Abstract
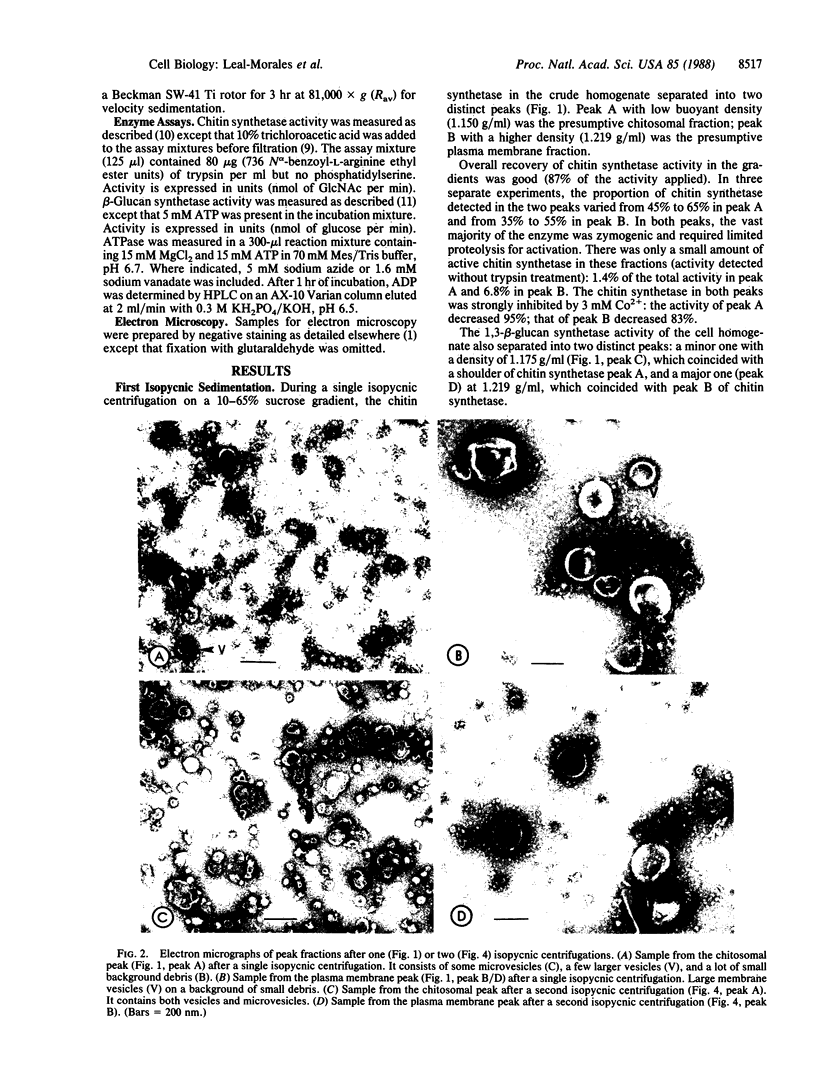
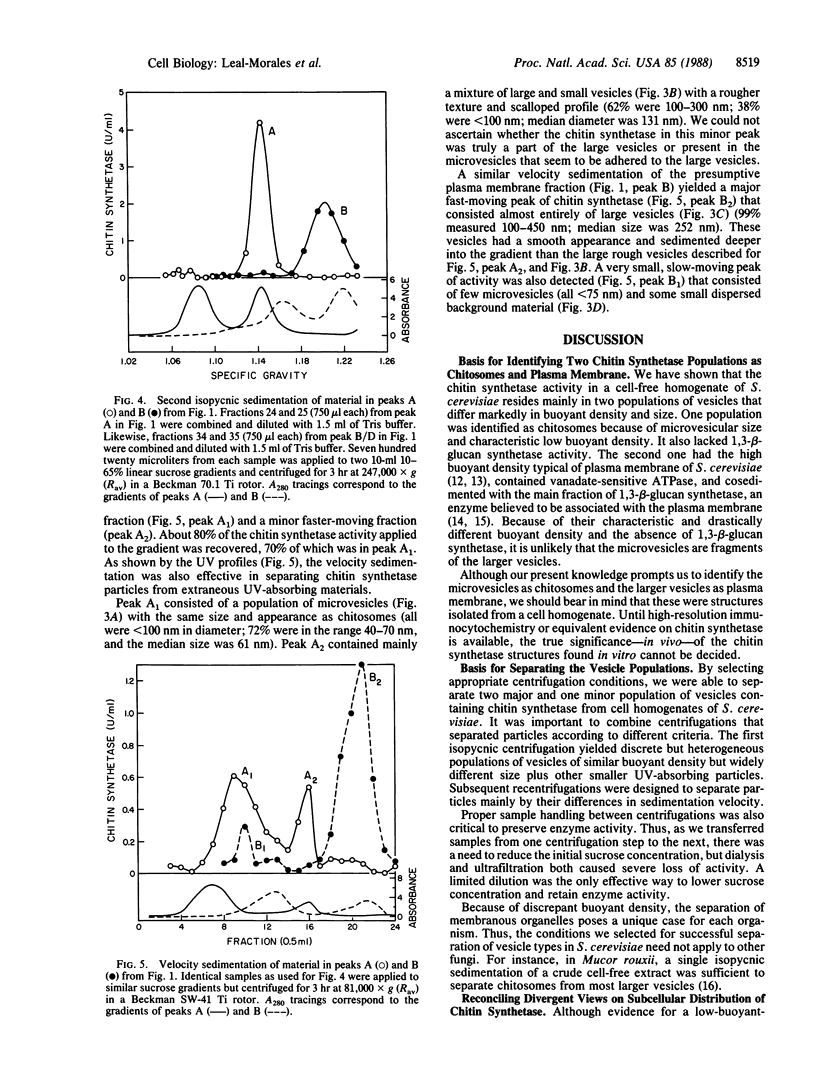
We describe an improved method for fractionating cell-free extracts of Saccharomyces cerevisiae to separate its membranous components by a combination of isopycnic and velocity sedimentations. These procedures were used to examine the subcellular distribution of chitin synthetase (chitin-UDP acetylglucosaminyltransferase; EC 2.4.1.16) in homogenates from exponentially growing walled cells of a wild-type strain of yeast. Chitin synthetase (Chs1) activity was mainly found in two distinct vesicle populations of nearly equal abundance but with markedly different buoyant densities and particle diameters. One population contained 45-65% of the total chitin synthetase and was identified as chitosomes because of microvesicular size (median diameter = 61 nm) and characteristic low buoyant density (1.15 g/ml); it also lacked 1,3-beta-glucan synthetase activity. The second population (35-55%) was identified as plasma membrane because of its high buoyant density (1.22 g/ml), large vesicle size (median diameter = 252 nm), and presence of vanadate-sensitive ATPase. This fraction cosedimented with the main peak of 1,3-beta-glucan synthetase. A third, minor population of chitin synthetase particles was also detected. Essentially all of the chitin synthetase in the two vesicle populations was zymogenic; therefore, we regard these vesicles as precursors of the final active form of chitin synthetase whose location in the cell has yet to be unequivocally determined.
Full text
PDF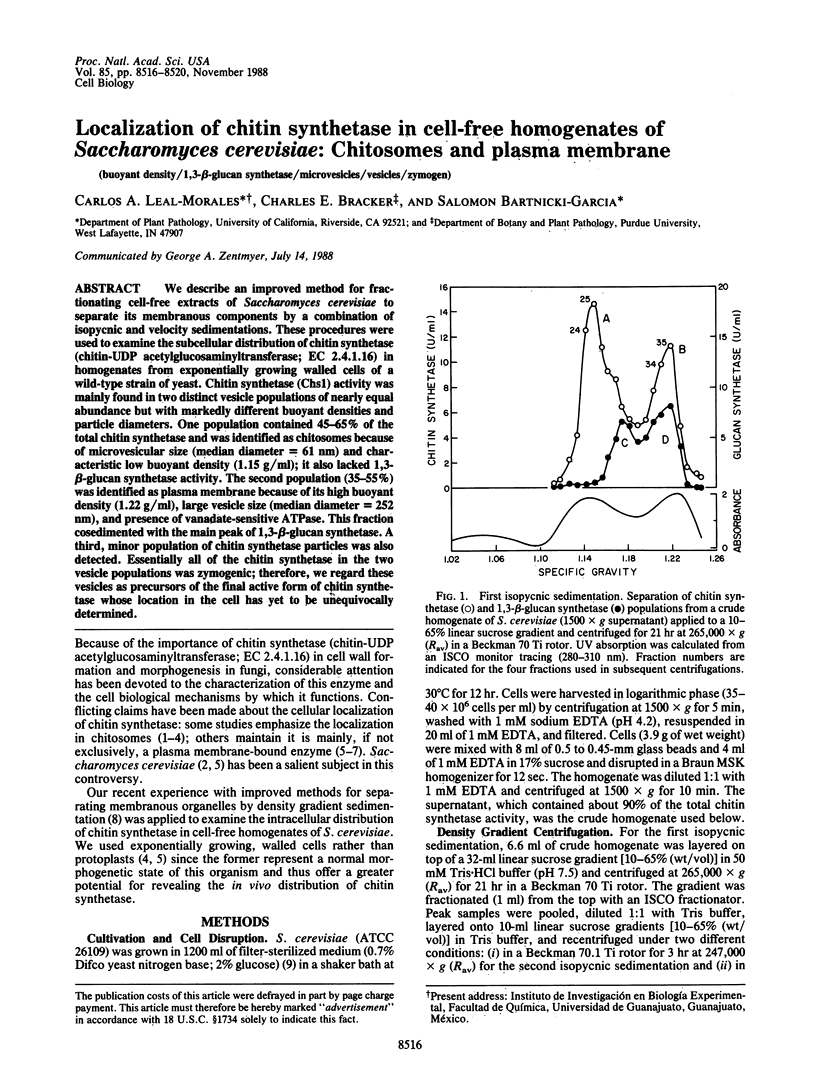


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bracker C. E., Ruiz-Herrera J., Bartnicki-Garcia S. Structure and transformation of chitin synthetase particles (chitosomes) during microfibril synthesis in vitro. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4570–4574. doi: 10.1073/pnas.73.12.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulawa C. E., Slater M., Cabib E., Au-Young J., Sburlati A., Adair W. L., Jr, Robbins P. W. The S. cerevisiae structural gene for chitin synthase is not required for chitin synthesis in vivo. Cell. 1986 Jul 18;46(2):213–225. doi: 10.1016/0092-8674(86)90738-5. [DOI] [PubMed] [Google Scholar]
- Durán A., Bowers B., Cabib E. Chitin synthetase zymogen is attached to the yeast plasma membrane. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3952–3955. doi: 10.1073/pnas.72.10.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M. S., Au-Young J., Cabib E. Modification of yeast plasma membrane density by concanavalin A attachment. Application to study of chitin synthetase distribution. J Biol Chem. 1985 Oct 15;260(23):12680–12684. [PubMed] [Google Scholar]
- Martínez J. P., Murgui A., Flores A., Sentandreu R. Subcellular fractionation of actively growing protoplasts of Saccharomyces cerevisiae. Biochim Biophys Acta. 1984 Sep 14;805(1):59–71. doi: 10.1016/0167-4889(84)90037-5. [DOI] [PubMed] [Google Scholar]
- Orlean P. Two chitin synthases in Saccharomyces cerevisiae. J Biol Chem. 1987 Apr 25;262(12):5732–5739. [PubMed] [Google Scholar]
- Ruiz-Herrera J., Bartnicki-Garcia S. Proteolytic activation and inactivation of chitin synthetase from Mucor rouxii. J Gen Microbiol. 1976 Dec;97(2):241–249. doi: 10.1099/00221287-97-2-241. [DOI] [PubMed] [Google Scholar]
- Sburlati A., Cabib E. Chitin synthetase 2, a presumptive participant in septum formation in Saccharomyces cerevisiae. J Biol Chem. 1986 Nov 15;261(32):15147–15152. [PubMed] [Google Scholar]
- Serrano R. Characterization of the plasma membrane ATPase of Saccharomyces cerevisiae. Mol Cell Biochem. 1978 Nov 30;22(1):51–63. doi: 10.1007/BF00241470. [DOI] [PubMed] [Google Scholar]
- Shematek E. M., Braatz J. A., Cabib E. Biosynthesis of the yeast cell wall. I. Preparation and properties of beta-(1 leads to 3)glucan synthetase. J Biol Chem. 1980 Feb 10;255(3):888–894. [PubMed] [Google Scholar]
- Willsky G. R. Characterization of the plasma membrane Mg2+-ATPase from the yeast, Saccharomyces cerevisiae. J Biol Chem. 1979 May 10;254(9):3326–3332. [PubMed] [Google Scholar]
- Yuh-Nung-Jan Properties and cellular localization of chitin synthetase in Phycomyces blakesleeanus. J Biol Chem. 1974 Mar 25;249(6):1973–1979. [PubMed] [Google Scholar]