:1377–1385. doi: 10.1152/jn.1996.75.4.1377. [DOI] [PubMed] [Google Scholar]
- 40.Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- 41.Foldy C, Neu A, Jones MV, Soltesz I. Presynaptic, activity-dependent modulation of cannabinoid type 1 receptor-mediated inhibition of GABA release. J. Neurosci. 2006;26:1465–1469. doi: 10.1523/JNEUROSCI.4587-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlief ML, Wilson RI. Olfactory processing and behavior downstream from highly selective receptor neurons. Nat. Neurosci. 2007;10:623–630. doi: 10.1038/nn1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.