Abstract
The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
The conditioned rewarding effects of novelty compete with those of cocaine for control over choice behavior using a place-conditioning task. The purpose of the present study was to use multiple doses of cocaine to determine the extent of this competition and to determine whether novelty's impact on cocaine reward was maintained over an abstinence period. In Experiment 1, rats were conditioned with cocaine (7.5, 20, or 30 mg/kg, IP) to prefer one side of an unbiased place conditioning apparatus relative to the other. In a subsequent phase, all rats received alternating daily confinements to the previously cocaine-paired and unpaired sides of the apparatus. During this phase, half the rats had access to a novel object on their initially unpaired side; the remaining rats did not receive objects. The ability of novelty to compete with cocaine in a drug-free and cocaine-challenge test was sensitive to cocaine dose. In Experiment 2, a place preference was established with 10 mg/kg cocaine and testing occurred after 1, 14, or 28 day retention intervals. Findings indicate that choice behaviors mediated by cocaine conditioning are reduced with the passing of time. Taken together, competition between cocaine and novelty conditioned rewards are sensitive to drug dose and retention interval.
Keywords: reward competition, Pavlovian conditioning, sensation seeking, exploratory behavior, conditioned place preference
In the case of drug abuse, one aspect of addiction may involve the excessive choice of a drug over other aspects of the addict's life (Winger, Woods, Galuska, & Wade-Galuska, 2005). Thus, interventions strategies may benefit by understanding the influence of associative learning on choice behavior. From a conditioning perspective, learning histories that include drug-associated stimuli may influence choice behavior during treatment or abstinence. The place conditioning procedure allows one to study the learning processes involved in associatively-motivated choice behavior (Bardo & Bevins, 2000). One way to compare the impact of conditioned associations on choice behavior is with a variation of the traditional place conditioning procedure. Rather than comparing a drug to saline, as is typically done, a known rewarding stimulus (e.g., cocaine) can be compared to some other value of the same stimuli or to a different stimulus (i.e., another cocaine dose or different drug) (Barr, Paredes, & Bridger, 1985; Bevins, 2005; Groblewski, Bax, & Cunningham, 2008; Patkina & Zvartau, 1998). Recently, several laboratories have also studied associatively-motivated choice behaviors with comparisons between drug and non-drug rewarding stimuli, including cocaine vs. pups (Mattson, Willams, Rosenblatt, & Morrell, 2001), cocaine vs. social interaction (Thiel, Okun, Neisewander, 2008), and cocaine vs. novel objects (Reichel & Bevins, 2008).
The interaction between conditioned cocaine reward and novelty seeking is particularly interesting given that individuals that seek highly rewarding stimuli are more vulnerable to drug abuse (Zuckerman & Kuhlman, 2000). This correlation between drug use and the sensory/novelty-seeking characteristic has been attributed to an overlap in the rewarding properties of abused drugs and response to novel situations (Bardo, Donohew, & Harrington, 1996; Bardo & Dwoskin, 2004; Bevins, 2001). Preclinical animal research shows that presentation of novel stimuli or placement in a novel environment can decrease drug intake (Cain, Smith, & Bardo, 2004; Thompson & Ostlund, 1965). In a place conditioning procedure, presentation of a novel object can potentiate the rewarding effects of a low dose of cocaine (Bevins, 2001). More so, our laboratory recently demonstrated that the conditioned rewarding effects of novelty can alter choice involving drug reward (Reichel & Bevins, 2008).
In these studies, rats were initially conditioned with cocaine (7.5 mg/kg, IP) to prefer one side of an unbiased place conditioning apparatus. In the subsequent phase, all rats received alternating daily confinements (10 min) to the previously cocaine-paired and unpaired (i.e., saline) sides of the apparatus. Consequently, when rats were placed on the previously cocaine-paired side, they were subjected to procedural extinction. That is, rats experienced the drug-paired environment (conditioned stimulus, CS) without the physiological effects of the drug (unconditioned stimulus, US) (Rescorla, 2004; Pavlov, 1927). During this phase, half the rats had access to a novel object on their initially unpaired side (i.e., Novelty rats), whereas the other half did not have objects (i.e., Control rats). Rats were tested in a drug-free and cocaine state. On the drug-free test, the cocaine-conditioned preference was eliminated for the Novelty rats, but not for Control rats. This pattern of choice behavior persisted even in the cocaine state. Thus, pairing the previously unpaired environment with novel objects shifted a preference away from the cocaine-paired environment during drug-free and drug-challenge tests.
The competition previously described was only characterized with one cocaine dose at one retention interval. The limit of this competition is unknown. It is possible that higher doses of cocaine may be more resistant to competition by novelty or that competition will not exist after longer retention intervals. Therefore, the purpose of this current study was twofold. First, in Experiment 1, we characterized the relation between conditioned novelty and cocaine reward by assessing competition across a range of cocaine doses (i.e., 7.5, 20, or 30 mg/kg). This range of doses was chosen based on preliminary data from our laboratory showing reliable cocaine place condition with these doses. Recent clinical studies in human cocaine users report consumption ranges form 0.54 to 12 g per week (Colzato, van den Wildenberg, & Hommel, 2008; Garcia-Rodriguez et al., 2009; Pace-Schott et al., 2008). Using an average body weight of 70 kg this range converts to 7.7 to 171.42 mg/kg/week. In regards to our cocaine doses rats received 30 mg/kg (4 injections of 7.5 mg/kg) to 120 mg/kg (4 injections of 30 mg/kg) over 8 days. Keeping in mind species differences and administration routes, our dose range can be considered low to moderate in relation to human consumption. Second, in Experiment 2, we determined whether novelty's ability to compete with cocaine persisted or if the initial drug preference returned after a period of abstinence. Previously, we reported that novelty reward can compete with cocaine reward when tested 24 to 48 h later (drug-free and drug-challenge tests, respectively) (Reichel & Bevins, 2008). This question is of particular interest because long-term intervention strategies are essential to alleviate the relapse of drug-seeking behaviors.
General Method
Subjects
Experimentally naïve male Sprague-Dawley rats (N=143) were 250-300 g at the time of delivery. They were individually housed in a temperature- and humidity-controlled colony on a 12:12 light: dark cycle in plastic cages 48.3 × 24.1 × 21.0 cm (l × w × h) with stainless steel lids. Water and food were available continuously in the home cage. The experimental protocols followed the “Guide for the Care and Use of Laboratory Animals” (National Research Council, 1996) and were approved by the University of Nebraska-Lincoln Institutional Animal Care and Use Committee.
Apparatus
Experiments were conducted in two identical aluminum and Plexiglas chambers having two distinct compartments (each compartment = 40 × 16 × 20 cm [l × w × h]) separated by a smaller area for placement. Different floor types created distinct end compartments. One floor had approximately 340 1.3-cm holes drilled into a 16-gauge aluminum sheet. The other floor had 1-cm rods made from stainless steel. The center placement area was created by removable pieces of metal to form a placement area (6.5 × 15.5 × 19.5 cm [l×w×h]). During conditioning, a solid aluminum floor the same length as that used in the center compartment (6.5 cm) was placed in each end chamber nearest the wall blocking access to the center compartment. This maneuver reduced the novelty of this floor on post-conditioning choice tests. The novel objects used were a white sock (about 40 cm long), a white PVC pipe (8 cm long; 10.5 cm diameter), a plastic scouring pad (9 cm diameter) attached to a paint roller (7.5 cm long, 4 cm diameter), and a sheet of newspaper wadded into a ball (cf. Bevins et al., 2002; Reichel & Bevins, 2008).
Drugs
(–)-Cocaine hydrochloride was purchased from Sigma Chemicals (St. Louis, MO), dissolved in saline (0.9% NaCl) and injected intraperitoneally (IP) at a volume of 1 ml/kg.
Statistical Analysis
Dependent measures
The main dependent measure during habituation and all ensuing test sessions was time spent (in sec) in each compartment. Sessions were videotaped and observed later. Criterion for a rat to be considered in compartment required the forelegs, head, and shoulders to be positioned inside the compartment. A secondary measure was horizontal activity (defined as the number of times the rat's front paws crossed a center line that bisected the end compartments) in each end compartment. The total number of line crosses during the session defined activity. Observers' naïve to the experimental conditions conducted interobserver reliability checks on both measures. A total of 34 time and activity observations in common were analyzed with Pearson-product moment correlation analysis (rs≥.90, ps=.001)
Data Analysis
On the habituation day, paired t-tests were used to analyze the time spent and activity counts in each end compartment (i.e., rods versus holes) to demonstrate the unbiased construction of our apparatus. For all other test days, 2- or 3-way analysis of variance (ANOVA) was used to analyze time spent in each end compartment and activity counts. Type I error rate was controlled for by Tukey Honestly Significant Difference (HSD) post-hoc comparisons. Statistical significance was declared at p<.05 for all tests and only significant values are reported.
Experiment 1: The Extent that Novelty Conditioned Reward Competes with Cocaine Conditioned Reward
Habituation
To confirm the unbiased construction of the apparatus and provide experience with the later testing procedure, rats were set onto the center placement area and allowed free access to explore both end compartments for 10 min. Conditioning groups were then assigned in an unbiased fashion (cf. Bevins & Cunningham, 2006).
Cocaine Place Conditioning
These procedures were previously described in Reichel & Bevins (2008). All placements into the compartments were counterbalanced according to rods/holes, spatial orientation, and whether drug pairing occurred on the first or second day of conditioning (Bevins & Cunningham, 2006). On the first conditioning day, half the rats were injected with cocaine (7.5, 20, or 30 mg/kg, IP) immediately before placement into their paired compartment. The other half were administered saline before placement into the unpaired compartment. On the second day of conditioning, rats that were injected with cocaine on the previous day received saline; rats that received saline were injected with cocaine and placed in the opposite compartment. Alternating daily placements lasted for 8 consecutive days and were separated by 24 h. On the test day, rats were placed into the center placement area after an injection of saline and allowed to roam the apparatus for 10 min.
Competing Conditioning
The competing conditioning phase occurred 24 hrs after the last conditioning session (see Figure 1, for a schematic representation). Rats were assigned to Control or Novelty groups with a restriction that the groups did not differ on the initial place conditioning score. Alternating daily placements were preceded by saline injections and lasted for 10 min. When rats were restricted to their previously cocaine-paired compartment, they experienced the drug-associated cues in the absence of the drug effect (i.e., extinction). This type of procedural extinction refers to re-exposure to the CS (i.e., exteroceptive cues of the paired environment) without the US (Pavlov, 1927; Rescorla, 2004). The rats in these experiments were placed on extinction of the cocaine-associated cues of the paired environment when they were confined to this environment during the novelty-conditioning phase and did not receive drug. When rats were placed on the previously saline-paired side, rats in the Novelty group had access to a different novel object on each placement. Control rats did not have access to objects. Importantly, both groups received procedural extinction on the previously cocaine-paired side, but only the Novelty group experienced the novel objects on the previously saline-paired side.
Figure 1.
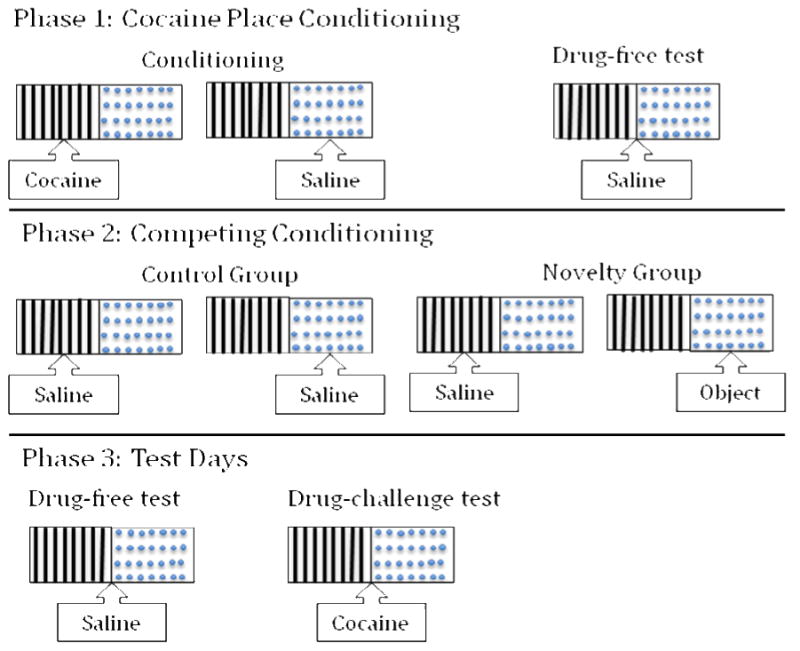
Compartment placements throughout the experimental phases are represented with this schematic representation. Rats were conditioned and assigned to groups in an unbiased fashion. All compartment placements were counterbalanced according to rods/holes, spatial orientation, and whether drug and novelty pairings occurred on the first or second day if conditioning.
Drug-free and Drug-challenge Test Days
The drug-free test was conducted 24 hr after completion of the competing conditioning phase. On the drug-free test day, rats were given saline, placed in the center compartment, and allowed to explore both sides of the apparatus for 10 min. On the drug-challenge test day (24 hr after the drug-free test), rats were injected with their conditioning dose of cocaine before placement in the center compartment and allowed free access to the entire apparatus for 10 min. Since our primary interest was the choice behaviors in a drug-free state, this test preceded the drug-challenge test for all rats.
Results
Habituation
Prior to cocaine conditioning, rats did not exhibit a bias toward one particular stimulus (holes vs. rods) over the other. The mean (± SEM) time spent in the holes versus rods compartment was 268.4 (± 3.89) and 260.4 (± 3.73), respectively. Activity on the holes side was 32.3 (±.81) and on rods was 33.2 (±.77).
Place Conditioning
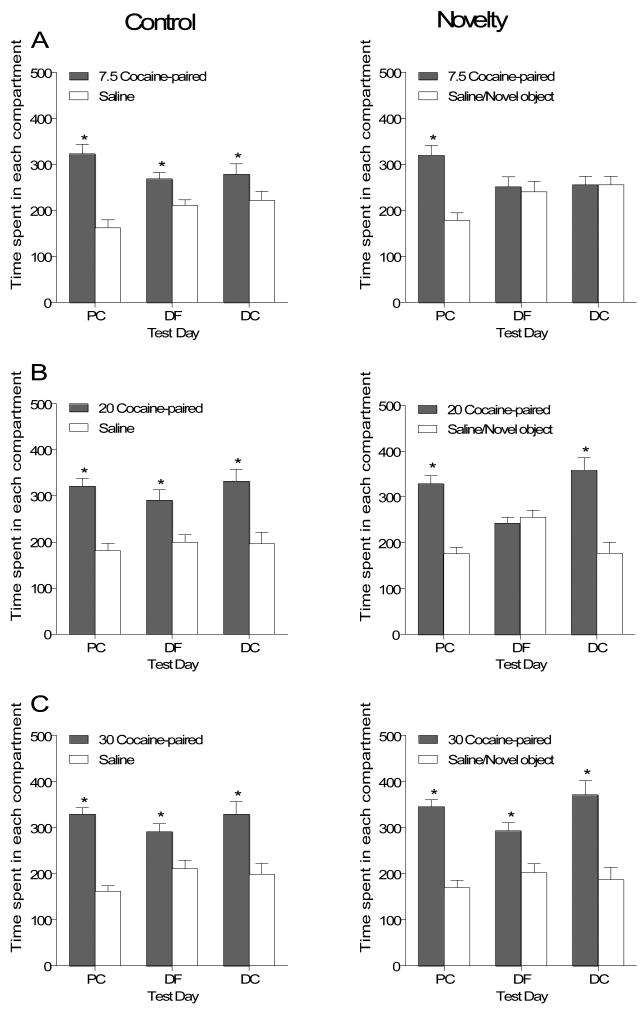
Figure 2 shows the seconds spent in each compartment on the three test days for Control (left panel) and Novelty (right panel) rats. For Control rats (n=12), conditioning with 7.5 mg/kg cocaine (Figure 2A, upper left graph) resulted in more time spent in the cocaine-paired compartment on all three test days. This observation was supported by a main effect of Side, F(1,11)=25.66, p<.001. Novelty rats (n=11, Figure 2A, upper right graph) conditioned with 7.5 mg/kg cocaine, only preferred the cocaine-paired side on the initial test of cocaine place conditioning [Side × Test interaction, F(2,20)=4.72, p<.021 and Tukey post hoc (p<.05)].
Figure 2.
Mean time (± SEM) spent in seconds for rats in the Control and Novelty groups conditioned with 7.5, 20, and 30 mg/kg cocaine on the three tests of place conditioning in Experiment 1. Panel A shows data for rats conditioned with 7.5 mg/kg cocaine. Panel B represents data from the 20 mg/kg conditioning group and Panel C from the 30 mg/kg group. The Control group is represented in the left column and the Novelty group on the right. PC= place conditioning, DF= drug-free test, DC= drug challenge test.
* Indicates significant difference between compartments.
Figure 2B shows the time spent in each end compartment for rats conditioned with 20 mg/kg cocaine. Control rats (n=12, center left graph) also preferred the cocaine-paired side on all three test days [main effect of Side, F(1,11)=22.14, p<.001]. For Novelty rats (n=12, center right graph) conditioned with 20 mg/kg cocaine compartment preference varied over test days as indicated by a Side × Test interaction, F(2,22)=9.99, p<.001. This group preferred the drug-paired side on the initial test of cocaine place conditioning and on the drug-challenge test day (Tukey, p<.05). The main effects of Side, F(1,11)=17.23, p<.002, and Test, F(2,22)= 6.29, p<.007, were also significant.
Figure 2C shows the data for rats conditioned with 30 mg/kg cocaine. Both groups, Control (n=12, lower left graph) and Novelty (n=12, lower right graph) preferred the drug-paired side on all three test days [main effects of Side [Control, F(1,11)=31.19, p<.001; Novelty, F(1,11)=30.38, p<.001].
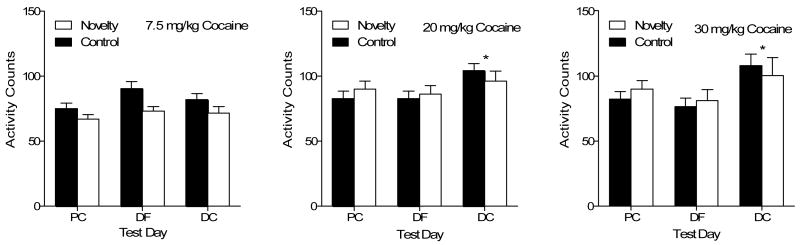
Activity
Figure 3 shows the activity data for the Novelty and Control groups conditioned with the three different cocaine doses. The two-way interaction of Cocaine × Test was significant, F(4,130)= 3.24, p<.014, as were the main effects of Test, F(2,130)= 7.90, p<.004, and Cocaine, F(2,65)= 5.45, p<.006. Post-hoc Tukey test show that drug challenges with 20 and 30 mg/kg cocaine increased activity in comparison to the initial test of place conditioning and the drug-free test (p<.05).
Figure 3.
Mean activity counts (± SEM) for rats conditioned with 7.5, 20, or 30 mg/kg cocaine in the Control and Novelty groups for Experiment 1. PC= place conditioning, DF= drug-free test, DC= drug challenge test.
* Indicates significant difference on the drug challenge test in comparison to both other tests for rats in both groups.
Discussion
The ability of novelty to compete with cocaine in a drug-free and cocaine-challenge test was sensitive to cocaine dose and drug state. Importantly, this experiment identified the limitations of novelty competition because preferences formed with higher doses of cocaine were more resistant to competition by novelty reward. In fact, Control and Novelty rats conditioned with 30 mg/kg cocaine preferred the cocaine-paired side whether tested in a drug or drug-free state and both groups conditioned with 20 mg/kg cocaine preferred the cocaine-paired side on the drug-challenge test. However, the conditioned rewarding effects of novelty competed against the previously established 7.5 and 20 mg/kg cocaine-conditioned preference in the drug-free state. This competition remained even in the cocaine state for 7.5 mg/kg cocaine. Lower doses of cocaine were more sensitive to novelty competition. This outcome is consistent with a study by Cain and colleagues using amphetamine self-administration in rats. In that experiment, they found that the opportunity to interact with a novel object during self-administration of low (0.003 and 0.01 mg/kg/infusion) amphetamine doses reduced the number of drug infusions, but the novel object had no impact on responding at higher doses (0.03 and 0.056 mg/kg/infusion).
The differences in compartment choice in the present study were not attributable to cocaine's locomotor activating effects because on all three test days the groups never differed on activity measures. This comparison is methodologically important because of concerns about the influence of motor activity on the expression of a compartment preference, particularly when drug is on board during testing. Indeed, on drug-challenge tests 20 and 30 mg/kg cocaine increased activity counts relative to the drug-free tests. Despite this increase, compartment preferences were expressed for both Control and Novelty groups with these doses of cocaine. This pattern dispels any concern about changes in sensory-motor processing having interfered with a subject's ability to approach and maintain contact with the stimulus in the present experiment (Gremel & Cunningham, 2007).
The finding that competition involving the conditioned rewarding effects of novelty was sensitive to cocaine dose is notable given that a place conditioning task is typically insensitive to dose effects. Indeed, one limitation of the place conditioning procedure is that conditioned choice is often “all-or-none” (e.g., Carr et al., 1989; Bardo & Bevins, 2000). That is, once the dose of drug of interest has crossed some threshold for reward (i.e., conditions a place preference), higher doses do not typically generate greater preferences for the paired environment (Bardo et al., 1995; Bardo & Bevins, 2000; Bevins, 2005; O'Dell et al., 1996; Meuller & Stewart, 2000). Presumably, this “all-or-none” pattern reflects the unchanging comparison between conditioned drug reward (paired) and similar familiarization and injection number as the paired environment without the reward (unpaired). By changing the nature of the comparison to a choice between cocaine or novelty conditioned rewards, this study reveals differences that may not be detectable in a standard place conditioning protocol.
Previously, we demonstrated that novelty competed with the conditioned rewarding effects of 7.5 mg/kg cocaine (Reichel & Bevins, 2008). One account of this finding is that novelty and cocaine reward are indistinguishable and generalized to each one another on test day for the novelty group. However, the dissociation between cocaine doses discounts such an account. In phase one of our experiment, cocaine enters into a conditioned association with the environment and saline does not. Thus, the choice was between an environment paired with cocaine versus an environment that has been equally exposed with saline injections. Such a comparison results in more time spent in the cocaine-paired environment on tests. During the novelty-conditioning phase, another stimulus with conditioned rewarding value (i.e., novel objects) is presented so that all ensuing tests are relying on a comparison between conditioned rewards (see Bevins, 2005; Bevins & Bardo, 2000 for theoretical accounts of the learning that occurs in place conditioning). Changing the nature of the tests prompts the possibility that the conditioned rewarding effects of cocaine and those of novelty generalized to both compartments on test day. That compartment preferences were dissociated between the 7.5 and 20 mg/kg cocaine treated Novelty groups demonstrates that rats were able to distinguish between environments even though both environments were associated with an appetitive stimulus, diminishing the likelihood that stimulus generalization was responsible for the choice competition exhibited in this experiment. In other words, the conditioned association between the environment (i.e., end compartment) and the reward contained specific information about the nature of the reward (novelty vs. 7.5, 20, or 30 mg/kg cocaine). Such a conclusion is consistent with a growing body of research on Pavlovian conditioning processes involving non-drug USs (cf. Corbit & Balleine 2005; Delamater & Holland 2008; Konorski 1967).
In sum, this experiment demonstrated that the introduction of an alternative reward during extinction training (i.e., during the second experimental phase) can shift compartment preferences depending upon cocaine dose (7.5, 20, or 30 mg/kg) and its presence in the central nervous system (drug-free vs. drug-challenge tests). Further, this experiment expanded on ways to circumvent the limitation of the “all-or-none” effect common to place conditioning by demonstrating differences in the associative strength of 7.5, 20, and 30 mg/kg cocaine. Importantly, these findings were not due to a stimulus generalization account or by differences in activity impacting compartment choice.
Experiment 2: Retention of Conditioned Novelty Reward
The previous experiment determined the extent that novelty conditioned reward competed with cocaine conditioned stimuli. Importantly, this competition differed according to the dose of cocaine. Competition was complete with 7.5 mg/kg cocaine on both the drug and drug-free tests. For the 20 mg/kg group competition only existed on the drug-free test. Due to this dissociation, we incorporated another dose of cocaine that is widely used in the field to even more fully characterize the competition seen between novelty and cocaine-conditioned reward. Thus, Experiment 2 established the initial place preference with10 mg/kg cocaine and determined whether novelty's ability to compete with cocaine persists or if the initial drug preference returned after a period of abstinence.
The conditioned rewarding effects of novelty compete with those of cocaine when tested 24 to 48 h (drug-free and drug-challenge tests, respectively) later (Reichel & Bevins, 2008). Learning theories based on the primacy and recency effects predict that this competition might not survive a long delay between the last novelty exposure and subsequent testing. According to the recency effect, training histories occurring closer in time have a greater impact on behavior relative to earlier training histories (e.g., Miller & Escobar, 2003). According to this theory Novelty rats would be expected to spend more time in the novelty-paired compartment because learning that occurred in Phase 2 (i.e., Novelty conditioning) would be more stable since this information occurred temporally closer to test day than learning that occurred in Phase 1 (i.e., Cocaine conditioning). However, it is possible that Novelty rats may spend more time in the cocaine-paired compartment after longer retention intervals because as retention intervals increase the recency effect subsides and is often replaced by a primacy effect (Miller & Escobar, 2003; Urushihara, Wheeler, & Miller, 2004). With this in mind, the purpose of this experiment was to test for the competition between cocaine and novelty conditioned rewards at 1, 14, and 28 day retention intervals.
Habituation, Cocaine Place Conditioning, and Competing Conditioning
The procedures used in these phases were identical to those described for Experiment 1 except that rats were assigned to one of 3 retention conditions (1, 14, or 28 days) and the dose of cocaine was 10 mg/kg.
Drug-free and Drug-challenge Test Days
The procedures used to test for preference were similar to those described previously except the drug-free tests occurred 1, 14, or 28 days after completion of the competing conditioning phase; the drug-challenge tests occurred 24 h after the drug-free retention test (i.e., day 2, 15, or 29).
Data Analysis
One of the goals of this experiment was to extend the findings of the dose effect (i.e., first experiment) to 10 mg/kg cocaine. To this end, rats assigned to the shortest retention interval (i.e., 1 and 2 day drug-free and drug-challenges, respectively) were conditioned and tested separately than the longer retention interval groups; therefore, these data are presented separately. The data were analyzed as previously described.
Results
Habituation
Prior to cocaine conditioning, rats did not exhibit a bias toward one particular stimulus (holes vs. rods) over the other. The mean time (± SEM) spent in the holes and rods compartments was 259.2 (± 6.58) and 264.2 (± 5.45), respectively. Activity scores were 35.3 (± 1.05) on the holes and 36.6 (± 1.28) on the rods.
Place Conditioning
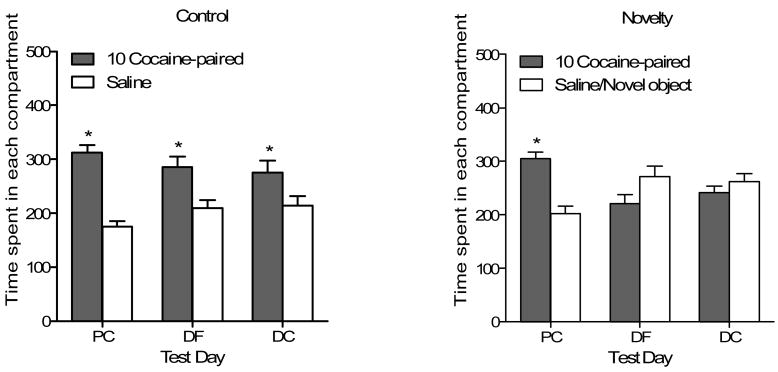
Figure 4 shows the time spent in each compartment for rats conditioned with 10 mg/kg cocaine and tested 24 and 48 h (drug-free and drug-challenge, respectively) after competing conditioning. Control rats (n=13, left graph) preferred the cocaine-paired compartment on all three test days [main effect of Side, F(1,12)=13.65, p<.003]. Novelty rats (n=13, right graph), in contrast, only preferred the cocaine-paired side on the initial test of cocaine (10 mg/kg) place conditioning [Side × Test interaction, F(2,24)=13.83, p<.001]. Tukey post-hoc comparisons show that more time was spent in the cocaine-paired compartment than the unpaired compartment on the initial day of place conditioning (p<.05).
Figure 4.
Mean time (± SEM) spent in seconds in each compartment for rats conditioned with 10 mg/kg cocaine in the Control (left graph) and Novelty (right graph) conditions on the three tests of place conditioning in Experiment 2. PC= place conditioning, DF= drug-free test, DC= drug challenge test.
* Significant difference between compartments.
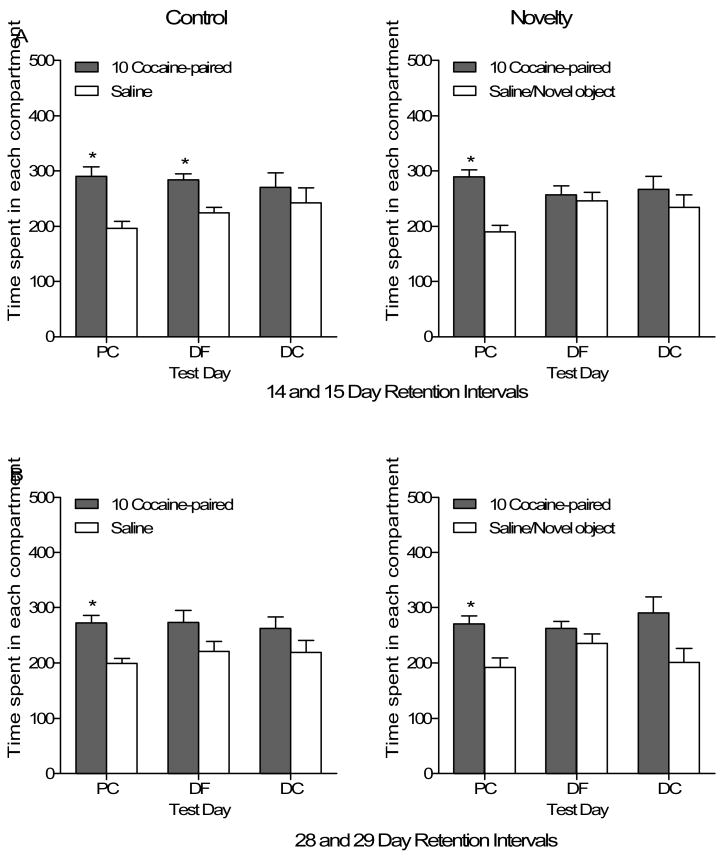
Figure 5 shows the time spent in each compartment for rats that were tested at longer retention intervals. The top panel (Figure 5A) shows the data for rats that were tested at 14 and 15 days (drug-free and drug-challenge, respectively) after completing the novelty conditioning phase. Control rats (n=12, upper left graph) preferred the cocaine-paired compartment on the initial test of place conditioning and the drug-free challenge, which is supported by a main effect of Side, F(1,11)=5.3, p=.042. A paired t-test between time spent in the paired vs. unpaired compartment confirmed that on the drug-challenge test time was distributed equally between compartments, t<1. Novelty rats (n=12, upper right graph), in contrast, only preferred the cocaine-paired side on the initial test of cocaine (10 mg/kg) place conditioning [Side × Test interaction, F(2,22)=3.45, p=.05, Tukey post-hoc p<.05].
Figure 5.
Time spent (mean ± SEM) in each compartment for rats in the Control and Novelty groups for the three tests of place conditioning in Experiment 2. Drug-free testing occurred 14 and 28 days after conditioning and drug-challenge testing occurred 15 and 29 days after conditioning. Panel A depicts data from rats tested at 14 and 15 day retention intervals and Panel B shows the 28 and 29 day retention intervals. Control groups are in the left column and Novelty groups are in the right. PC= place conditioning, DF= drug-free test, DC= drug challenge test.
* Indicates significant difference between compartments.
The lower panel (Figure 5B) shows the amount of time spent in each compartment for rats that were tested at 28 and 29 days (drug-free and drug-challenge, respectively) after novelty conditioning. Control rats (n=11, lower left graph) did not maintain compartment preferences as there were no significant effects. Likewise, Novelty rats (n=12, lower right graph), did not maintain compartment preferences as the main effect of Side and Side × Test interaction were not significant. To confirm that preferences existed on the test of cocaine place conditioning separate paired t-tests were conducted for each group. Indeed, both groups preferred the cocaine-paired side, Control, t(10)=3.60, p<.005; Novelty, t(11)=2.76, p<.02.
Activity
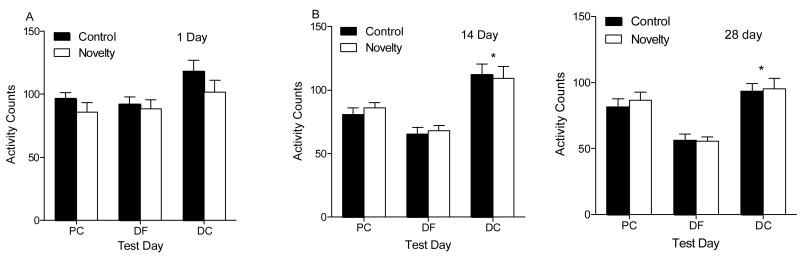
Figure 6A and B shows the activity data for the Novelty and Control groups tested at the three retention intervals. For rats in the shortest retention group (left graph), there was a main effect of test, F(2,48)=7.13, p=.002. However, post hoc comparisons did not reveal any group differences. For rats tested at the longer retention intervals, there was a main effect of Test, F(2,48)=61.16, p=.001. Specifically, a cocaine challenge occurring 15 or 29 days after Novelty conditioning resulted in elevated activity in comparison to a drug-free challenge on the preceding day (Tukey, p<.05).
Figure 6.
This figure shows activity scores (mean ± SEM) for rats tested at different retention intervals on the three tests of conditioning in Experiment 2. Figure 5a depicts the shortest retention interval in which drug-free and drug-challenge tests occurred 24 and 48 hrs, respectively, after the novelty-conditioning phase. Figure 5b shows activity scores for the longer retention intervals. Specifically, drug-free testing occurred 14 and 28 days after conditioning and drug-challenge testing occurred 15 and 29 days after conditioning. PC= place conditioning, DF= drug-free test, DC= drug challenge test.
* Indicates significant difference on the drug challenge test in comparison to the drug-free test for both groups.
Discussion
In the previous experiment, the doses of cocaine tested were 7.5, 20, and 30 mg/kg cocaine. That experiment identified the upper limit (i.e., no competition at 30 mg/kg cocaine) of the extent that the conditioned rewarding effects of novelty could compete with those of cocaine, and identified a dissociation in that competition between 7.5 and 20 mg/kg cocaine. Specifically, novelty competed with 7.5 mg/kg cocaine on both tests, yet novelty competed with 20 mg/kg cocaine only on the drug-free test. The current experiment suggests that the conditioned rewarding effects of 10 mg/kg cocaine are more similar to 7.5 than 20 mg/kg cocaine. That is, novelty competed with the conditioned rewarding effects of 10 mg/kg cocaine on the drug-free and drug-challenge test. Thus, evidence of competition on both test days for the 7.5 and 10 mg/kg group suggests that these two moderate doses of cocaine have similar appetitive and stimulus properties as measured in this version of a place conditioning task. Conversely, this pattern of results with 10 mg/kg cocaine differs from 20 mg/kg; the conditioned rewarding effects of this higher dose were more robust than novelty when tested in the cocaine state.
Novelty conditioned reward also competed with the conditioned rewarding effects of cocaine after a 14 day retention interval on the drug-free test. Even though these rats spent similar amounts of time in both compartments on the drug-challenge test, the interpretation of this test is negated because the Control group did not maintain a preference for the cocaine compartment in the drug state. Perhaps a weakened compartment preference could not survive additional extinction of the cocaine-paired cues occurring on the drug-free test. The Control rats did, however, retain a cocaine preference 14 days but not 28 days post-novelty conditioning. Recall that the retention interval in our study refers to the time between the novelty conditioning phase and the subsequent test days. Thus, the retention interval for cocaine place conditioning in this study is the retention interval plus 1 test day plus the 8 days of novelty conditioning—23 and 37 days. This finding is consistent with Mueller and Stewart's (2000) report that cocaine conditioned place preferences are maintained 4 (28 days) but not 6 (42 days) weeks post conditioning. We should also note that retention of cocaine place conditioning was likely weakened by the brief extinction (i.e., CS presented without the US) built into the experimental design. Rats in the Control and Novelty conditions during the novelty-conditioning phase received four 10-min sessions of the previously cocaine-paired floor in the absence of any cocaine. Thus, the impact of the prolonged retention interval in combination with some extinction of the floor CS-cocaine association likely weakened the compartment preference for the cocaine-paired side.
A general question prompted by the present research is why the behavioral effects of the conditioned rewarding effects of novelty or cocaine do not survive indefinitely—especially novelty, given that there was no extinction (of the novelty paired compartment) experience before testing. There are two notable theoretical accounts that could explain the lack of effect of either novelty or cocaine after a long retention interval: stimulus generalization or the context-change account of forgetting. According to the stimulus generalization account, recall of detailed stimulus properties within a learning situation is transient because specific attributes of stimuli are forgotten (Riccio, Ackil, & Burch-Vernon, 1992). This loss of detail regarding the learning situation leads to greater generalization across learning situations. Albeit speculative, applied to the present experiment there could be recall of a context-reward association remaining, but not recall of the specific flooring that is the only distinct stimulus in our conditioning situation.
Alternatively, the context-change account suggests that perception of contextual cues present at the time of conditioning change with the passage of time. This shift is responsible for what appears to be forgetting of stimulus attributes (Bouton et al., 1999). This account conceptualizes the context as including the internal state of the animal at the time of conditioning and testing (Bouton et al., 1999). For example, rats tested at longer retention intervals are typically older, larger and have experienced differences in handling than rats tested at shorter retention intervals, which may change perception of the testing context. This problem can be overcome by testing subjects at the same time; however the age at which conditioning occurs varies thus introducing a different problem. Seemingly, any exploration of retention intervals is fraught with either a “day-of-test” or “day-of-training” confound (Bouton et al., 1999). Regardless, in the current experiment perceived aspects of the conditioning and testing experience may have changed over time from both external and internal sources, which may have impacted compartment choice on the drug-free tests.
Albeit speculative, the theoretical accounts described in the previous paragraph can be related to a neural account proposed by Rosenbaum, Winocur, and Moscovitch (2001). According to that neural theory, as context specific memories become consolidated by the hippocampus they loose specificity over time (Rosenbaum et al., 2001). More so, with the passage of time, Rosenbaum et al. (2008) posit that the link between the event and the actual context in which an event occurred becomes less important than the memory itself. A place conditioning procedure relies on the formation of a conditioned association between the rewarding aspects of the stimulus of interests and the contextual cues of the environment. In our study, the contexts become associated with the rewarding aspects of cocaine and novelty. Over time, it is possible that the link between a preference for the paired environment and the actual physical feature of the context becomes less important than the memory of the preference itself. Although not tested, in our experiment this neural account takes into consideration a change in perception of conceptual cues and generalization among external stimuli.
To conclude, this experiment demonstrated that the conditioned rewarding effects of novelty competed with those of cocaine following a 14-day retention interval in the drug-free state. The lack of competition following a 28-day retention interval is subject to interpretation by different theoretical accounts. When considered together, the pattern of compartment preferences expressed in this experiment suggests that our model is limited as designed. Despite this limitation in design, we have identified a window of opportunity for conditioned rewards to compete for control over choice behavior. Strategies to increase this window should be of interest and will likely prove useful for studying competition among conditioned reward (see below).
General Discussion
This study demonstrates that the conditioned rewarding effects of novelty can compete with those of moderate cocaine doses (e.g., 7.5 and 10 mg/kg cocaine) whether or not the drug is present in the central nervous system. However, with higher and presumably more rewarding doses competition only occurs in the absence of drug (20 mg/kg) or not at all (30 mg/kg). Importantly, this competition does not seem to be permanent. These experiments varied cocaine dose and retention interval while holding the novelty stimulus constant during the second experimental phase. Throughout both experiments the order, number, and time of the novel object presentations were consistent between experiments and our previous report (Reichel & Bevins, 2008). And, indeed, these procedures reliably condition compartment preferences in our laboratory (Besheer, Jensen, & Bevins, 1999).
The present research examined the import of intensity or salience of the cocaine US while holding constant the intensity of the novel object US. Experiments that change the saliency of the novelty US by varying the intensity of the novel object exposure may result in more robust competition between the two rewards and have an even more profound influence on choice behaviors maintained by drug conditioning. Indeed, reducing the intensity of the novel objects by reducing the amount of access time decreased preferences conditioned by novel objects (Bevins et al., 2002). Whether the converse is true in regards to reward competition remains unexplored.
In the current experiments, rats experienced the rewarding aspects of systemic cocaine by alternating daily placements into the chamber. Thus, an association is presumably formed between the physiological effects of cocaine (US) and the features of the environment (i.e., the CS). In contrast, to experience novelty reward the rat must perceive, approach, and interact with the object. Thus, an association may occur between the rewarding aspects of novelty (US) and the features of the objects (CS) and/or the environment (CS) in this situation. In other words, adding the object on a conditioning day may be thought of as creating a compound CS composed of the environment CS plus the object CS. In essence, the stimulus aspects of the object on a given day may somewhat overshadow conditioning to the environment CS. Further, on the test day only the environment CS is assessed. If so, such overshadowing and then testing of only part of the relevant stimulus elements functioning as a CS limits the impact of conditioned novelty reward on choice in the present protocol.
Exposure to novel stimuli during treatment may have clinical utility by acting as an effective substitute for drug reward (Bevins, 2001; Cain, Saucier, & Bardo, 2005; Dellu et al., 1996; D'Silva, Harrington, Palmgreen, Donohew, & Lorch, 2001). The ability of novelty to compete with cocaine may have use as a behavioral substitution strategy because environment–drug associations formed while abusing the substance continually impact choice behaviors. Providing alternative-learning histories, including new non- drug associations during the intervention program may change choice behavior after an environment–drug association is formed. These studies indicate that novelty as a behavioral substitution strategy may have more success with mild users rather than heavier users. More so, implementing novelty may be more effective in the earlier rather than later stages of treatment.
Alternative choices presented to the addict during times of abstinence may increase the likelihood of discontinued drug use. The use of novel experiences as an adjunct for behavior treatments, like contingency management, may be one option available to promote non-drug choices in addicts. This approach may be particularly relevant to individuals classified as high novelty- and/or sensation-seekers (Cloninger, 1987; Zuckerman, 1994). People that seek out novel and high-risk situations (i.e., high sensation seekers) report high incidences of drug use (Palmgreen et al., 2001; Stephenson, 2003; Zuckerman & Kuhlman, 2000). More so, individuals fitting into this category (i.e., high-sensation seekers) generally participate in more extracurricular activities than those classified as low-sensation seekers (D'Silva et al., 2001). In fact high-sensation seekers tend to choose leisure activities classified as action-adventure (e.g., scuba diving, mountain climbing, white water rafting, kayaking, rock climbing, canoeing, snow skiing) and conflict-combat (e.g., survival games, role playing, martial arts, paint ball) related activities (D'Silva et al., 2001). In regards to the present discussion, treatment programs implementing novel rewards targeted to those individuals that have high novelty/sensation seeking tendencies may offer addicts the opportunity (e.g., with vouchers) to participate in one of the activities mentioned previously in the hopes of maintaining abstinence. In conclusion, these studies provide empirical support for the idea that drug treatment programs may use novelty to enhance intervention programs by providing new learning histories that are incompatible with drug use.
Acknowledgments
We thank Jessica Barr, Erin Ryan, and Andrea Wilson for their assistance scoring the video tapes and conducting reliability checks. This research was supported by a NIH National Research Service Award (DA023283) and an American Psychological Association Dissertation Research Award to C.M. Reichel. Additional support for the research was provided by DA017086 and DA018114.
References
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153(1):31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behavioural Brain Research. 1996;77:23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Dwoskin LP. Biological connection between novelty- and drug-seeking motivational systems. In: Bevins RA, Bardo MT, editors. Nebraska symposium on motivation: Vol 50 Motivational factors in the etiology of drug abuse. Lincoln, NE: University of Nebraska Press; 2004. pp. 127–158. [PubMed] [Google Scholar]
- Bardo MT, Rowlett JT, Harris MJ. Conditioned place preference using opiate and stimulant drugs: A meta-analysis. Neuroscience and Biobehavioral Reviews. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Barr GA, Paredes W, Bridger WH. Place conditioning with morphine and phencyclidine: Dose dependent effects. Life Sciences. 1985;36(4):363–368. doi: 10.1016/0024-3205(85)90122-5. [DOI] [PubMed] [Google Scholar]
- Bevins RA. Novelty seeking and reward: Implications for the study of high-risk behaviors. Current Directions in Psychological Science. 2001;10(6):189–193. [Google Scholar]
- Bevins RA. The reference-dose place conditioning procedure yields a graded dose-effect function. International Journal of Comparative Psychology. 2005;18:101–111. [Google Scholar]
- Bevins RA, Cunningham CL. Place conditioning: A methodological analysis. In: Anderson M, editor. Tasks and Techniques: A Sampling of Methodologies for the Investigation of Animal Learning, Behavior, and Cognition. Hauppauge NY: Nova Science Publisher; 2006. [Google Scholar]
- Bouton ME, Nelson JB, Rosas JM. Stimulus generalization, context change, and forgetting. Psychological Bulletin. 1999;125(2):171–181. doi: 10.1037/0033-2909.125.2.171. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Peck CA. Spontaneous recovery in cross-motivational transfer (counter conditioning) Animal Learning & Behavior. 1992;20(4):313–321. [Google Scholar]
- Cain ME, Smith CM, Bardo MT. The effect of novelty on amphetamine self-administration in rats classified as high and low responders. Psychopharmacology. 2004;176(2):129–138. doi: 10.1007/s00213-004-1870-2. [DOI] [PubMed] [Google Scholar]
- Carr GD, Phillips AG, Fibiger HC. Independence of amphetamine reward from locomotor stimulation demonstrated by conditioned place preference. Psychopharmacology. 1988;94:221–226. doi: 10.1007/BF00176849. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal. Archives of general psychiatry. 1987;44(6):573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Colzato LS, van den Wildenberg WPM, Hommel B. Reduced spontaneous eye blink rates in recreational cocaine users: Evidence for dopaminergic hypoactivity. PLoS ONE. 2008;3(10):e3461. doi: 10.1371/journal.pone.0003461. 101.1371/journal.pone0003461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of Pavlovian-instrumental transfer. Journal of Neuroscience. 2005;25:962–70. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamater AR, Holland PC. The influence of CS-US interval on several different indices of learning in appetitive conditioning. J Exp Psychol: Anim Behav Process. 2008;34:202–22. doi: 10.1037/0097-7403.34.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellu F, Piazza PV, Mayo W, LeMoal M, Simon H. Novelty-seeking in rats – biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34(3):136–145. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- D'Silva MU, Harrington NG, Palmgreen P, Donohew LD, Lorch EP. Drug use prevention for the high sensation seeker: The role of alternative activities. Substance Use & Misuse. 2001;36(3):373–385. doi: 10.1081/ja-100102631. [DOI] [PubMed] [Google Scholar]
- Garcia-Rodriquez O, Secades-Villa R, Higgins ST, Fernandez-Hermida JR, Carballo JL, Perez JME, Diaz SA. Effects of voucher-based intervention on abstinence and retention in an outpatient treatment for cocaine addiction: A randomized controlled trial. Experimental and Clinical Psychopharmacology. 2009;17(3):131–138. doi: 10.1037/a0015963. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL. Role of test activity in ethanol-induced disruption of place preference expression in mice. Psychopharmacology. 2007;191(2):195–202. doi: 10.1007/s00213-006-0651-5. [DOI] [PubMed] [Google Scholar]
- Groblewski PA, Bax LS, Cunningham CL. Reference-dose place conditioning with ethanol in mice: Empirical and theoretical analysis. Psychopharmacology. 2008 doi: 10.1007/s00213-008-1251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konorski J. Integrative activity of the brain. Chicago: University of Chicago Press; 1967. [Google Scholar]
- Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimli: pups and cocaine throughout the postpartum period. Behavioral Neuroscience. 2001;115(3):683–694. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- Miller RR, Escobar M. Learning: Laws and models of basic learning. In: Gallistel CR, editor. Steven's handbook of experimental psychology: Vol 3 Learning, motivation, and emotion. Vol. 3. New York: Wiley; 2003. pp. 42–102. [Google Scholar]
- Mueller D, Stewart J. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behavioural Brain Research. 2000;115(1):39–47. doi: 10.1016/s0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Khroyan TV, Neisewander JL. Dose-dependent characterization of the rewarding and stimulant properties of cocaine following intraperitoneal and intravenous administration in rats. Psychopharmacology. 1996;123(2):144–153. doi: 10.1007/BF02246171. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Morgan PT, Malison RT, Hart CL, Edgar C, Walker M, Stickgold R. Cocaine users differ from normals on cognitive tasks which show poorer performance during drug abstinence. The American Journal of Drug and Alcohol Abuse. 2008;34(1):109–121. doi: 10.1080/00952990701764821. [DOI] [PubMed] [Google Scholar]
- Palmgreen P, Donohew L, Lorch EP, Hoyle RH, Stephenson MT. Television campaigns and adolescent marijuana use: Tests of sensation seeking targeting. American Journal of Public Health. 2001;91(2):292–296. doi: 10.2105/ajph.91.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patkina NA, Zvartau EE. Caffeine place conditioning in rats: comparison with cocaine and ethanol. European Neuropsychopharmacology. 1998;8(4):287–291. doi: 10.1016/s0924-977x(97)00086-2. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. London: Oxford University Press; 1927. [Google Scholar]
- Reichel CM, Bevins RA. Competition between the conditioned rewarding effects of cocaine and novelty. Behavioral Neuroscience. 2008;122(1):140–150. doi: 10.1037/0735-7044.122.1.140. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Spontaneous Recovery. Learning and Memory. 11(5):501–509. doi: 10.1101/lm.77504. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasky WF, editors. Classical Conditioning II. Appleton-Century-Crofts; New York: 1972. pp. 64–99. [Google Scholar]
- Riccio DC, Ackil J, Burch-Vernon A. Forgetting of stimulus attributes: methodological implications for assessing associative phenomena. Psychological Bulletin. 1992;112:433–445. doi: 10.1037/0033-2909.112.3.433. [DOI] [PubMed] [Google Scholar]
- Riccio DC, Richardson R, Ebner DL. The contextual change paradox is still unresolved: comment on Bouton, Nelson, and Rosas. Psychological Bulletin. 1999;125(2):187–189. doi: 10.1037/0033-2909.125.2.187. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Winocur G, Moscovitch M. New views on old memories: re-evaluating the role of the hippocampal complex. Behavioural Brain Research. 2001;127:183–197. doi: 10.1016/s0166-4328(01)00363-1. [DOI] [PubMed] [Google Scholar]
- Stephenson MT. Mass media strategies targeting high sensation seekers: What works and why. American Journal of Health Behavior. 2003;27(supplement 3):S233–S238. doi: 10.5993/ajhb.27.1.s3.7. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: a model revealing an interaction between cocaine and social context rewards in rats. Drug and Alcohol Dependence. 2008;96(3):202–212. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T, Ostlund W. Susceptibility to readdiction as a function of the addiction and withdrawal environments. Journal of Comparative and Physiological Psychology. 1965;60:388–392. doi: 10.1037/h0022588. [DOI] [PubMed] [Google Scholar]
- Urushihara K, Wheeler DS, Miller RR. Outcome pre- and postexposure effects: Retention interval interacts with primacy and recency. Journal of Experimental Psychology: Animal Behavior Processes. 2004;30(4):283–298. doi: 10.1037/0097-7403.30.4.283. [DOI] [PubMed] [Google Scholar]
- Winger G, Woods JH, Galuska CM, Wade-Galuska T. Behavioral perspectives on the neuroscience of drug addiction. Journal of Experimental Analysis of Behavior. 2005;84(3):667–681. doi: 10.1901/jeab.2005.101-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Behavioral expressions and biosocial bases of sensation seeking. New York: Cambridge University Press; 1994. [Google Scholar]
- Zuckerman M, Kuhlman DM. Personality and risk-taking: Common biosocial factors. Journal of Personality. 2000;68(6):999–1029. doi: 10.1111/1467-6494.00124. [DOI] [PubMed] [Google Scholar]