Abstract
Recent evidence suggests that complement and Toll-like receptors (TLRs) crosstalk to coordinate innate immunity. We report a novel immune subversion mechanism involving microbial exploitation of the ability of complement and TLRs for communication. Porphyromonas gingivalis, a major oral and systemic pathogen expressing complement C5 convertase-like activity, was shown to synergize with C5a for cAMP elevation resulting in macrophage immunosuppression and enhanced pathogen survival in vitro and in vivo. The cAMP synergy strictly required TLR2 signaling and a pertussis toxin- and thapsigargin-sensitive C5a receptor pathway, whereas protein kinase A and glycogen synthase kinase-3β acted as downstream effectors. Antagonistic blockade of the C5a receptor abrogated this evasive strategy and may thus have important therapeutic implications in periodontitis and atherosclerosis, where P. gingivalis is implicated. This first demonstration of complement-TLR crosstalk for immunosuppressive cAMP signaling indicates that pathogens may not simply undermine complement and/or TLRs as separate entities, but may also exploit their crosstalk pathways.
INTRODUCTION
Although traditionally perceived as an antimicrobial enzyme system in serum, complement is now recognized as a central component of host defense impacting both innate and adaptive immunity (1). More recently, complement was suggested to crosstalk with another major innate defense system, the Toll-like receptors (TLRs), to apparently coordinate the host response to infection (2, 3). Not surprisingly, given its importance in fighting pathogens, complement constitutes a key target of immune evasion by microbes which cause persisting infections (4). Here we describe a novel strategy of immune subversion, involving microbial exploitation of the fifth complement component (C5) for corrupting TLR immunity via a hitherto unknown mechanism of complement-TLR crosstalk.
The pathogen involved in these subversive interactions, Porphyromonas gingivalis, is a gram-negative anaerobic bacterium. This organism is strongly associated with periodontitis, a highly prevalent oral chronic inflammatory disease, and is moreover implicated in systemic conditions such as atherosclerosis and aspiration pneumonia (5). Although P. gingivalis overall inhibits the complement cascade regardless of the initiation pathway involved, curiously enough, this pathogen selectively generates biologically active C5a (6, 7). C5a generation by P. gingivalis is mediated by its Arg-specific cysteine proteinases (RgpA and RgpB gingipains) which act in a C5 convertase-like manner (6, 7). Interestingly, upon release of C5a from C5, the C5b remnant is proteolytically destroyed by P. gingivalis (6) to apparently prevent activation of the terminal complement pathway, which leads to the formation of the membrane attack complex (1). Since C5a is a powerful chemoattractant and activator of phagocytes (8), it seems counterproductive for a pathogen to actively contribute to C5a generation. An intriguing question, therefore, is whether there is any survival advantage for P. gingivalis to specifically generate C5a in its periodontal niche, where complement proteins are abundantly present at up to 70% of their concentration in serum (7).
Below we present evidence that P. gingivalis paradoxically employs the proinflammatory C5a for targeted immune suppression of macrophages through a novel crosstalk mechanism between the C5a receptor (C5aR) and TLR2, the predominant TLR utilized by this organism in vitro and in vivo (9, 10). This is the first report for a pathogen capable of proactively instigating and exploiting crosstalk signaling between complement and TLRs, rather than undermining either system independently as previously shown for a number of other microbes (4, 11).
RESULTS
C5a and subversion of macrophage function
We were prompted to investigate whether C5a signaling is advantageous to P. gingivalis by earlier observations that its enzymatic activity selectively generates functional C5a, despite overall inhibiting the complement cascade (6, 7). We first examined whether C5a influences the macrophage intracellular killing of P. gingivalis. Strikingly, the ability of this pathogen to survive intracellularly in mouse macrophages was significantly promoted by C5a, but not by the related anaphylatoxin C3a (Fig. 1, A and B). This unexpected promicrobial effect of C5a was enhanced with increasing concentrations of C5a (fig. S1A) and was also observed in interferon (IFN)-γ–primed macrophages (Fig. 1, C and D). The elevated viable cell counts of P. gingivalis in C5a-treated macrophages could not be attributed to possible differences in the initial bacterial loads, since P. gingivalis phagocytosis was not significantly affected by the absence or presence of C5a or C3a (fig. S2A). Consistent with this, the expression of macrophage receptors which coordinately mediate P. gingivalis uptake, such as CD14, TLR2, and CD11b/CD18, was essentially unaffected by C5a (fig. S2, B and C).
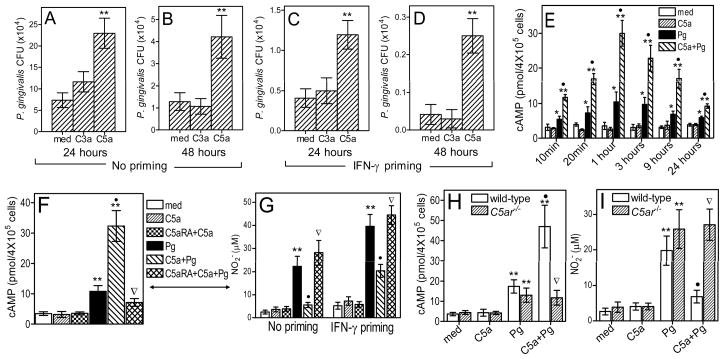
Figure 1.
Immunosubversive effects of C5a on macrophages. (A–D) Peritoneal mouse macrophages were left untreated (A,B) or primed with 100 ng/ml IFN-γ (C,D) overnight, washed, and incubated with P. gingivalis (Pg; MOI=10:1) in the presence or absence of C3a (200 nM) or C5a (50 nM). Viable counts of internalized bacteria at 24 hours (A and C) or 48 hours (B and D) post-infection were determined by CFU enumeration. (E) Macrophages were incubated with medium only or with Pg in the presence or absence of C5a for the indicated times and assayed for induction of intracellular cAMP. (F) Similar experiment as in E, involving 1-hour incubation and the use of a specific C5a receptor antagonist (C5aRA; 1 μM), as indicated. (G) Unprimed or IFN-γ–primed macrophages were assayed for NO2− after 24-hour incubation with or without Pg and/or C5a, which acted in the absence or presence of C5aRA. (H–I) Similar experiments for induction of cAMP (H) and NO2− (I) using macrophages from both wild-type and C5aR-deficient (C5ar−/−) mice. Data are means ± SD (n = 3) from typical experiments performed three (A–D, F, G) or two (E, H–I) times yielding consistent results. *, P < 0.05 and **, P < 0.01 vs. medium (med) control treatments. •, P < 0.01 in C5a+Pg vs. Pg alone. Inverted triangles indicate significant (P < 0.01) reversal of C5a effects by C5aRA or C5aR deficiency.
We next investigated the mechanism(s) underlying C5a-mediated inhibition of the macrophage intracellular killing capacity. In this regard, we hypothesized that the combined action of C5a and P. gingivalis on macrophages may induce immunosuppressive signaling. We first used real-time quantitative PCR to determine whether C5a upregulates the expression of negative regulators of TLR signaling in P. gingivalis-stimulated macrophages. Although the bacterium alone upregulated the expression of some of the investigated regulators, including the suppressor of cytokine signaling-1, the interleukin-1 receptor-associated kinase M, and the ubiquitin-editing enzyme A20, no synergistic or additive effects were seen in the concomitant presence of P. gingivalis and C5a (fig. S3). Therefore, these regulatory molecules are not likely involved in C5a-mediated suppression of macrophage killing of P. gingivalis. Moreover, although induction of cAMP can induce immunosuppressive signaling (12), C5a by itself failed to induce a cAMP response in macrophages (Fig. 1E). Strikingly, however, C5a synergized with P. gingivalis resulting in >3-fold elevation of the intracellular cAMP levels relative to P. gingivalis stimulation alone (Fig. 1E). The synergy was observed as early as 10 min after cell stimulation, peaked at 1 hour, but significantly elevated cAMP levels were sustained for at least 24 hours (Fig. 1E). This upregulatory effect of C5a was dose-dependent (fig. S1B) and was totally abrogated by a C5aR antagonist (C5aRA), the cyclic hexapeptide AcF(OP(D)ChaWR) (Fig. 1F), indicating that C5a acted through the classic C5aR (CD88), rather than the alternative C5a-like receptor 2.
Given that P. gingivalis is exquisitely resistant to killing by the oxidative burst (13), we investigated whether C5a interferes with induction of nitric oxide as a possible mechanism for its promicrobial effect. The underlying rationale was that P. gingivalis is sensitive to nitric oxide-mediated killing (14, 15). Indeed, C5a significantly inhibited, via a C5aR-dependent mechanism, the production of nitric oxide in P. gingivalis-stimulated macrophages, even in cells primed with IFN-γ (Fig. 1G). The C5aR specificity of the C5a-driven augmentation of cAMP and suppression of nitric oxide in P. gingivalis-challenged macrophages was confirmed by lack of these effects in C5aR-deficient (C5ar−/−) macrophages (Fig. 1, H and I, respectively). The inhibitory effect of C5a on nitric oxide was dose-dependent (fig. S4, A and B), although it progressively declined with increasing delay of C5a addition to the P. gingivalis-infected macrophages (fig. S4, C and D), suggesting a requirement for an early crosstalk between C5a-and P. gingivalis-induced signaling. On the other hand, when C5a was added together with P. gingivalis, the inhibitory C5a effect was maintained for at least 48 hours (fig. S4, E and F). The Fig. 1 findings suggest that C5aR activation by C5a results in suppression of P. gingivalis intracellular killing associated with elevation of cAMP and reduction of nitric oxide. Cause-and-effect relationships were established in subsequent experiments (below).
C5a immunosubversive effects are strictly dependent on cAMP-PKA signaling
We investigated whether the C5a-mediated inhibition of nitric oxide production depends upon the ability of C5a to stimulate synergistic elevation of cAMP. Indeed, the inhibitory C5a effect on nitric oxide was reversed in macrophages pretreated with inhibitors of cAMP synthesis (SQ22536) or of PKA (H89 and PKI 6-22) but not of irrelevant kinases (chelerythrin or KT5823) (Fig. 2A), indicating that the C5a effect is mediated by cAMP-dependent PKA signaling. Importantly, the upregulation of nitric oxide levels by inhibitors of cAMP or of PKA was linked to significantly reduced intracellular survival of P. gingivalis in those same cells (Fig. 2B). Moreover, macrophage pretreatment with C5aRA counteracted the protective effect of C5a on P. gingivalis intracellular viability, whereas L-NAME (nitric oxide synthesis inhibitor) mimicked C5a and overrode the C5aRA effect (Fig. 2C). In contrast, D-NAME, an inactive enantiomer control, had no effect in that regard (Fig. 2C). Interestingly, the ability of inhibitors of cAMP or of PKA to reverse the immunosuppressive C5a effect progressively declined with increasing delay of their addition to the culture system (Fig. 2D). Therefore, P. gingivalis needs to immediately activate cAMP-dependent PKA signaling to suppress the macrophage killing capacity, consistent with the requirement for early availability of C5a in order to disable P. gingivalis-challenged macrophages (fig. S4, C and D).
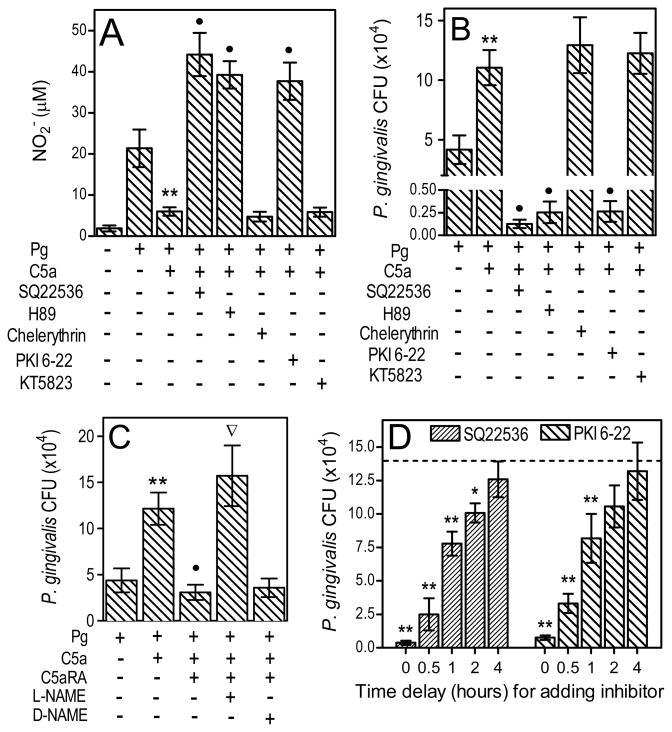
Figure 2.
C5a-mediated inhibition of nitric oxide and promotion of P. gingivalis survival is cAMP- and PKA-dependent. (A and B) Mouse macrophages were pretreated or not with SQ22536 (cAMP synthesis inhibitor; 200 μM), H89 (PKA inhibitor; 5 μM), chelerythrin (protein kinase C inhibitor; 5 μM), PKI 6-22 (peptide inhibitor of PKA; 1 μM), or KT5823 (peptide inhibitor of protein kinase G; 1 μM), and then infected with P. gingivalis (Pg; MOI=10:1) with or without C5a (50 nM), as indicated. (C) Macrophages were pretreated with 1 mM L-NAME (or D-NAME) and/or 1 μM C5aRA and then infected with Pg with or without C5a. (D) Macrophages were incubated with Pg and C5a in the absence or presence of SQ22536 or PKI 6-22, added prior to Pg and C5a (“0 time delay”) or with increasing delay times, as indicated. NO2− production (A) and viable counts of internalized bacteria (B–D) were determined at 24 hours postinfection. In D, the dashed line indicates Pg CFU in the absence of inhibitors (13.7±2.7[×104] CFU). Results are means ± SD (n = 3) from typical experiments performed at least twice with consistent results. *, P < 0.05 and **, P < 0.01 vs. corresponding controls. •, P < 0.01 in C5a+Pg plus inhibitor or antagonist vs. C5a+Pg only. In C, the inverted triangle shows significant (P < 0.01) reversal of the C5aRA effect.
In vivo exploitation of C5aR signaling for inhibition of nitric oxide and promotion of microbial survival
To determine if C5aR signaling promotes P. gingivalis virulence also in vivo, we investigated the pathogen’s ability to survive in mice after intraperitoneal infection, in the absence or presence of C5aRA. At 24 hours postinfection, the peritoneal lavage fluid from C5aRA-treated mice contained significantly lower P. gingivalis CFU compared to control mice (>95% reduction; Fig. 3A). Consistent with this, C5ar−/− mice were superior to wild-type controls in controlling the P. gingivalis infection (Fig. 3A). The wild-type control mice were additionally found to be bacteremic for P. gingivalis (4 out of 5 mice in this group had positive blood cultures 24 hours postinfection), whereas no bacteremia could be detected in C5ar−/− or C5aRA-treated wild-type mice, further indicating that C5aR signaling promotes P. gingivalis virulence. Additional support that the reduced peritoneal bacterial burden in the absence of C5aR signaling reflects increased P. gingivalis killing (rather than P. gingivalis escaping and taking up residence in internal organs) was obtained by lack of P. gingivalis CFU detection in homogenates of several organs examined (spleen, kidney, liver, and lungs) from either C5ar−/− or wild-type mice. The ability of C5aRA-treated mice for enhanced clearance of P. gingivalis correlated with elevated nitric oxide production (relative to control mice), whereas L-NAME counteracted both effects (Fig. 3, B and C). Therefore, as shown in vitro, the in vivo exploitation of C5aR signaling by P. gingivalis for enhanced survival involves a nitric oxide-dependent mechanism.
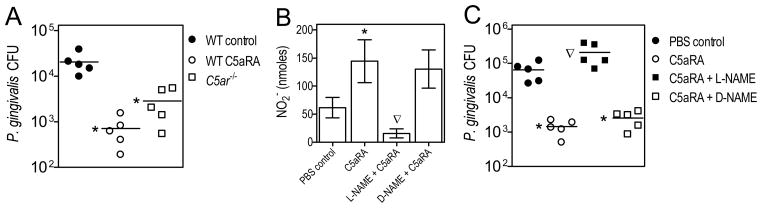
Figure 3.
P. gingivalis exploits C5aR signaling to inhibit nitric oxide production and promote its survival in vivo. (A) Wild-type (WT) mice were i.p. pretreated with C5aRA (1 mg/Kg body weight) or PBS control, followed by i.p. infection of these mice, as well as mice deficient in C5aR (C5ar−/−), with 5×107 CFU P. gingivalis. (B and C) Wild-type mice were i.p. pretreated or not with C5aRA with or without L-NAME or D-NAME (0.1 ml of 12.5 mM solution, corresponding to 0.34 mg per mouse) followed by P. gingivalis i.p. infection. Peritoneal fluid was collected 24 hours postinfection and used to determine viable P. gingivalis CFU (A and C) and NO2− production (B). Data are from typical experiments performed twice yielding consistent findings and represent means ± SD (n = 5) or are shown for each individual mouse with horizontal lines denoting mean values. *, P < 0.01 vs. controls. The inverted triangles show significant (P < 0.01) reversal of the C5aRA effects.
Synergistic activation of the cAMP-PKA pathway requires C5aR-TLR2 crosstalk
A systematic analysis of crosstalk in intracellular signaling pathways has revealed that receptor-mediated elevation of intracellular Ca2+ may potentiate cAMP induction by appropriate stimuli (16). If the synergistic effect of C5a on cAMP induction (Fig. 1E) depends upon its Ca2+-mobilizing activity, then this synergy should be inhibited by thapsigargin, an inhibitor of the endoplasmic reticulum Ca2+-ATPase which blocks the C5a-induced intracellular Ca2+ response (17). Indeed, macrophage pretreatement with thapsigargin abrogated the synergistic C5a effect on P. gingivalis-induced cAMP, whereas EGTA, which chelates extracellular Ca2+, had a relatively minimal and statistically insignificant effect (Fig. 4A). Significant reversal of the C5a effect on cAMP induction was also seen in cells pretreated with pertussis toxin (Fig. 4A), suggesting Gαi-coupled C5aR signaling (18).
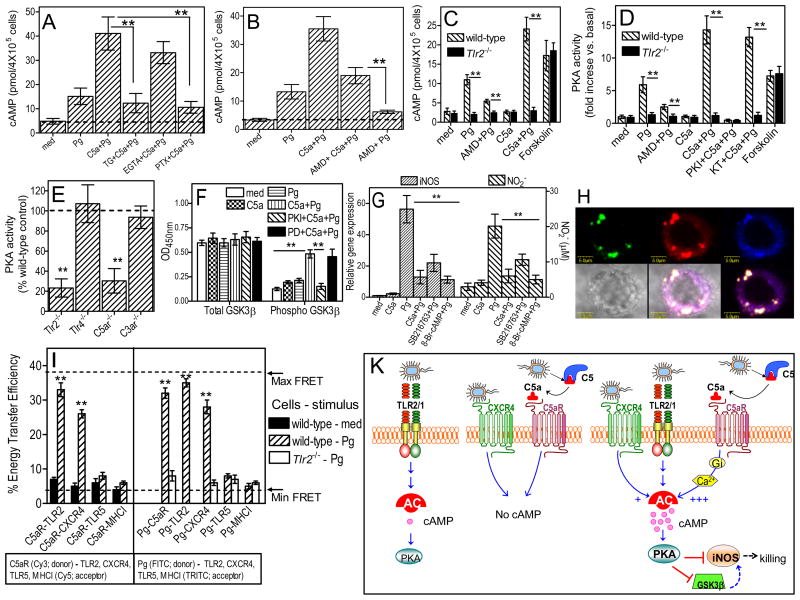
Figure 4.
Synergistic activation of the cAMP-PKA pathway requires C5aR-TLR2 crosstalk. Macrophages pretreated with 1 μM thapsigargin (TG), 5 mM EGTA, 100 ng/ml pertussis toxin (PTX) (A) or 1μg/ml AMD3100 (B–D) were stimulated with P. gingivalis (Pg; MOI=10:1; 1 hour) with or without 50 nM C5a and assayed for cAMP (A–C) or PKA activity (D). PKA assay specificity was confirmed using PKI-6-22 and an irrelevant kinase inhibitor (KT5823). Forskolin (20 μM; 10-min) served as positive control in experiments with Tlr2−/− macrophages (C and D). (E) PKA activities in freshly explanted peritoneal macrophages from Pg-infected mice (activities of indicated receptor-deficient cells expressed as % wild-type activity). (F) Macrophages pretreated with 1 μM PKI-6-22 or 25 μM PD98059 (PD; control) were stimulated with Pg, with or without C5a, and assayed for GSK3β Ser9-phosphorylation and total GSK3β. (G) Macrophages stimulated with Pg with or without C5a (50 nM), SB216763 (10 μM), or 8-Br-cAMP (100 μM) were assayed for iNOS expression (4 hours) or NO2− (24 hours). (H) Confocal colocalization of P. gingivalis (green), C5aR (red), and TLR2 (blue), as better shown in the bottom right merge image. (I) FRET between the indicated donors and acceptors measured from the increase in donor (Cy3 or FITC) fluorescence after acceptor (Cy5 or TRITC) photobleaching. Data are means ± SD (n=3 except for E, n=5) from typical experiments performed at least twice with consistent results. *, P<0.05; **, P <0.01 between the indicated groups or vs. controls (E and I). (K) Pg induces weak TLR2-dependent cAMP induction (left), whereas CXCR4 or C5aR signaling alone fails to induce cAMP (middle). However, Pg-induced TLR2 signaling with concomitant activation of C5aR and, to a lesser extent, CXCR4 synergistically enhances the immunosuppressive cAMP-PKA pathway that inactivates GSK3β and impairs iNOS-dependent killing.
In the absence of C5a, the ability of P. gingivalis to induce cAMP depends on its interaction with the CXC-chemokine receptor 4 (CXCR4) (15). We thus initially speculated that the synergistic C5a effect on cAMP induction could involve a crosstalk between C5aR and CXCR4. Although CXCR4 blockade by AMD3100 (at 1 μg/ml which completely inhibits the CXCR4-P. gingivalis interaction (15)) modestly attenuated the synergistic C5a effect on cAMP production, the synergism was still profoundly manifested (>6-fold difference between AMD+C5a+Pg vs. AMD+Pg; Fig. 4B). Moreover, P. gingivalis failed to elevate intracellular cAMP in CXCR4-transfected CHO-K1 cells, although it induced cAMP production in cells cotransfected with CXCR4 and TLR2 (fig. S5). Therefore, CXCR4 is not directly involved in cAMP induction but cooperates in that regard with TLR2, which on its own induces a rather weak cAMP response (fig. S5). We next showed that the synergistic C5a effect on cAMP induction actually involves a crosstalk with TLR2.
Indeed, the ability of C5a to synergistically induce cAMP and activate PKA in P. gingivalis-stimulated wild-type macrophages was utterly absent in similarly stimulated Tlr2−/− macrophages, which displayed only background activity levels (Fig. 4, C and D). However, the inherent capacity of Tlr2−/− macrophages to elevate intracellular cAMP and activate PKA was confirmed by including a forskolin control (direct adenylate cyclase activator) (Fig. 4, C and D). This novel concept of C5aR-TLR2 crosstalk for synergistic cAMP-dependent PKA activation is consistent with additional findings from an in vivo experiment. Indeed, the PKA activity detected in freshly explanted peritoneal macrophages from P. gingivalis-infected mice was significantly reduced by TLR2 or C5aR deficiency, but not by TLR4 or C3aR deficiency, relative to cells from wild-type mice (Fig. 4E).
We also showed that another synergistic interaction downstream of this receptor crosstalk involved PKA-dependent phosphorylation of glycogen synthase kinase-3β (GSK3β) on Ser9 (Fig. 4F), an event that inactivates this kinase which would otherwise positively regulate cell activation (19). Indeed, although C5a or P. gingivalis by themselves only slightly increased Ser9-phosphorylation of GSK3β, their combination displayed a synergistic effect which was inhibited by PKI 6-22 (but not by PD98059 control, an inhibitor of mitogen-activated protein kinase kinase) (Fig. 4F). Importantly, the GSK3β inhibitor SB216763 mimicked the inhibitory C5a effect on P. gingivalis-induced iNOS expression and nitric oxide production, as did 8-Br-cAMP (PKA agonist; positive control) (Fig. 4G). Thus, GSK3β appears to regulate iNOS and nitric oxide downstream of PKA in C5a plus P. gingivalis-challenged macrophages.
The C5aR-TLR2 crosstalk is also consistent with confocal microscopy findings revealing for the first time colocalization of the two receptors in P. gingivalis-stimulated macrophages (Fig. 4H), and with fluorescence resonance energy transfer (FRET) experiments indicating that C5aR, TLR2, and P. gingivalis come into molecular proximity (Fig. 4I). Indeed, FRET analysis revealed significant energy transfer between Cy3-labeled C5aR and Cy5-labeled TLR2 in P. gingivalis-stimulated but not resting macrophages (Fig. 4I). No significant energy transfer was detected between Cy3-labeled C5aR and Cy5-labeled TLR5 or MHC Class I (controls) under the same conditions (Fig. 4I). Moreover, significant energy transfer was observed between FITC-labeled P. gingivalis and TRITC-labeled C5aR or TLR2 (but not TLR5 or MHC Class I) (Fig. 4I). However, unlike TLR2 which can directly be engaged by P. gingivalis (9, 20), C5aR appeared to associate indirectly with P. gingivalis in a TLR2-dependent way; indeed, the P. gingivalis-C5aR FRET association was abrogated in Tlr2−/− macrophages (Fig. 4I). Taken together, the findings from Fig. 4 firmly establish a crosstalk between C5aR and TLR2 for synergistic induction of cAMP signaling.
FRET analysis further revealed that in P. gingivalis-challenged macrophages, C5aR also associates with CXCR4 (Fig. 4I), suggesting coassociation of all three receptors (CXCR4, TLR2, C5aR). These interactions likely occur in lipid rafts since all three receptors (but not TLR5 or MHC Class I) come within FRET proximity with an established lipid raft marker (GM1 ganglioside) in P. gingivalis-stimulated macrophages, unless the rafts are disrupted by methyl-β-cyclodextrin (fig. S6). Although the C5aR-TLR2 crosstalk can proceed independently of CXCR4 and potently upregulate cAMP (Fig. 4B), maximal cAMP induction requires cooperation of all three receptors (Fig. 4K model).
DISCUSSION
A molecular crosstalk between the complement system and the TLRs seems essential to appropriately coordinate the early innate response to infection (2, 3). Here, we addressed the intriguing possibility that at least some of the complement-TLR interplay may be instigated by pathogens, such as P. gingivalis, for promoting their adaptive fitness. The necessity for this evasion mechanism may be related to the fact that P. gingivalis cannot antagonize TLR2 activation at the receptor level, as it does with TLR4 (21). Therefore, it can be stated that this pathogen has evolved a subversive C5aR-TLR2 crosstalk mechanism for blunting the TLR2 antimicrobial response (Fig. 4K), as an alternative to direct TLR2 antagonism. Notably, P. gingivalis does not rely on immunological mechanisms for C5aR activation, since it can activate this receptor through gingipain-mediated local generation of C5a (6) (Fig. 4K). We confirmed and expanded the biochemical demonstration of C5a generation by purified gingipains acting on purified C5 substrate (6), by estimating that P. gingivalis generates high levels of C5a (32.7 ± 4.3 nM) upon 30-min incubation in heat-inactivated human serum. Notably, unlike C5a, C3a is extensively degraded and inactivated by P. gingivalis (6). Since C3a (but not C5a) exerts direct bactericidal effects (22), C3a destruction by P. gingivalis may serve to protect this pathogen.
The striking ability of C5a to synergize for cAMP production with P. gingivalis in a pertussis toxin-sensitive and TLR2-dependent way could be explained as follows. The Giβγ subunits, released upon activation of the pertussis toxin-sensitive Gαi subunit, can potently regulate adenylate cyclase (AC) activity, either positively or negatively depending on the enzyme isoform (23). Thus, although Giβγ cannot stimulate AC by themeselves, they can dramatically upregulate the activity of several AC isoforms in the presence of an appropriate stimulus. Such stimulus is apparently provided by P. gingivalis activation of TLR2. Importantly, the AC isoforms which are positively regulated by Giβγ are not those that are sensitive to the inhibitory action of Gαi (23). Since the ability of C5a to synergize with P. gingivalis for cAMP production is additionally dependent on intracellular Ca2+, Giβγ may possibly mediate their stimulatory effects on AC activity through their Ca2+-mobilizing effects.
A major mechanism underlying the regulatory effects of cAMP on cell activation involves the ability of cAMP-dependent PKA to phosphorylate the cAMP response element-binding protein (CREB), which effectively competes with the p65 subunit of nuclear factor-κB for limiting amounts of common transcriptional cofactors (12). Besides being under nuclear factor-κB control, the iNOS is additionally regulated by IFN-γ; interestingly, however, PKA also inhibits the IFN regulatory factor-1 that is required for the synergistic IFN-γ contribution to iNOS transcription (24, 25). Moreover, as supported by the figure 4F data, PKA can phosphorylate and inactivate GSK3β, thus abrogating its stimulatory effect on proinflammatory gene expression (19). Since PKA activation causes greater iNOS inhibition than GSK3β inactivation (Fig. 4G), it is likely that PKA may inibit iNOS also in a GSK3β-independent way (Fig. 4K).
Although modest TLR-induced cAMP induction may control excessive proinflammatory signaling, sustained high levels of cAMP instigated by pathogens (and thus out of host control) may impair host defense. P. gingivalis is the first pathogen shown to exploit complement and TLRs to cause cAMP-dependent immune subversion in vitro and in vivo. It should be noted, however, that the interaction of C5a with P. gingivalis-challenged macrophages did not induce a generalized or nonspecific macrophage immunosuppression, since C5a actually enhanced P. gingivalis-induced interleukin-6 (IL-6) production (fig. S7). This sophisticated subversive crosstalk instigated by P. gingivalis (Fig. 4K) serves in lieu of “built-in” adenylate cyclase which is not expressed by this bacterium, in contrast to Bordetella pertussis which disables human or mouse phagocytes by means of its own adenylate cyclase (26).
Macrophages can interact with P. gingivalis not only in periodontal tissues but also in the setting of systemic inflammatory diseases such as atherosclerosis (5, 13, 27). Our previous findings that P. gingivalis persists intracellularly in macrophages for at least 72h (28) were confirmed by an independent group, which additionally showed that up to 25% of the cells undergo necrosis by 72h and release cellular contents (29). It is thus conceivable that viable P. gingivalis could be released from necrotic macrophages, especially in the presence of C5a which dramatically promotes its intracellular persistence. This possibility becomes intriguing in view of epidemiological and mechanistic links between periodontitis and atherosclerosis (5, 27). However, whether the documented localization of viable P. gingivalis bacteria in atherosclerotic plaques (30) can be attributed to relocation of infected macrophages from periodontal tissues is currently uncertain. Nevertheless, the pathogen’s capacity to exit initially infected host cells and then enter and multiply within new hosts, including vascular cells, has been documented (31, 32).
C5aR activation in macrophages was also shown to inhibit TLR4-induced mRNA expression of IL-12p35, IL-12/IL-23p40, and IL-23p19, and production of IL-12p70 and IL-23 protein, through C5a-induced phosphatidylinositol-3 kinase and extracellular signal-regulated kinase 1/2 signaling (2, 3, 33). The physiological significance of these C5a regulatory effects is likely to attenuate potential tissue damage mediated by various T cell effector subsets (e.g., Th1 and Th17, regulated by IL-12 and IL-23, respectively), as seen in various pathological inflammatory conditions (34). However, undesirable outcomes may arise when C5a is not produced physiologically but rather through the uncontrolled action of microbial enzymes. In this context, pathogen-induced generation of C5a may modify TLR signaling and skew the T helper response in ways that could interfere with protective immunity. Therefore, on the basis of our findings and the reports on IL-12 and IL-23 regulation by C5a, it becomes evident that pathogens may exploit TLR-C5aR crosstalk in various ways.
In summary, this work constitutes the first report of complement-TLR crosstalk for synergistic cAMP induction which disables macrophages. From a therapeutic viewpoint, C5aR blockade effectively deprived this pathogen of crucial survival tactics and may thus confer protection against periodontitis and associated systemic diseases like atherosclerosis. Since C5a can be generated by both complement and non-complement C5 convertases that also include microbial enzymes (6, 35, 36), it becomes important to identify other pathogens that exploit C5a-mediated subversive crosstalk signaling with TLRs. This will have important implications for novel counter-strategies to neutralize microbial virulence. Our findings further suggest that, in the course of evolution, chronically persisting pathogens may not have simply “learned” to breach complement and the TLRs separately, but, as hereby exemplified by P. gingivalis, to also exploit their communication hubs.
MATERIALS AND METHODS
Reagents
SQ22536, H89, SB216367, 8-Br-cAMP, AMD3100, forskolin, L-NAME (N(G)-nitro-L-arginine methyl ester), D-NAME (N(G)-nitro-D-arginine methyl ester), and EGTA were purchased from Sigma-Aldrich. Chelelythrin, PKI 6-22, KT5823, and thapsigargin were obtained from Calbiochem. PD98059 was from Cell Signaling Technology. Mouse-specific monoclonal antibodies to TLR2 [clone 6C2] was from e-Bioscience, TLR5 [85B152.5] from Abcam, and C5aR (20/70) from Cedarlane Laboratories or Hycult. Mouse IFN-γ was from the R&D Systems. Mouse C5a was purchased from Cell Sciences or the R&D Systems and C3a from the R&D Systems. The cyclic hexapeptide AcF(OP(D)ChaWR) (acetylated phenylalanine (ornithine-proline-(D)cyclohexylalanine-tryptophan-arginine)), a specific and potent C5a receptor (CD88) antagonist, was synthesized in the laboratory of one of the co-authors (JDL), as previously described (37, 38). C5a and C3a were used at concentrations up to 100 nM and 200 nM, respectively, which are widely used in in vitro experiments (2, 3). Moreover, these concentrations are consistent with observations that under inflammatory conditions, C5a and C3a may reach serum levels as high as 100 nM and 400 nM, respectively, although even higher levels may be generated at local sites of inflammation (8, 39). All reagents were used at optimal concentrations determined in preliminary or published studies by our laboratories (15, 38, 40). When appropriate, dimethyl sulfoxide (DMSO) was included in medium controls and its final concentration was ≤ 0.2 %.
Bacteria and mammalian cells
P. gingivalis ATCC 33277 was grown anaerobically from frozen stocks on modified Gifu anaerobic medium (GAM)-based blood agar plates for 5–6 days at 37°C, followed by anaerobic subculturing for 18–24 hours at 37°C in modified GAM broth (Nissui Pharmaceutical). Thioglycollate-elicited macrophages were isolated from the peritoneal cavity of wild-type or mice deficient in TLR2, TLR4, C3aR, or C5aR (The Jackson Laboratory) (3, 9), in compliance with established federal guidelines and institutional policies. The macrophages were cultured at 37°C and 5% CO2 in RPMI 1640 (InVitrogen) supplemented with 10% heat-inactivated FBS, 2 mM L-glutamine, 100 units/ml penicillin G, 100 μg/ml streptomycin, and 0.05 mM 2-ME. None of the experimental treatments, including treatments with C5a up to 100 nM, affected cell viability (monitored by the CellTiter-Blue assay; Promega) compared to medium-only treatments.
Intracellular survival assay
The viability of phagocytosed P. gingivalis was monitored by an antibiotic protection-based intracellular survival assay, as previously described (28). Briefly, mouse peritoneal macrophages were allowed to phagocytose P. gingivalis (MOI = 10:1; 5×106 bacteria and 5×105 cells) for 30 min at 37°C. This was followed by washing to remove extracellular nonadherent bacteria and 1-hour treatment with antibiotics (300 μg/ml gentamicin and 200 μg/ml metronidazole) to eliminate residual or extracellular adherent bacteria. The macrophages were subsequently cultured overnight (for a total of 24 hours) or for 48 hours. Immediately after, the macrophages were washed and lysed in sterile distilled water and viable counts of internalized P. gingivalis were determined by plating serial dilutions of macrophage lysates on blood agar plates subjected to anaerobic culture (28).
Cell signaling and activation assays
Induction of nitric oxide production was assessed by measuring the amount of NO2− (stable metabolite of nitric oxide) in stimulated culture supernatants using a Griess reaction-based assay kit (R&D Systems), as previously performed (15). Levels of cAMP in activated cell extracts were measured using a cAMP enzyme immunoassay kit (CaymanChemical) (40). PKA activity in lysates of activated cells was determined using the ProFluor™ PKA assay, according to the instructions of the manufacturer (Promega) (15). Phosphorylation of GSK3β on Ser9 and total GSK3β were monitored using FACE™ GSK3β ELISA kits (Active Motif).
In vivo infection
Upon i.p. infection of mice with P. gingivalis (5×107 CFU), peritoneal lavage was performed 24 hours postinfection and the peritoneal fluid was used to enumerate recovered CFU (following anaerobic growth on blood agar plates) and measure production of NO2− (15). All animal procedures were approved by the Institutional Animal Care and Use Committee and performed in compliance with established federal and state policies.
Quantitative real-time PCR
Gene expression in resting or activated mouse macrophages was quantified using quantitative real-time PCR. Briefly, RNA was extracted from cell lysates using the PerfectPure RNA cell kit (5 Prime, Fisher) and quantified by spectrometry at 260 and 280 nm. The RNA was reverse-transcribed using the High-Capacity cDNA Archive kit (Applied Biosystems) and quantitative real-time PCR with cDNA was performed using the ABI 7500 Fast System, according to the manufacturer’s protocol (Applied Biosystems). TaqMan probes, sense primers, and antisense primers for expression of a house-keeping gene (GAPDH) or iNOS (or genes shown in fig. S3) were purchased from Applied Biosystems.
Confocal microscopy
To examine colocalization of P. gingivalis with C5aR and TLR2, mouse macrophages were grown on chamber slides and exposed to FITC-labeled P. gingivalis for 10 min. The cells were then fixed, permeabilized, stained with Texas Red-labeled anti-C5aR plus allophycocyanin-labeled anti-TLR2, and mounted with coverslips for imaging on an Olympus FV500 confocal microscope (28).
Fluorescence resonance energy transfer (FRET)
Upon stimulation for 10 min at 37°C with P. gingivalis, mouse macrophages were labeled with a mixture of Cy3-conjugated (donor) and Cy5-conjugated (acceptor) antibodies, as indicated in Fig. 4I. In other experiments shown in Fig. 4I, FITC-labeled P. gingivalis was used as donor and TRITC-labeled receptors served as acceptors. The cells were washed and fixed, and energy transfer between various donor-acceptor pairs was calculated from the increase in donor fluorescence after acceptor photobleaching (9, 41). The maximum (max) and minimum (min) energy transfer efficiencies in the experimental system were determined in control experiments as the energy transfer between two different epitopes on the same molecule or between molecules that do not engage in heterotypic associations, and their values are denoted by dashed lines in Fig. 4I. The conjugation of antibodies to Cy3 or Cy5 was performed using kits from Amersham Biosciences.
Statistical analysis
Data were evaluated by analysis of variance and the Dunnett multiple-comparison test using the InStat program (GraphPad Software, San Diego, CA). Where appropriate (comparison of two groups only), two-tailed t tests were performed. P < 0.05 was taken as the level of significance. All experiments were performed at least twice for verification.
Supplementary Material
Figure S1. C5a dose-dependently promotes the intracellular survival of P. gingivalis and the cAMP response.
Figure S2. C5a does not affect P. gingivalis phagocytosis.
Figure S3. Relative expression of negative regulators of TLR signaling in P. gingivalis-stimulated macrophages in the absence or presence of C5a.
Figure S4. C5a inhibits nitric oxide production in a dose- and time-dependent way
Figure S5. TLR2-dependent cAMP production by P. gingivalis.
Figure S6. Association of TLR2, C5aR, and CXCR4 with GM1 (lipid raft marker) in P. gingivalis-stimulated macrophages.
Figure S7. Upregulation of IL-6 production by C5a in P. gingivalis-stimulated macrophages.
Acknowledgments
This study was supported by U.S. Public Health Service Grants AI068730 and GM062134 (to JDL) & DE015254 and DE018292 (to GH), and by funds from the Wellcome Trust (to KT).
Footnotes
Publisher's Disclaimer: This manuscript has been accepted for publication in Science Signaling. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencesignaling.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS
REFERENCES AND NOTES
- 1.Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. 2007;171:715–727. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawlisch H, Belkaid Y, Baelder R, Hildeman D, Gerard C, Kohl J. C5a negatively regulates toll-like receptor 4-induced immune responses. Immunity. 2005;22:415–426. doi: 10.1016/j.immuni.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Kimura Y, Fang C, Zhou L, Sfyroera G, Lambris JD, Wetsel RA, Miwa T, Song WC. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110:228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambris JD, Ricklin D, Geisbrecht BV. Complement evasion by human pathogens. Nat Rev Microbiol. 2008;6:132–142. doi: 10.1038/nrmicro1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 6.Wingrove JA, DiScipio RG, Chen Z, Potempa J, Travis J, Hugli TE. Activation of complement components C3 and C5 by a cysteine proteinase (gingipain-1) from Porphyromonas (Bacteroides) gingivalis. J Biol Chem. 1992;267:18902–18907. [PubMed] [Google Scholar]
- 7.Popadiak K, Potempa J, Riesbeck K, Blom AM. Biphasic effect of gingipains from Porphyromonas gingivalis on the human complement system. J Immunol. 2007;178:7242–7250. doi: 10.4049/jimmunol.178.11.7242. [DOI] [PubMed] [Google Scholar]
- 8.Ward PA. The dark side of C5a in sepsis. Nat Rev Immunol. 2004;4:133–142. doi: 10.1038/nri1269. [DOI] [PubMed] [Google Scholar]
- 9.Hajishengallis G, Tapping RI, Harokopakis E, Nishiyama SI, Ratti P, Schifferle RE, Lyle EA, Triantafilou M, Triantafilou K, Yoshimura F. Differential interactions of fimbriae and lipopolysaccharide from Porphyromonas gingivalis with the Toll-like receptor 2-centred pattern recognition apparatus. Cell Microbiol. 2006;8:1557–1570. doi: 10.1111/j.1462-5822.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 10.Burns E, Bachrach G, Shapira L, Nussbaum G. Cutting Edge: TLR2 is required for the innate response to Porphyromonas gingivalis: Activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J Immunol. 2006;177:8296–8300. doi: 10.4049/jimmunol.177.12.8296. [DOI] [PubMed] [Google Scholar]
- 11.Finlay BB, McFadden G. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell. 2006;124:767–782. doi: 10.1016/j.cell.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 12.Parry G, Mackman N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-κB-mediated transcription. J Immunol. 1997;159:5450–5456. [PubMed] [Google Scholar]
- 13.Hajishengallis G. Porphyromonas gingivalis-host interactions: open war or intelligent guerilla tactics? Microbes Infect. 2009;11:637–645. doi: 10.1016/j.micinf.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gyurko R, Boustany G, Huang PL, Kantarci A, Van Dyke TE, Genco CA, Gibson FC., 3rd Mice lacking inducible nitric oxide synthase demonstrate impaired killing of Porphyromonas gingivalis. Infect Immun. 2003;71:4917–4924. doi: 10.1128/IAI.71.9.4917-4924.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hajishengallis G, Wang M, Liang S, Triantafilou M, Triantafilou K. Pathogen induction of CXCR4/TLR2 cross-talk impairs host defense function. Proc Natl Acad Sci U S A. 2008;105:13532–13537. doi: 10.1073/pnas.0803852105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Natarajan M, Lin KM, Hsueh RC, Sternweis PC, Ranganathan R. A global analysis of cross-talk in a mammalian cellular signalling network. Nat Cell Biol. 2006;8:571–580. doi: 10.1038/ncb1418. [DOI] [PubMed] [Google Scholar]
- 17.Moller T, Nolte C, Burger R, Verkhratsky A, Kettenmann H. Mechanisms of C5a and C3a complement fragment-induced [Ca2+]i signaling in mouse microglia. J Neurosci. 1997;17:615–624. doi: 10.1523/JNEUROSCI.17-02-00615.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monk PN, Scola AM, Madala P, Fairlie DP. Function, structure and therapeutic potential of complement C5a receptors. Br J Pharmacol. 2007;152:429–448. doi: 10.1038/sj.bjp.0707332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davey M, Liu X, Ukai T, Jain V, Gudino C, Gibson FC, 3rd, Golenbock D, Visintin A, Genco CA. Bacterial fimbriae stimulate proinflammatory activation in the endothelium through distinct TLRs. J Immunol. 2008;180:2187–2195. doi: 10.4049/jimmunol.180.4.2187. [DOI] [PubMed] [Google Scholar]
- 21.Coats SR, Jones JW, Do CT, Braham PH, Bainbridge BW, To TT, Goodlett DR, Ernst RK, Darveau RP. Human Toll-like receptor 4 responses to P. gingivalis are regulated by lipid A 1- and 4′-phosphatase activities. Cell Microbiol. 2009;11:1587–1599. doi: 10.1111/j.1462-5822.2009.01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nordahl EA, Rydengard V, Nyberg P, Nitsche DP, Morgelin M, Malmsten M, Bjorck L, Schmidtchen A. Activation of the complement system generates antibacterial peptides. Proc Natl Acad Sci U S A. 2004;101:16879–16884. doi: 10.1073/pnas.0406678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sunahara RK, Taussig R. Isoforms of mammalian adenylyl cyclase: Multiplicities of signaling. Mol Interv. 2002;2:168–184. doi: 10.1124/mi.2.3.168. [DOI] [PubMed] [Google Scholar]
- 24.Martin E, Nathan C, Xie QW. Role of interferon regulatory factor 1 in induction of nitric oxide synthase. J Exp Med. 1994;180:977–984. doi: 10.1084/jem.180.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delgado M, Ganea D. Inhibition of IFN-γ-induced janus kinase-1-STAT1 activation in macrophages by vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. J Immunol. 2000;165:3051–3057. doi: 10.4049/jimmunol.165.6.3051. [DOI] [PubMed] [Google Scholar]
- 26.Confer D, Eaton J. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982;217:948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- 27.Gibson FC, 3rd, Hong C, Chou HH, Yumoto H, Chen J, Lien E, Wong J, Genco CA. Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109:2801–2806. doi: 10.1161/01.CIR.0000129769.17895.F0. [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Shakhatreh M-AK, James D, Liang S, Nishiyama S-i, Yoshimura F, Demuth DR, Hajishengallis G. Fimbrial proteins of Porphyromonas gingivalis mediate in vivo virulence and exploit TLR2 and complement receptor 3 to persist in macrophages. J Immunol. 2007;179:2349–2358. doi: 10.4049/jimmunol.179.4.2349. [DOI] [PubMed] [Google Scholar]
- 29.Huang MT, Taxman DJ, Holley-Guthrie EA, Moore CB, Willingham SB, Madden V, Parsons RK, Featherstone GL, Arnold RR, O’Connor BP, Ting JP. Critical role of apoptotic speck protein containing a caspase recruitment domain (ASC) and NLRP3 in causing necrosis and ASC speck formation induced by Porphyromonas gingivalis in human cells. J Immunol. 2009;182:2395–2404. doi: 10.4049/jimmunol.0800909. [DOI] [PubMed] [Google Scholar]
- 30.Kozarov EV, Dorn BR, Shelburne CE, Dunn WA, Jr, Progulske-Fox A. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler Thromb Vasc Biol. 2005;25:e17–e18. doi: 10.1161/01.ATV.0000155018.67835.1a. [DOI] [PubMed] [Google Scholar]
- 31.Yilmaz O, Verbeke P, Lamont RJ, Ojcius DM. Intercellular spreading of Porphyromonas gingivalis infection in primary gingival epithelial cells. Infect Immun. 2006;74:703–710. doi: 10.1128/IAI.74.1.703-710.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Michel R, Cohen J, Decarlo A, Kozarov E. Intracellular survival and vascular cell-to-cell transmission of Porphyromonas gingivalis. BMC Microbiol. 2008;8:26. doi: 10.1186/1471-2180-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.la Sala A, Gadina M, Kelsall BL. G(i)-protein-dependent inhibition of IL-12 production is mediated by activation of the phosphatidylinositol 3-kinase-protein 3 kinase B/Akt pathway and JNK. J Immunol. 2005;175:2994–2999. doi: 10.4049/jimmunol.175.5.2994. [DOI] [PubMed] [Google Scholar]
- 34.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 35.Huber-Lang M, Younkin EM, Sarma JV, Riedemann N, McGuire SR, Lu KT, Kunkel R, Younger JG, Zetoune FS, Ward PA. Generation of C5a by phagocytic cells. Am J Pathol. 2002;161:1849–1859. doi: 10.1016/S0002-9440(10)64461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fick RB, Jr, Robbins RA, Squier SU, Schoderbek WE, Russ WD. Complement activation in cystic fibrosis respiratory fluids: in vivo and in vitro generation of C5a and chemotactic activity. Pediatr Res. 1986;20:1258–1268. doi: 10.1203/00006450-198612000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Finch AM, Wong AK, Paczkowski NJ, Wadi SK, Craik DJ, Fairlie DP, Taylor SM. Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. J Med Chem. 1999;42:1965–1974. doi: 10.1021/jm9806594. [DOI] [PubMed] [Google Scholar]
- 38.Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, Coukos G, Lambris JD. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9:1225–1235. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takabayashi T, Vannier E, Clark BD, Margolis NH, Dinarello CA, Burke JF, Gelfand JA. A new biologic role for C3a and C3a desArg: regulation of TNF-α and IL-1β synthesis. J Immunol. 1996;156:3455–3460. [PubMed] [Google Scholar]
- 40.Liang S, Wang M, Triantafilou K, Triantafilou M, Nawar HF, Russell MW, Connell TD, Hajishengallis G. The A subunit of Type IIb enterotoxin (LT-IIb) suppresses the proinflammatory potential of the B subunit and its ability to recruit and interact with TLR2. J Immunol. 2007;178:4811–4819. doi: 10.4049/jimmunol.178.8.4811. [DOI] [PubMed] [Google Scholar]
- 41.Triantafilou K, Triantafilou M, Dedrick RL. A CD14-independent LPS receptor cluster. Nat Immunol. 2001;2:338–345. doi: 10.1038/86342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. C5a dose-dependently promotes the intracellular survival of P. gingivalis and the cAMP response.
Figure S2. C5a does not affect P. gingivalis phagocytosis.
Figure S3. Relative expression of negative regulators of TLR signaling in P. gingivalis-stimulated macrophages in the absence or presence of C5a.
Figure S4. C5a inhibits nitric oxide production in a dose- and time-dependent way
Figure S5. TLR2-dependent cAMP production by P. gingivalis.
Figure S6. Association of TLR2, C5aR, and CXCR4 with GM1 (lipid raft marker) in P. gingivalis-stimulated macrophages.
Figure S7. Upregulation of IL-6 production by C5a in P. gingivalis-stimulated macrophages.