Abstract
Background
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease that predominantly affects women. Despite Klinefelter's syndrome (47,XXY) and SLE coexisting in isolated cases, no association has been established with SLE or any other autoimmune disease. Methods: Sex chromosome genotyping was performed in 981 SLE patients (213 were men). A first group of 843 SLE patients from 378 multiplex families and a second group of 138 men with non-familial SLE were evaluated. Fluorescent in situ hybridization (FISH) and karyotyping in transformed B cell lines enumerated chromosomes for selected cases.
Results
Of 213 men with SLE, five had Klinefelter's syndrome (or 1 in 43). Four of them were heterozygous at X markers. FISH and karyotyping confirmed Klinefelter’s syndrome in the fifth. An overall rate of 235 47,XXY per 10,000 male SLE patients (95%CI: 77 to 539) was found, a dramatic increase over the known prevalence of Klinefelter's syndrome in an unselected population (17 per 10,000 live male births). Asking men with SLE about fertility was highly sensitive (100%) for Klinefelter’s syndrome. All 768 SLE women were heterozygous at X.
Conclusions
47,XXY Klinefelter's syndrome, often subclinical, is increased in men with SLE by ~14-fold, compared to its prevalence in men without SLE. Diagnostic vigilance for 47,XXY males in SLE is warranted. These data are the first to associate Klinefelter's syndrome with an autoimmune disease found predominantly in women. The risk of SLE in Klinefelter's syndrome is predicted to be similar to the risk in normal 46,XX women and ~14-fold higher than in 46,XY men, consistent with SLE susceptibility being partly explained by a X chromosome gene dose effect.
Systemic lupus erythematosus (SLE) is uncommon in men, being ten-fold more prevalent in women, a difference usually attributed to sex hormones (reviewed in 1). SLE has been reported in 30 men with Klinefelter’s syndrome since 1969 (2–6) with two others having anti-U1RNP and “mixed connective tissue disease” (7,8). Klinefelter’s syndrome, resulting from a 47, XXY karyotype, is present in 17 of 10,000 live male births (with a 95% confidence interval (95%CI) from 14 to 20 per 10,000 as calculated from published data (9–11)). Klinefelter’s syndrome is characterized by abnormal sexual development at puberty from sex hormone metabolic differences with small testes, gynecoid body habitus, absent secondary sexual characteristics, gynecomastia, impotence, and sterility. Many 47,XXY men remain undiagnosed until well after puberty.
In a study of 22 men with SLE, no instance of Klinefelter’s syndrome was found (12). Of 500 patients with Klinefelter’s syndrome, none had or developed SLE after a decade of observation (13). These efforts failed to support an association between Klinefelter’s and SLE, but are small sample sizes for uncommon diseases.
As anticipated, ~90% of the patients in our SLE genetic studies are women (14). We explored both familial and non-familial instances of men with SLE to determine whether the prevalence of Klinefelter’s syndrome is increased in men with SLE.
Methods
Clinical
Upon obtaining informed consent, patients satisfying classification criteria for SLE (15–16) and their families (14) were enrolled. 1257 participants taken from multiplex lupus families (75 SLE men, 768 SLE women and 414 non-SLE men) were obtained from the Lupus Genetics Studies at the Oklahoma Medical Research Foundation in Oklahoma. Ninety-eight of the 138 non-familial SLE male cases were part of the PROFILE cohort (17), and the remaining 40 non-familial SLE male cases came from simplex families from the lupus genetic studies based in Oklahoma (Table 1). Data included questionnaire responses, an interview, and medical record review. One SLE male patient was encountered with known Klinefelter’s syndrome, diagnosed at age 17 years, coincident with the onset of SLE. Androgen therapy led to apparent improvement with no evidence of an SLE recurrence for the subsequent 15 years (Scofield, Bruner, Harley, manuscript in preparation).
Table 1.
Sex chromosome findings in SLE patients.
| Genotype (or Karyotype*) | ||||
|---|---|---|---|---|
| 46,XY | 47,XXY | 46,XX | 45,XO | |
| Group 1 | ||||
| Familial SLE (Oklahoma) | ||||
| SLE Men | 74 | 2* | ||
| SLE Women | 768 | 0 | ||
| Non-SLE Men | 414 | 0 | ||
| Group 2 | ||||
| Non-familial SLE (Oklahoma) | ||||
| SLE Men | 38 | 1*† | ||
| Non-familial SLE (PROFILE) | ||||
| SLE Men | 96 | 2 | ||
| Total SLE men (Groups 1 & 2) | 207 | 5 | ||
47,XXY established by FISH and karyotype for three SLE men.
This patient (see Figure 2) is 47,XXY with a duplicated X chromosome. All other sex chromosome designations are deduced from genotyping.
Genotyping and karyotyping
Each SLE patient in the first group was genotyped at a panel of up to 16 microsatellite nucleotide repeats on the X chromosome with two from the pseudoautosomal regions. Each male in the study was also typed at two Y chromosome markers, YS390 and YS389. Chromosomes from lymphoblastoid cell lines of three patients were analyzed by trypsin-Giemsa banding (GTG-banding) (18) and fluorescent in situ hybridization (FISH) analysis with X and Y centromere specific DNA probes (Vysis, Inc., Downers Grove, IL). The confirmatory cohort of 138 non-familial male SLE cases was typed at seven X and two Y chromosome markers. In both sets of microsatellite typing experiments, markers were chosen that spanned the length of the X chromosome. The previously diagnosed Klinefelter’s male SLE patient in the latter cohort was typed at 256 single nucleotide polymorphisms (SNPs) from the non-pseudoautosomal regions of the X chromosome, using the 10K GeneChip™ Array (Affymetrix Inc., Santa Clara, CA).
Statistical analysis
Bayes’ theorem [P(B|A)=[P(A|B)*P(B)]÷P(A)] was used to estimate the frequency of SLE in Klinefelter’s syndrome (where A=the frequency of Klinefelter’s, B=the frequency of SLE in men, and P(B|A) indicates the probability of SLE within the group with Klinefelter’s syndrome). 95% binomial confidence intervals (95% CI) were calculated with the raw data and then converted to per 10,000 for ease of presentation.
Role of the funding source
The funding sources had no role in study design; collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Results
In 378 families with two or more SLE patients (843 patients total), there were 76 men with SLE. 74 of these men with SLE were monozygous at each of the X chromosome microsatellites tested (Table 1). One male SLE patient was heterozygous at 14 and the other at 12 of the 16 X chromosome markers. Both had allele assignments identical to their mothers (Figure 1). The two Y chromosome markers, and hence also the Y chromosomes, were present in both of the phenotypic males with heterozygous X chromosomes. A diagnosis of Klinefelter’s syndrome or 47,XXY had not been previously considered for either of these men. Cytogenetics and FISH analyses confirmed that both patients have the Klinefelter’s syndrome karyotype, 47,XXY. Genotyping studies in 414 men without SLE in these families showed no evidence for more than one X chromosome.
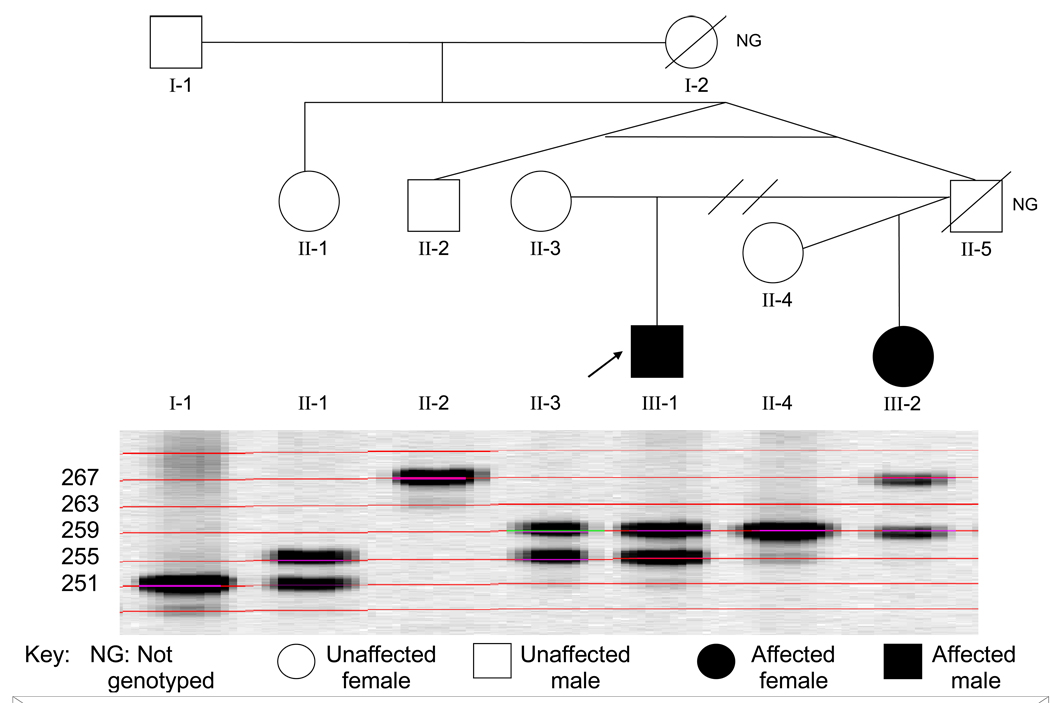
Figure 1.
Pedigree with an SLE male (arrow) who is heterozygous at an X chromosome marker. The marker shown (GATA144D04) is located at 44.7 Mb on the X chromosome. The SLE patient is also heterozygous at 13 of another 15 X chromosome markers (data not shown). The male SLE patient has alleles at this marker identical to his mother, and thus inherited both maternal X chromosomes. His father was not typed, but the father’s identical twin brother is homozygous with an allele distinct from the patient and mother.
All 768 women with SLE studied from the 378 families were heterozygous at multiple X chromosome microsatellite markers, providing no evidence for Turner’s syndrome with SLE in this population.
Thus, 2 of the 76 men with SLE in multiplex families have Klinefelter’s syndrome. This gives a prevalence of 263 per 10,000 men with SLE (95%CI: 32 to 918), which is >15 times higher than the rate of Klinefelter’s syndrome in the general population and is well above the expected Klinefelter’s population rate of 17 in 10,000 (9–11). Thus, Klinefelter’s syndrome occurred more frequently than expected (P=0.007 by Fisher’s Exact Test) in these men with SLE.
In an effort to test and replicate our findings, we typed an independent group of 138 men with non-familial SLE. Of these, 136 were homozygous at the X and Y markers. However, in one of these, Klinefelter’s syndrome had been diagnosed several years previously. Karyotype and FISH analysis of a lymphoblastoid cell line from this patient revealed 47,XXY (Figure 1), consistent with meiosis II non-disjunction resulting in a duplication of a maternal X chromosome, which is present in about 18% of Klinefelter’s syndrome (19). Ascertainment for study entry had been for SLE with the presence or absence of Klinefelter’s syndrome having no consequence upon the decision to enter the study. The other two men were homozygous at the Y markers and heterozygous at multiple X chromosome markers (Table 1). One had two alleles at 6 and the other at 7 of 7 X chromosome microsatellite markers. Thus, the association of Klinefelter’s syndrome and SLE was confirmed in 3 of 138 (217 per 10,000 (95% CI: 45 to 622)). The known rate of Klinefelter’s −17 per 10,000 live male births (9–11) – is excluded from the confidence intervals.
Given the estimates of the prevalence rates for Klinefelter’s syndrome in the original and replication groups, a combined rate was obtained (5 of 213 or 235 47,XXY men for every 10,000 men with SLE, 95%CI: 77 to 539), a >13-fold higher prevalence of Klinefelter’s syndrome than found in the male population (9–11), p<0.001 by Fisher’s Exact Test.
The prevalence of SLE is between 1 in 485 for African-American women and 1 in 2762 for European-American women (20,21). Our SLE patients are 26.8% African-American and 55.9% European-American. This ethnic distribution predicts a prevalence of one SLE patient in 1324 women. Meanwhile, ethnic differences in male SLE prevalence rates are not reliable because of the small samples studied (22,23). Since lupus is 10 times more common in women than men, we therefore predict an estimated prevalence of ~1 in 13,240 for the SLE males studied. The rate of Klinefelter’s syndrome among men with SLE (5 in 213) has been presented above. We also know the incidence of Klinefelter’s syndrome in the population to be 17 in 10,000 (9–11). Therefore, the rate of SLE among Klinefelter’s syndrome patients is estimated from Bayes’ Theorem as 1 in 960 (See Figure 3), since measures of the other three variables are available,. This number approximates the rate of SLE in women and is much higher, nearly 14-fold higher, than the rate of SLE in normal 46,XY men (22,23).
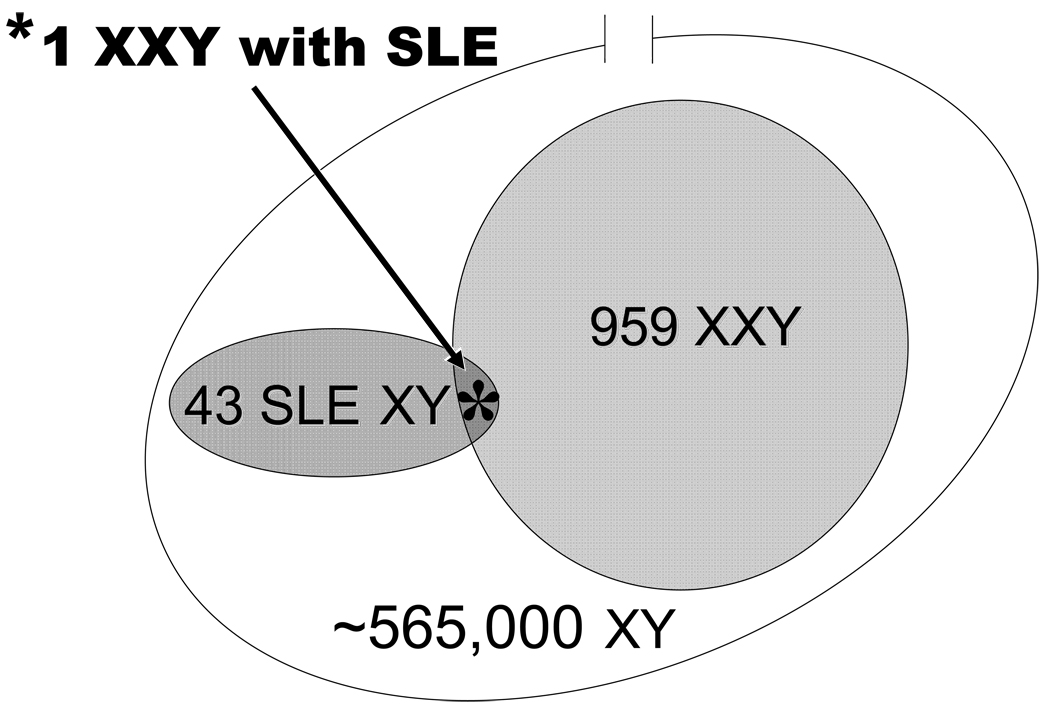
Figure 3.
The prevalence of 47,XXY in SLE males. We observe one male with Klinefelter’s (KS) in 43 SLE men. The known rate of Klinefelter’s syndrome in the general population is 17 in 10,000. Using Bayes’ Theorem, we estimate that one male will have SLE for every 960 Klinefelter’s men. These SLE men and Klinefelter’s men would be contained in a sample of ~565,000 [(960*10,000) / 17 = 564,705] men with the proportional racial composition of the study.
The 135 men with SLE enrolled in this study by the Oklahoma genetic studies were asked “Are you infertile?” upon entry into the study. All three men subsequently found to have Klinefelter’s syndrome, along with six other men answered this question with a response other than ‘No’. Two stated ‘Yes’ and the third answered he ‘did not know’. Thus, this simple clinical question was 100% sensitive and 33% specific for identification of Klinefelter’s syndrome in men with SLE (p=0.00021 by Fisher’s Exact test comparing Klinefelter’s men with the other men with SLE).
Discussion
Klinefelter’s syndrome and SLE, two very different conditions, occur in the population with similar prevalence rates. Herein, we show that these diseases occur together more often than expected by chance alone (Figure 3). There are reasons to suspect that Klinefelter’s syndrome and SLE might be associated. First, numerous case reports document the co-existence of the two diseases (1–8,12,13,24). Second, sex hormone similarities between women and men with Klinefelter’s are associated with SLE (1,4,25,26). This argument is bolstered by data from some animal models of SLE demonstrating increased susceptibility with estrogen and protection with androgens (reviewed in 1). Third, Klinefelter’s syndrome is also associated with several conditions related to gender. For example, Klinefelter’s syndrome men die of breast cancer at a similar rate as women (27). About two in 50 men with breast cancer have Klinefelter’s syndrome (28), a rate similar to that found for male SLE herein. There may be an increase in rheumatoid factor in the serum of patients with Klinefelter’s syndrome (29). Testosterone treatment reverses immune activation abnormalities in Klinefelter’s syndrome (30), and has also led to therapeutic improvement of SLE in individual case reports (5,31), as observed in one of our patients (Scofield, Bruner, Harley, unpublished data). These observations may be related to the beneficial effect of the mild androgen dehydroepiandrosterone (DHEA or prasterone) upon mild SLE in women (32).
X chromosome polymorphisms do not detect 47,XXY caused by duplication of the X chromosome and produced by a maternal meiosis II nondisjunction, which we discovered in one man from his clinical presentation. Any subclinical 47,XXY caused by this mechanism would not have been detected by X chromosome polymorphism screening, as applied to some of our sample. The actual population prevalence of 47,XXY in our male SLE sample may be higher, therefore, than the 5 in 213 we detected. However, the best estimate suggests that only 18% of patients with Klinefelter’s syndrome have this mechanism of supernumerary X chromosomes (19). Therefore, there is a small but finite possibility of an additional undiscovered Klinefelter’s man among the SLE men studied by genotyping of the X chromosome only. Of course, typing of X chromosome markers is not the usual clinical modality of diagnosis for Klinefelter’s syndrome, but karyotype and FISH, the usual clinical tests, require cells, which were not available in some of subjects. In other work, we have typed several hundred X chromosome single nucleotide polymorphisms. We find heterozygocity in from 40% to 60% of these markers in the men identified as 47,XXY in the present study by microsattelite markers. This rate of heterozygocity is similar to that found in normal women. Thus, we are confident that the techniques used herein have accurately identified men with Klinefelter’s syndrome as well as 46,XY men.
Klinefelter’s syndrome is a genetic abnormality in which androgen and estrogen levels are abnormal from at least the initiation of puberty (33). Klinefelter’s syndrome may specifically predispose men to SLE compared to other more common forms of hypogonadism, such as primary testicular failure. However, 5 of 35 men with SLE were found to have hypergonadotropic hypogonadism in one study but the etiology of the hypogonadism was not otherwise delineated (34). Another study found a high rate of hypogonadism in men with rheumatic diseases (only 2 of which had SLE). Of interest, of 13 men with rheumatic disease and untreated hypogonadism, 5 had Klinefelter’s syndrome, 2 had Kallman’s syndrome and 2 had idiopathic cryptorchism. Thus, 9 of 13 had a congenital from of hypogonadism (6). There are case reports of Klinefelter’s syndrome with other female-predominant autoimmune diseases (35,36). However, our data are the first to conclusively demonstrate an association of Klinefelter’s syndrome with a female-predominate autoimmune disease.
We estimate that one SLE patient will be found in every 960 Klinefelter’s males. This is much closer to the one SLE patient in 1324 women based on the ethnic distribution in our population than it is to the estimated prevalence of 1 in ~14,000 for the SLE males. Thus, the 47,XXY males have the 46,XX female risk of lupus and not the approximately 10-fold lower 46,XY male risk. This result is consistent with a gene dose effect for lupus risk originating from the X chromosome where XX (whether 46,XX or 47, XXY) confers a 10-fold higher risk than 46,XY.
Perhaps, subsequent studies of large numbers of women with SLE will estimate the relative risk for SLE in 45,XO, Turner’s syndrome. If the gene dose hypothesis is correct, then the rate of SLE 45,XO should be similar to the 46,XY male rate. Our sample of 768 SLE women is too small to determine whether this is the case. Turner’s syndrome is about four-fold less prevalent than Klinefelter’s at 4 per 10,000 (11,12). Even so, SLE in Turner’s syndrome is virtually unreported (37,38). In contrast, autoimmune thyroid disease has an increased prevalence in Turner’s syndrome, especially in those with an Xq isochromosome (39). These differences suggest that SLE and autoimmune thyroid disease, both female dominated autoimmune diseases, have distinct X chromosome dependent susceptibilities. In particular, our data imply that X chromosome monosomy, either congenital or acquired, may not be a risk factor for SLE, as has been suggested for primary biliary cirrhosis (40) or autoimmune thyroid disease (41).
The 46,XX and 47,XXY karyotypic risks for SLE appear to be similar. Consequently, some feature or features of Klinefelter’s syndrome must be sufficient to give the full female risk of SLE; and, applying parsimony, the many differences between normal 46,XX females and 47,XXY males are not sufficient to alter risk for SLE. The decreased estrogen in 47,XXY compared to 46,XX, for example, does not alter the SLE risk. Thus, our data support the notion that differences in estrogens alone do not explain the much lower incidence and prevalence of SLE in 46,XY men compared to 46,XX women. Some might construe the lack of an association of oral contraceptive use with exacerbation in established SLE (42) as being consistent with this interpretation.
Meanwhile, the increased androgens of 47,XXY compared to 46,XX do not protect from SLE. The other implication from the 47,XXY prevalence in male SLE is that the Y chromosome does not appear to influence the overall risk of SLE in men. The increased prevalence of 47,XXY in SLE supports the difference between the SLE prevalence of 46,XY men and 46,XX women being dominated by the X chromosome dose.
Our data show that the clinician treating male SLE, however, is working in a population enriched for 47,XXY and increased awareness of Klinefelter’s may improve diagnostic recognition. Three of three SLE men with Klinefelter’s syndrome answered the fertility question in a way that increased suspicion of infertility. There were only six other responses from other men. This question, which was asked and answered before this study was initiated, may be the most generally discriminating initial question for a man with SLE when screening to identify the 47,XXY SLE patients. Certainly, in any male SLE patient whose fertility is questionable, an evaluation for the physical and, perhaps, laboratory features of Klinefelter’s syndrome is suggested.
The increased prevalence of 47,XXY Klinefelter’s in males with SLE has genetic implications for understanding SLE by suggesting a gene dose effect at the X chromosome. In addition, diagnosing those who are 47,XXY from among the male SLE patients provides them access to potentially important medical management.
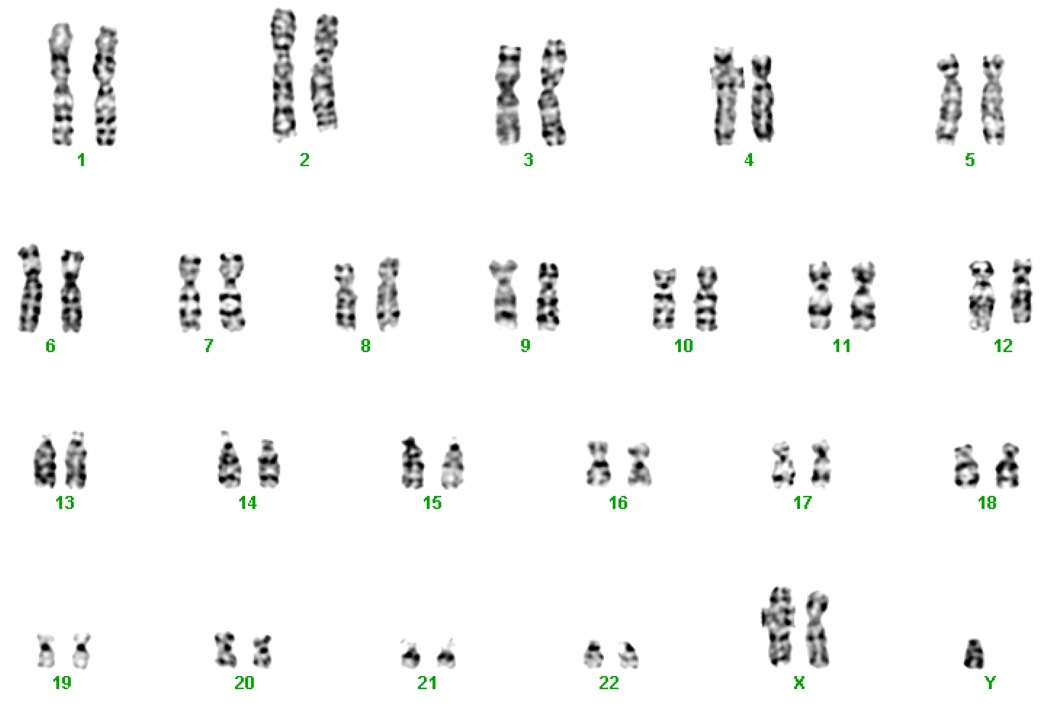
Figure 2.
The 47,XXY chromosomal karyotype of a male SLE patient. The diagnosis of Klinefelter’s syndrome in this patient was made clinically at about age 17. He is homozygous at all polymorphic X chromosome markers tested and therefore, must have Klinefelter’s syndrome as a result of a meiosis II non-disjunction in his mother.
Acknowledgments
This work has been supported by the NIH (AI24717, AI31584, AI54117, AI053747, AI062629, AR12253, AR24260, AR43727, AR48940, AR049084, AR049743, AR053734, DE015223, RR015577, RR019369, RR020143), the Alliance for Lupus Research, the Hopkins General Clinical Research Center (M01-RR-00052) and the U.S. Department of Veteran Affairs as well as the General Clinical Research Centers at Northwestern University Feinberg School of Medicine (MO1-RR00048) and the University of Oklahoma Health Sciences Center (MO1-RR014467). This investigation was conducted in part in a facility constructed with support from Research Facilities Improvement Program (grant C06 RR14570-01) from the National Center for Research Resources, National Institutes of Health. Dr. Harley is a Mary Kirkland Scholar. The authors are grateful for the help and cooperation of the SLE patients and their families and the referral of cases from support groups and physicians. The statistical advice of Barbara Neas, PhD, technical assistance of Michele Calvo, Parvathi Viswanathan, David Hutchings, Carisa Cooney, and Carrie Thornton, and critical reading of the manuscript by Michael Lockshin are all appreciated.
References
- 1.Lahita RG. Sex hormones and systemic lupus erythematosus. Rheum Dis Clin N Am. 2000;26:951–968. doi: 10.1016/s0889-857x(05)70178-2. [DOI] [PubMed] [Google Scholar]
- 2.Ortiz-Neu C, Leroy CE. The coincidence of Klinefelter’s syndrome and systemic lupus erythematosus. Arthritis Rheum. 1969;12:241–246. doi: 10.1002/art.1780120312. [DOI] [PubMed] [Google Scholar]
- 3.Gilliland WR, Stashower ME. Klinefelter’s syndrome and systemic lupus erythematosus. Clin Exp Rheumatol. 2000;18:107–109. [PubMed] [Google Scholar]
- 4.Lahita RG, Bradlow HL. Klinefelter’s syndrome: hormone metabolism in hypogonadal males with systemic lupus erythematosus. J Rheumatol. 1987;14:154–157. [PubMed] [Google Scholar]
- 5.Bizzarro A, Valentini G, Di Martino G, Daponte A, De Bellis A, Iacono G. Influence of testosterone therapy on clinical and immunologic features of autoimmune diseases associated with Klinefelter’s syndrome. J Clin Endo Metabol. 1986;64:32–36. doi: 10.1210/jcem-64-1-32. [DOI] [PubMed] [Google Scholar]
- 6.Jimenez-Balderas FJ, Tapia-Serrano R, Fonseca ME, et al. High frequency of association of rheumatic/autoimmune diseases and untreated male hypogonadism with severe testicular dysfunction. Arthritis Res. 2001;3:362–367. doi: 10.1186/ar328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeuchi Y, Murata Y, Sintani J, et al. Klinefelter’s syndrome accompanied by mixed connective tissue disease and diabetes mellitus. Internal Med. 1999;38:875–881. doi: 10.2169/internalmedicine.38.875. [DOI] [PubMed] [Google Scholar]
- 8.Ishihara K, Yoshimura M, Nakao H, Kanakura Y, Kanayama Y, Matsuzawa Y. T cell abnormalities in mixed connective tissue disease complicated with Klinefelter’s syndrome. Internal Med. 1994;33:714–717. doi: 10.2169/internalmedicine.33.714. [DOI] [PubMed] [Google Scholar]
- 9.Brown WM. Sex chromosome aneuploidy in man and its frequency, with special reference to mental subnormality and criminal behavior. Int Rev Exp Pathol. 1969;7:31–97. [PubMed] [Google Scholar]
- 10.Neilen J, Wohlert M. Chromosome abnormalities found among 34 910 newborn children: results from a 13-year incidence study from Arhus, Denmark. Hum Genet. 1991;87:81–83. doi: 10.1007/BF01213097. [DOI] [PubMed] [Google Scholar]
- 11.Hamerton JL, Canning N, Ray M, Smith S. A cytogenetic survey of 14 069 newborn infants. Clin Genet. 1975;8:223–243. doi: 10.1111/j.1399-0004.1975.tb01498.x. [DOI] [PubMed] [Google Scholar]
- 12.Dubois EL, Kaplan BJ. SLE and Klinefelter's syndrome. Lancet. 1976;1:93. doi: 10.1016/s0140-6736(76)90191-4. [DOI] [PubMed] [Google Scholar]
- 13.Price WH, MacLean N, Littlewood AP. SLE and Klinefelter’s syndrome. Lancet. 1976;1:807. doi: 10.1016/s0140-6736(76)91646-9. [DOI] [PubMed] [Google Scholar]
- 14.Moser KL, Neas BR, Salmon JE, et al. Genome scan of human systemic lupus erythematosus: Evidence for linkage on chromosome 1q in African-American pedigrees. Proc Natl Acad Sci USA. 1998;95:14869–14874. doi: 10.1073/pnas.95.25.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 16.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 17.Alarcon GS, McGwin G, Jr, Petri M, Reveille JD, Ramsey-Goldman R, Kimberly RP PROFILE Study Group. Baseline characteristics of a multiethnic lupus cohort: PROFILE. Lupus. 2002;11(2):95–101. doi: 10.1191/0961203302lu155oa. [DOI] [PubMed] [Google Scholar]
- 18.Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971;2:971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs PA, Bacino C, Hassold T, Morton NE, Keston M, Lee M. A cytogenetic study of 47,XXY males of known origin and their parents. Ann Hum Genet. 1988;52:319–325. doi: 10.1111/j.1469-1809.1988.tb01111.x. [DOI] [PubMed] [Google Scholar]
- 20.Hopkinson ND, Doherty M, Powell RJ. Clinical features and race-specific incidence/prevalence rates of systemic lupus erythematosus in a geographically complete cohort of patients. Ann Rheum Dis. 1994;53:675–680. doi: 10.1136/ard.53.10.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson AE, Gordon C, Palmer RG, Bacon PA. The prevalence and incidence of systemic lupus erythematosus in Birmingham, England. Arthritis Rheum. 1995;38:551–558. doi: 10.1002/art.1780380415. [DOI] [PubMed] [Google Scholar]
- 22.Lopez P, Mozo L, Gutierrez C, Suarez A. Epidemiology of systemic lupus erythematosus in a northern Spanish population: gender and age influence on immunological features. Lupus. 2003;12:860–865. doi: 10.1191/0961203303lu469xx. [DOI] [PubMed] [Google Scholar]
- 23.Petri M. Epidemiology of systemic lupus erythematosus. Best Prac Res Clin Rheumatol. 2002;16:847–858. doi: 10.1053/berh.2002.0259. [DOI] [PubMed] [Google Scholar]
- 24.Rovensky J. Rheumatic diseases and Klinefelter's syndrome. Autoimmun Rev. 2006;6:33–36. doi: 10.1016/j.autrev.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Lahita RG. The importance of estrogen in systemic lupus erythematosus. Clin Immunol Immunopathol. 1992;63:17–18. doi: 10.1016/0090-1229(92)90086-4. [DOI] [PubMed] [Google Scholar]
- 26.Lahita RG. Sex steroids and SLE: metabolism of androgens to estrogen. Lupus. 1992;1:125–127. doi: 10.1177/096120339200100301. [DOI] [PubMed] [Google Scholar]
- 27.Price WH, Clayton JF, Wilson J, Collyer S, De May R. Causes of death in X chromatin positive males (Klinefelter’s syndrome) J Epidemiol Comm Health. 1985;39:330–336. doi: 10.1136/jech.39.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langlands AO, MacLean N, Kerr GR. Carcinoma of the male breast: Report of a series of 88 cases. Clin Radiol. 1976;27:21–25. doi: 10.1016/s0009-9260(76)80008-6. [DOI] [PubMed] [Google Scholar]
- 29.Engelberth O, Charvat J, Jezkova Z, Raboch J. Autoantibodies in chromatin-positive men. Lancet. 1966;2:1164. [Google Scholar]
- 30.Kocar IH, Yesilova Z, Ozata M, Turan M, Sengul A, Ozdemir I. The effect of testosterone replacement treatment on immunological features of patients with Klinefelter’s syndrome. Clin Exp Immunol. 2000;121:448–452. doi: 10.1046/j.1365-2249.2000.01329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen NJ, Kovacs WJ. Case report: testosterone treatment of systemic lupus erythematosus in a patient with Klinefelter's syndrome. Am J Med Sci. 1995;310 doi: 10.1097/00000441-199510000-00006. 158-6. [DOI] [PubMed] [Google Scholar]
- 32.Petri MA, Mease PJ, Merrill JT, et al. Effects of prasterone on disease activity and symptoms in women with active systemic lupus erythematosus. Arthritis Rheum. 2004;50(9):2858–2868. doi: 10.1002/art.20427. [DOI] [PubMed] [Google Scholar]
- 33.Smyth CM, Bremner WJ. Klinefelter Syndrome. Arch Intern Med. 1998;158:1309–1314. doi: 10.1001/archinte.158.12.1309. [DOI] [PubMed] [Google Scholar]
- 34.Mok CC, Lau CS. Profile of sex hormones in male patients with systemic lupus erythematosus. Lupus. 2000;9:252–257. doi: 10.1191/096120300680198926. [DOI] [PubMed] [Google Scholar]
- 35.Tojo K, Kaguchi Y, Tokudome G, Kawamura T, Abe A, Sakai O. 47 XXY/XY mosaic Klinefelter’s syndrome presenting with multiple endocrine abnormalities. Intern Med. 1996;35:396–402. doi: 10.2169/internalmedicine.35.396. [DOI] [PubMed] [Google Scholar]
- 36.Sarri C, Cote GB, Mengreli C, Lambadidis I, Pantelakis S. Hypothyroidism and sex chromosomes. J Med Genet. 1988;25:247–249. doi: 10.1136/jmg.25.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takegami T, Nakao K, Nagayama Y, et al. A case of SLE associated with Turner’s syndrome of 45,XO/46,XXq mosaicism. Nippon Na Gakkai. 1980;69:861–866. doi: 10.2169/naika.69.861. [DOI] [PubMed] [Google Scholar]
- 38.Itani S, Hoshino T. Unusual X chromosome, anomary 45, X, X, t (X;X) (q26;q26) and systemic lupus erythematosus in a woman with gonadal dysgenesis. Jap J Hum Genet. 1979;24:175. [Google Scholar]
- 39.Elsheikh M, Wass JA, Conway GS. Autoimmune thyroid syndrome in women with Turner's syndrome - the association with karyotype. Clin Endocrinol. 2001;55:223–226. doi: 10.1046/j.1365-2265.2001.01296.x. [DOI] [PubMed] [Google Scholar]
- 40.Invernizzi P, Miozzo M, Battezzati PM, Bianchi I, Grati FR, Simoni G, Selmi C, Watnik M, Gershwin ME, Podda M. Frequency of monosomy X in women with primary biliary cirrhosis. Lancet. 2004;363:533–535. doi: 10.1016/S0140-6736(04)15541-4. [DOI] [PubMed] [Google Scholar]
- 41.Invernizzi P, Miozzo M, Selmi C, Persani L, Battezzati PM, Zuin M, Lucchi S, Meroni PL, Marasini B, Zeni S, Watnik M, Grati FR, Simoni G, Gershwin ME, Podda M. X chromosome monosomy: a common mechanism for autoimmune disease. J Immunol. 2005;175:575–578. doi: 10.4049/jimmunol.175.1.575. [DOI] [PubMed] [Google Scholar]
- 42.Petri M, Buyon JP, Kim M, et al. Combined oral contraceptives (OC) are not associated with an increased rate of flare in SLE patients in SLENA. Arthritis Rheum. 2004;50:S239. [Google Scholar]