Abstract
The blood brain barrier (BBB) evolved to preserve the microenvironment of the highly excitable neuronal cells to allow for action potential generation and propagation. Intricate molecular interactions between two main cell types, the neurons and the glial cells, form the underlying basis of the critical functioning of the nervous system across species. In invertebrates, interactions between neurons and glial cells are central in establishing a functional BBB. However, in vertebrates, the BBB formation and function is coordinated by interactions between neurons, glial cells, and endothelial cells. Here we review the neuron-glial interaction–based blood barriers in invertebrates and vertebrates and provide an evolutionary perspective as to how a glial-barrier system in invertebrates evolved into an endothelial barrier system. We also summarize the clinical relevance of the BBB as this protective barrier becomes disadvantageous in the pharmacological treatment of various neurological disorders.
Keywords: septate junctions, tight junctions, endothelial cells, astrocytes, neurovascular unit, Drosophila
INTRODUCTION
Proper neuronal function necessitates a highly regulated extracellular environment, where the concentrations of sodium, potassium, and calcium ions need to be maintained within very narrow ranges. The central nervous system (CNS) is extremely sensitive to a wide range of substances that are otherwise readily metabolized without causing harm to the peripheral organ system. As a result, it is essential that the interface between the CNS and the peripheral circulatory system functions as a dynamic regulator of ion balance, a facilitator of nutrient transport, and a barrier to potentially harmful molecules (Hawkins & Davis 2005). Thus the structural aspects of the cerebral microcirculation, historically referred to as the blood-brain barrier (BBB), perform all these functions.
More than 100 years ago, Paul Ehrlich (1885) observed that certain pharmacologically active molecules and dyes injected into the bloodstream rapidly diffused into most organs with the exception of the CNS, composed of brain, spinal cord, and retina, because these were highly impermeable to most small molecules. Ehrlich proposed that the CNS environment possessed specialized properties that allow selective entry of only a small fraction of circulating factors. These and related findings formed the concept of a BBB that functions as a barricade to block blood-borne materials from entering the CNS microenvironment and also prevents permeability in the outward direction such that chemicals released from the nerve cells do not mix with blood (Goldmann 1913). Thus the concept of a vascular BBB, which also functions as a brain-blood barrier, was born (Bradbury 1979). Researchers now accept that several blood-CNS barriers exist, including blood-cerebrospinal fluid barrier and blood-retinal barrier (Risau & Wolburg 1990, Strazielle et al. 2004).
Advances in microscopy revealed that, in contrast with other vascular beds, the brain endothelial cells lining the vascular wall are tightly linked with junctional complexes to prevent free diffusion of blood-borne substances into the brain parenchymal space. Electron microscopic studies helped in identifying ultrastructural properties of the brain endothelium as the actual BBB. Brain endothelial cells have specialized intercellular tight junctions (TJs) of extremely high electrical resistance that provide a diffusion barrier between the blood and brain. These earlier studies using electron-dense tracers revealed that the BBB is an endothelial barrier present in capillaries that course through the brain (Reese & Karnovsky 1967, Brightman & Reese 1969).
One essential function of this BBB in vertebrates and invertebrates is to establish a stable ionic microenvironment that ensures appropriate firing of neurons and action potential propagation. In addition, the BBB is of outstanding clinical relevance because its breakdown leads to severe pathology as seen in several cerebrovascular disorders. Although this barrier is essential for the normal operation of the nervous system, it also prevents the entry of possible therapeutic molecules into the brain, making it a stubborn obstacle for treatment. Despite BBB’s importance, fundamental questions still remain about the cellular and molecular mechanisms that control its establishment, integrity, maintenance, and function.
A comparatively recent approach toward understanding the BBB has been in exploring the specific interactions between the neurons, astrocytes, and brain endothelium that might regulate BBB function. Here we review how neuron-glial interactions set up the blood barriers in invertebrates and vertebrates, the key structural attributes of the barrier, the molecular components that establish and/or maintain the barrier, and the pathological conditions resulting from a breach of the BBB, thus highlighting its outstanding importance across species.
NEURON-GLIAL INTERACTIONS IN BLOOD BARRIERS OF INVERTEBRTAES
Unlike the higher vertebrates where the BBB is formed by endothelial cells lining cerebral microvessels, the barrier in invertebrates, such as insect and crustacean, is formed by glial cells. An understanding of the glial cells in invertebrates is thus a prerequisite for knowing the blood barriers. Furthermore, studies on invertebrate glial cells, such as insect glia, can give information and provide insights that are either difficult to obtain or are unavailable from the study of vertebrate glia alone. Several common properties have emerged wherever comparisons between invertebrate and vertebrate glia have been possible, indicating that studies in a range of animal models can add to our understanding of the glial architecture and function (Bellen & Schulze 2003). For example, in the mature Dipteran Drosophila melanogaster nervous system, cell body glia perform modulatory functions, similar to vertebrate astrocytes; longitudinal glia ensheath CNS axons, similar to vertebrate oligodendrocytes; peripheral glia ensheath the nerves that project from CNS into the peripheral nervous system (PNS), similar to vertebrate Schwann cells; multiple glial subtypes remove apoptotic corpses from the CNS similar to vertebrate microglia; and perineurial glial cells establish the blood-nerve barrier (BNB) in Drosophila similar to BBB of vertebrates (Freeman et al. 2003). In both systems, neuron-glial interactions are known to be essential for many aspects of neuronal development, including regulation of neuronal precursor proliferation, axon pathfinding, axon fasciculation, and synaptogenesis.
The role of glia in the development of the embryonic insect nervous system has been studied in detail. Such extensive studies on developing insect glia have been feasible because of the transparency and accessibility of the embryo. The various insect glial cell types and their distribution and function are summarized in Table 1. In Drosophila, the availability of various genetic tools has allowed the most exhaustive and detailed analysis of glial lineages so far (Ito et al. 1995). Depending on the species and location, the invertebrate glia show a variety of morphologies and functions. Among invertebrate phyla, glial cells are absent from the Mesozoa, Porifera, Coelenterata, and Echinodermata but are present in Platyhelminthes, Aschelminthes, Annelida, Arthropoda, and Mollusca (reviewed in Radojcic & Pentreath 1979). The close glial ensheathment of neurons underlie neuron-glial interactions in mollusc, arthropod, and annelid ganglia (Figure 1). Some evidence demonstrates bidirectional signaling between neurons and glial cells and mutual dependence for their survival (Pentreath & Kai-Kai 1982, Fields & Stevens-Graham 2002).
Table 1.
Glial cell types in insects and vertebrates
| Glial cell type | Distribution | Function | Reference | |
|---|---|---|---|---|
| Insects | ||||
| CNS | 1. Surface-associated glia | CNS surface | Insulation of CNS | Ito et al. 1995 |
| (a) Perineurial glia | Pereanu et al. 2005 | |||
| (b) Subperineurial glia | ||||
| 2. Cell body glia | Interspersed between neuronal cell body in cortex | Encapsulate neuronal somata and neuroblast | Dumstrei et al. 2003 | |
| 3. Neuropile-associated glia | Cortex-neuropile interface | Extend processes around and into the neuropile | Ito et al. 1995 | |
| (a) Nerve root glia | Associated with nerve root | |||
| (b) Interface or longitudinal glia | Longitudinal connectives | |||
| (c) Midline glia | Commisural tract at midline | Ensheath commissural axons | Jacobs 2000 | |
| PNS | 4. Exit glia | Transition zone between CNS and PNS | Wrap axon bundles of peripheral nerve | Bellen & Schulze 2003 |
| 5. Peripheral glia | Peripheral nerve | Ensheathment of sensory and motor axons | Banerjee et al. 2006a | |
| 6. Perineurial glia | Peripheral nerve | Ensheath peripheral axons and peripheral glia and form SJs | Bellen & Schulze 2003, Banerjee et al. 2006a | |
| Vertebrates | ||||
| CNS | 1. Astrocytes | CNS surface | Ensheath neuronal cell bodies and synaptic contacts/BBB | Fields & Stevens-Graham 2002 |
| 2. Oligodendrocytes | CNS axons | Myelination of CNS axons | Fields & Stevens-Graham 2002 | |
| PNS | 3. Schwann cells | PNS axons | Myelination of PNS axons, ensheath small diameter unmyelinated axon bundles | Bhat 2003, Salzer 2003, Taveggia et al. 2005 |
Figure 1.
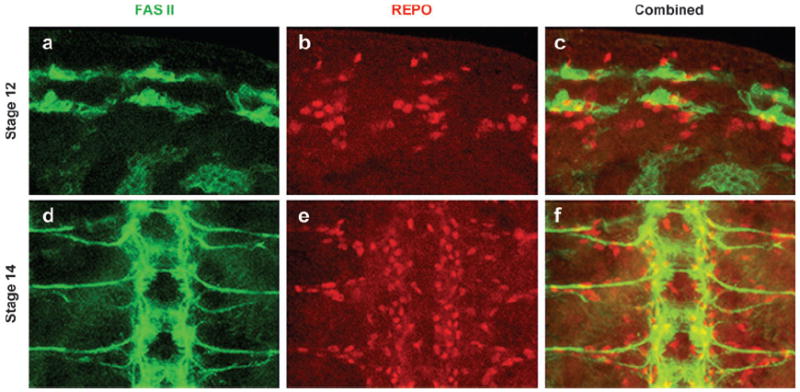
Distribution and migration of glial cells in the Drosophila embryos. (a–c) A portion of a stage 12 embryo immunostained against a motor neuron marker, anti-FAS II (green), and a glial marker, anti-REPO (red). The glial cells are in close proximity of the motor neurons in the neurectoderm, which produces both neuroblasts and glioblasts. (d–f) A portion of a stage 14 embryo showing migration of the glia toward the periphery. The FAS II positive axons have exited the CNS. The glial cells are lined along the axons and are still migrating to their final destination. These close interactions between neurons and glial cells eventually allow the ensheathment of neurons and establishment of the BBB.
Blood barriers in insects (Drosophila) arise temporally during their life cycle to partition PNS, CNS, and visual system neurons from direct access to circulating hemolymph (Carlson et al. 2000). The first barrier forms in the PNS where pleated-sheet septate junctions (SJs) bond cells of the nascent embryonic proprioreceptor neurons in the chordotonal organs during early embryogenesis (see below). At the end of the embryonic life, the CNS is protected by a functional BBB. Another blood barrier, the blood-eye barrier (BEB) arises during early pupal life and is maintained throughout the adult life. We briefly describe these three forms of blood barriers in the following sections.
Blood-Brain Barrier in the Central Nervous System
BBB is necessary where the nervous system is involved in complex sensory and motor processing because of the need for ionic homeostasis around central integrating synapses (Abbott et al. 1986a). Some insects have additional requirements for an effective barrier owing to the unusually high or fluctuating levels of K+ in their hemolymph (Hoyle 1952). Insects, higher arachnids, and decapod Crustacea possess a BBB (reviewed in Abbott et al. 1986b), which is an important component in the ensheathment of the nervous system.
The membrane specializations underlying the barrier are varied, e.g., TJs are present in cockroaches, locusts, and moths (Lane & Swales 1979, Swales & Lane 1983). SJs are present in flies; linker junctions in centipedes, millipedes, and Limulus; and novel restricting junctions in squid Sepia (Abbott et al. 1985, Lane 1989, Juang & Carlson 1992).
In avascular CNS of insects, the barrier resides in the glial epithelia, i.e., perineurial or sheath glia (Figure 2) (Swales & Lane 1985). This insulatory barrier is provided by pleated SJs (Banerjee et al. 2006a,b). The molecular components of these junctions include a number of proteins including Neurexin IV (Nrx IV), Contactin (Cont), Neuroglian (Nrg), and Gliotactin (Gli) (Auld et al. 1995, Baumgartner et al. 1996, Faivre-Sarrailh et al. 2004, Banerjee et al. 2006b). Loss of these proteins causes a breakdown of the BBB, leading to neuronal function failure (Figure 3). Several components of this junctional complex are conserved in vertebrates and play a role in the formation of axo-glial SJs at the paranodal areas flanking the nodes of Ranvier (Bhat et al. 2001, Bhat 2003, Salzer 2003). From a molecular standpoint, apart from the initially described SJ-specific proteins, Nrx IV and Gli, several proteins have been recently identified that contribute to the BBB formation and function (Bainton et al. 2005; Schwabe et al. 2005, Strigini et al. 2006).
Figure 2.
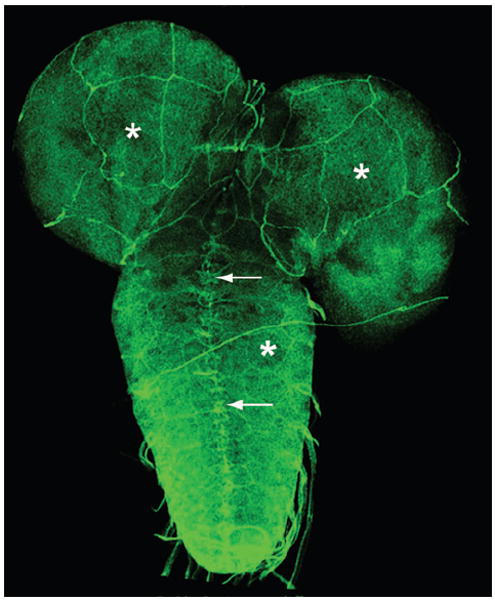
Perineurial glial cells and the BBB. Immunolocalization of Nrx IV in the larval ventral nerve cord and brain lobes of the CNS shows that the protein is expressed in midline glia (arrows) as well as at the edges of the perineurial glial cells required for the maintenance of the BBB in third instar larvae. These are very large cells that ensheath the entire CNS. Note the giant cells surrounding the brain lobes and also the nerve cord (asterisks). Anterior is up.
Figure 3.
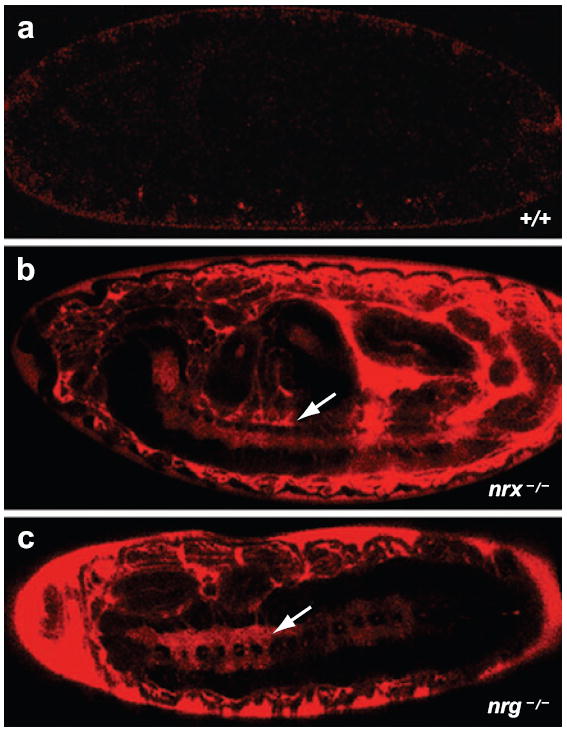
Breakdown of the BBB. (a) A wild-type stage 16 Drosophila embryo injected with a 10-KDa rhodamine-dextran dye shows the presence of functional SJs because the dye does not penetrate any of the organs. (b) neurexin IV (nrx–/–) and (c) neuroglian (nrg–/–) mutant embryos, which lack SJs, show a breakdown of the BBB as the dye penetrates the CNS (arrows) and other organs.
Blood-Nerve Barrier in the Peripheral Nervous System
The concept of the BNB is fairly new compared with BBB. In Drosophila PNS the BNB resides in the chordotonal organs and peripheral nerves. The BNB is the earliest barrier formed in Drosophila embryo and blocks the hemolymph from entering the PNS. Axonal insulation in Drosophila is accomplished by peripheral (inner) and perineurial (outer) glial cells (Bellen et al. 1998; Banerjee et al. 2006a,b). Similar to vertebrate oligodendrocytes, each inner glial cell of Drosophila can wrap around multiple axons. This wrapping is required for axonal ensheathment and axon-glial interaction establishment (Figure 4). The outer glial cell membrane wraps the inner glial cells and the axons, either singly or in fascicles (Banerjee et al. 2006a,b). Invertebrates and vertebrates have specialized junctions that are formed between either the outer and inner glial cells in Drosophila or between myelin loops and axons in vertebrates. In myelinated axons, these junctions display a unique ladder-like structure that creates an ionic barrier to protect the axonal ionic microenvironment and also serves as a fence to separate the ion channels at and around the node of Ranvier (Bhat et al. 2001, Bhat 2003, Rios et al. 2003). The BNB is an insulatory barrier formed by perineurial glia and the peripheral (inner) glial cells that wrap around each individual axon or fascicle. Thus the BNB is established by ensheathing glial cells in the Drosophila PNS (Banerjee et al. 2006a)
Figure 4.
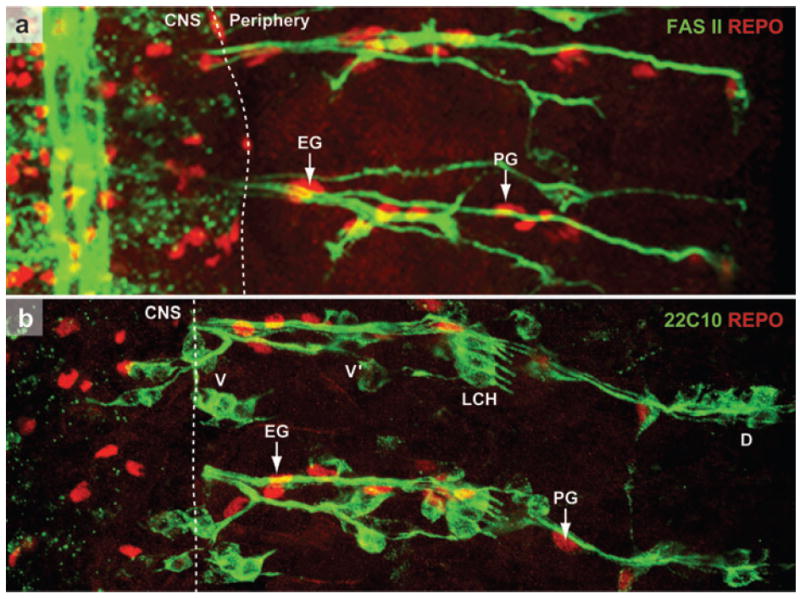
Distribution and final position of peripheral glia. (a) A portion of a stage 16 wild-type Drosophila embryo stained with a motor axon marker, Fasciclin II (FAS II, green) and a glial marker REPO (red). The axons have completed their exit from the CNS and have grown toward the periphery. The white dotted line indicates a presumptive boundary that separates the CNS from the periphery. Some of the glial cells that can be clearly identified in this focal plane are EG (exit glia) and PG (peripheral glia). (b) A portion of a stage 16 wild-type embryo stained with sensory neuron marker 22C10 (green) and REPO (red). The axons from the sensory neuronal clusters (dorsal, D; lateral chordotonal cluster, LCH; ventral prime, V′ and ventral, V) have made their way into CNS. The glial cells highlighted in (a) are also marked here. The sensory and motor axons in a segment use the same paths and are always insulated together. This ensheathment ensures a functional BNB in PNS. (For further details, see Klambt & Goodman 1991.)
The chordotonal sensory organs of the Drosophila PNS has neuronal and glial cell types and is an excellent model to study the BNB formation (Carlson et al. 2000, Banerjee et al. 2006a). The neuron-glial interactions resulting from the close association of the glial cell types, namely the cap cell, the scolopale cell, and the ligament cell, with the sensory neuron are central to the functioning of this sensory organ (Figure 5). Functional SJs established at the interface of the cap and scolopale form the underlying basis of the BNB (Carlson et al. 1997). Recent studies have identified junctional proteins Nrx IV, Cont, and Nrg as the molecular components of the BNB of both CO and peripheral nerves (Banerjee et al. 2006a).
Figure 5.
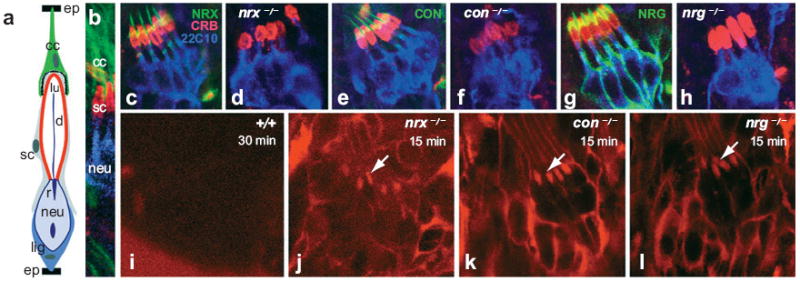
Disruption in the chordotonal organ (CO) morphology and BNB function in nrx IV, cont, and nrg mutants. (a) Schematic of a CO showing the various cell types. Cap cells (cc, green), scolopale (sc, red), and ligament (lig) are the three glial cell types. The neuron (neu, blue) has a rootlet (r) and its dendrite (d) projects into the lumen (lu) of the scolopale. The cc and the lig cell attachment sites of the CO to the epidermis (ep) are also shown. The presence of extensive SJs is apparent between the cap and scolopale cells, thus providing a functional BNB. (b) Wild-type CO triple stained with antiβ3tubulin (green) marking the cap cells, anti-Crb (red) marking the lumen of the scolopale, and anti22C10 (blue) marking the sensory neuron. (c–h) Wild-type COs (c, e, and g) stained with anti-CRB (red) and anti22C10 (blue) in combination with anti-Nrx IV (c, d), anti-Cont (e, f), and anti-Nrg (g–h) show a fusiform shape of the scolopales in the CO cluster. nrx IV (d), cont (f), and nrg (h) mutants as evident from the lack of staining of their respective antibodies show a defective morphology and a disarrayed organization of the cluster. (i–l) Dye exclusion assays performed on the wild-type embryos (i), nrx IV (j), cont (k) and nrg (l) mutant embryos. Confocal images after dye injection of the regions of the peripheral nervous system at the level of the COs. Wild-type embryos (i) excluded the dye from the COs even after 30 min of injection, indicating that a functional BNB is present. Under identical conditions nrx IV (j), cont (k), and nrg (l) mutant embryos failed to exclude the dye from the COs. Confocal images showed dye penetration into COs within 15 min after injection, indicating that the BNB has broken down in these mutants. Printed with permission from Banerjee et al. (2006a); copyright 2006 by the Society for Neuroscience.
Blood-Eye Barrier in Insect Ommatidia
Compound eyes of insects have several hundred ommatidia, each of which has an assembly of photoreceptor neurons and accessory cells (Wolff & Ready 1993). For the phototransduction machinery to function properly, each ommatidium is vested with a functional BEB. Initial studies on BEB in adult locust eyes showed the presence of a resistance barrier: When introduced in the circulating hemolymph, the dyes never entered the optic lobes, which confirmed the presence of a barrier (Shaw 1984). The discovery of tight and pleated-sheet SJs in the housefly provided an anatomical correlate of the BEB and clarified that TJs and SJs associated with neurons and glial cells formed a complex system of blood barriers in the insect eye (Chi & Carlson 1981, Lane 1981, Saint Marie & Carlson 1983). At the retina-brain border, a barrier is established by the fenestrated glial cells, which are linked to each other and to underlying glia via gap junctions (Carlson & Saint Marie 1990). The distal surface of these glial cells is greatly increased by deep folds, a requirement for vesicular trafficking, which is also supported by the expression of vesicle-specific proteins such as adaptin (Kretzschmar et al. 2000). The lamina glia electrically isolate the lamina not only from the retina but also from the other visual ganglia. The various glial types in the lamina cortex are extensively coupled with each other and also with neurons (Kretzschmar and Pflugfelder 2000). TJs between epithelial glial cells ensure isolation of each optic neuropil. This enveloping system seems to be responsible for the high electrical resistance between retina and lamina and may contribute to the large field potentials initiated in the lamina after light stimulation (Chi & Carlson 1980).
In Drosophila, BEB arises in the pupal stage and is assembled by pleated SJs that ramify throughout the visual system. The electrical activity of the photoreceptor neurons commences from the pupal stage and requires the establishment of a functional barrier that is maintained throughout the adult life (Carlson et al. 1998). This barrier acts as a partition to ensure that the ionic constitution of extra-neuronal fluid is distinct from the circulating hemolymph. Genetic analysis of the neuron-glial interactions in the Drosophila ommatidium has revealed that SJs and their components are also essential for BEB formation and function (S. Banerjee & M. Bhat, unpublished observations).
EVOLUTIONARY DIVERGENCE FROM A GLIAL BARRIER TO AN ENDOTHELIAL BARRIER
In considering the changes in CNS barriers over evolutionary time, examination of both invertebrate and vertebrate groups is instructive. The presence of a barrier system in insects, Crustacea, and Cephalopod mollusks, and its absence in the lower invertebrates, led researchers to suggest that a barrier is needed to perform complex integrative and analytical activities in the nervous system (Abbott et al. 1986a). Investigators proposed that the evolutionarily selective pressure and advantage to developing a barrier came from the need to preserve ionic homeostasis from any fluctuations around the synaptic active zones. Animals with better brain interstitial ionic homeostasis as a result of better barrier function would have gained selective evolutionary advantage. The presence of a glial barrier in invertebrates and a primitive vertebrate group (elasmobranch), together with the presence of a glial ependymal barrier in higher vertebrate brain in early embryonic stages, suggests that the glial barrier is the primitive or ancestral condition and that an endothelial barrier as found in higher vertebrates is a later evolutionary feature (Abbott 2005). Elasmobranch fish, such as sharks, have a BBB formed by perivascular glial end feet and not by the endothelium (Abbott et al. 1986a,b; 1992). A barrier at the level of the pericyte/smooth muscle layer in larger vessels may represent an intermediate condition, as observed in cephalopods (Abbott 1992). Astrocytic glial cells are closely associated with the brain endothelial barrier. During evolution, the barrier likely shifted from the glial to the endothelial barrier, in parallel with the increasing importance of the microvasculature and its regulation, although remnants of a glial barrier still remain in the modern mammalian CNS (Abbott et al. 1986a, Abbott 2005).
A shift to an endothelial barrier is one aspect of the greater division of labor between cell types seen in higher animal CNS. This shift is one of the key features of the modern multifunctional barrier system (Abbott et al. 1986a). In the endothelial barrier, glial cells are still important to inducing and maintaining the barrier, which is reflected by the close association and interaction between glial cells and the vasculature. Thus a breakdown of the barrier in some glial tumors and other CNS pathologies suggests a disruption of the functional interaction between the endothelial cells and their associated glial cells (reviewed in Abbott et al. 1992, 2006).
BLOOD-BRAIN BARRIER IN THE VERTEBRATES
The BBB is a selective barrier formed by the endothelial cells that line cerebral microvessels (Risau & Wolburg 1990, Abbott 2002). Close interactions between endothelial cells, astrocytes, neurons, and pericytes contribute to the formation and function of the BBB (Figure 6). The BBB acts as a physical, metabolic, and transport barrier restricting traffic of nutrients and other molecules (for review see Abbott 2005, Abbott et al. 2006). It limits and regulates the transit of small molecules through the interspaces between the endothelial cells. Strong evidence available from in vitro and in vivo studies indicates that astrocytes can modulate many BBB features, leading to tighter TJs for a physical barrier (Dehouck et al. 1990, Rubin et al. 1991), the expression and polarized localization of transporters for a transport barrier (Schinkel 1999), and specialized enzyme systems to function as a metabolic barrier (Abbott 2002, Haseloff et al. 2005).
Figure 6.
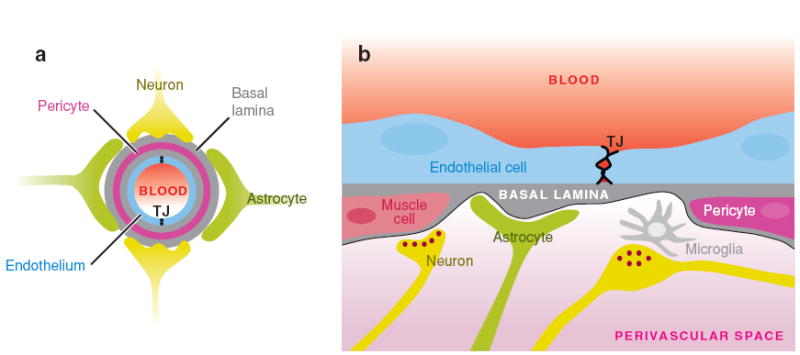
Schematic of the neurovascular unit. (a) A cross-section through a brain capillary shows adjacent endothelial cells connected by TJs that establish the BBB. The endothelial cell layer is surrounded by the basal lamina that separates the endothelium from the pericytes, astrocytes, and neurons. (b) A longitudinal section through a portion of a brain capillary reveals the presence of adjacent endothelial cells connected by TJs. Pericytes are present within the basal lamina in close proximity to the endothelial cells, whereas astrocytic endfeet are on the outer surface of the basal lamina. Microglia, nerve fibers, and neuromuscular synapses are found in the perivascular space. Panel b has been modified with permission from Abbott 2005; copyright 2005 by Springer Science and Business Media.
The other cell types present at the BBB, including pericytes, perivascular macrophages, and neurons, also contribute to barrier induction (Duport et al. 1998, Ramsauer et al. 2002, Zenker et al. 2003). Given the complexity of the BBB properties and the anatomical relationship of the associated cell types, it is not surprising to find synergistic inductive functions involving more that one cell type. For example, the pericytes form a meshwork on the outer surface and share a basal lamina with the endothelium and the end-feet of perivascular astrocytes form a covering of fine processes and lamellae interrupted by gaps (Kacem et al. 1998, Abbott 2005). Neuronal terminals, microglia, and other perivascular cells are found in the perivascular space, and smooth muscle layers, in some cases together with sheaths derived from the meninges, are present in arterioles, arteries, and larger veins (Weller et al. 1992). Thus one can see that interactions between many of these cell types can contribute to the properties of the BBB.
Organization of a Neurovascular Unit
A neurovascular unit is organized by complex associations between endothelial cells, extra-cellular matrix, basal lamina, pericytes, closely juxtaposed neurons, and astrocytes (Huber et al. 2001b) (Figure 6). The components of the neurovascular unit maintain dynamic interactions with each other and play an important role in cerebrovascular function (Rubin & Staddon 1999, Ballabh et al. 2004, McCarty 2005). Contact and communications between cells of the neurovascular unit regulate CNS development and synaptic activity and influence permeability properties of the BBB (Zonta et al. 2003, Iadecola 2004, Mulligan & MacVicar 2004). A major function of the neurovascular unit is to regulate the transport and diffusion properties of brain capillary endothelial cells that compose the BBB (Staddon & Rubin 1996).
Within the neurovascular organization, further modular structures, termed the gliovascular units, are detected where individual astrocytic glia support the function of particular neuronal populations and communicate with associated segments of the microvasculature (Anderson & Nedergaard 2003, Nedergaard et al. 2003). Several recent studies have highlighted the importance of this modular organization in disease pathology and the cell-cell interactions that result in modulating the BBB.
Astrocytes interact with the vasculature to form a gliovascular network and contribute toward organizing the brain’s structural architecture. The repeated astrocytic domains suggest an order of primary structure. A secondary structure to this primary repeated motif is the capillary microvasculature in the brain similarly organized with microvessels positioned along the interfaces between adjacent astrocytic domains and the endfeet of adjacent astrocytes. This arrangement provides a contiguous but nonoverlapping ensheathment around the capillaries (Nedergaard et al. 2003, Simard et al. 2003). Yet, another layer of structural complexity is imposed on the existing glial organization by the neurons that are dispersed among the astrocytic domains, surrounded by their glial processes (Figure 6).
Astrocytes were originally regarded as passive elements of the nervous system, mainly as supportive cells to neurons, as guiding structures during brain development, or as regulators of ionic homeostasis. Current opinion on astrocytic function, however, has changed, and astrocytes are shown as bidirectional communication partners in the CNS, receiving signals from neighboring neurons and responding to them with the release of neuroactive substances (Araque et al. 2000). The high degree of intercellular communication has led to the proposal that astrocytes are organized as networks that might be subjected to remodeling and to some plasticity (Giaume & McCarthy 1996, Kirchhoff et al. 2001). The extent and shape of astrocytic networks, in turn, are regulated by neurons (Giaume & McCarthy 1996, Rouach et al. 2000). The exact role of astrocytes in the formation and maintenance of the BBB and the cellular and molecular mechanisms of the interactions between endothelial cells and their neural environment remain to be elucidated.
The astrocytic network of fine processes enwrap synaptic terminals and are thereby in close apposition to neuronal signal transmission (Grosche et al. 1999, Ventura & Harris 1999) and express receptors for most neurotransmitters and neuromodulators (Verkhratsky & Kettenmann 1996). In addition, astrocytes are metabolically coupled to neuronal activity (Pellerin et al. 1998). Because some of the BBB characteristics in brain endothelial cells appear very early and prior to astrocyte differentiation and because astrocytes are also present in circumventricular organs that lack endothelial BBB, astrocytes are likely necessary, but not sufficient, in the formation and establishment of the barrier.
Astrocytic glial cells are highly fibrous and have considerable structural complexity, uniquely characterized by a dense array of processes interposed between neuronal elements, some of which contact and ensheath local vascular walls. The relative number of astrocytes with respect to neurons strikingly increase with phylogeny and brain complexity (for review see Nedergaard et al. 2003). The greater abundance of astrocytes with evolution could be due to increasingly sophisticated synaptic networking requiring greater degrees of modulation and control. Compelling evidence supports the concept that astrocytes directly signal to neurons to modulate synaptic strength in the CNS (Kang et al. 1998, Haydon 2001, Newman 2003) and regulate synaptogenesis (Pfrieger & Barres 1997). Astrocytes also convey signals from neurons to the vasculature, predicting that glutamate released during synaptic transmission, through activation of metabotropic glutamate receptors, triggers astrocytic Ca2+ signaling leading to arteriolar dilation and an increase in local blood flow (Anderson & Nedergaard 2003, Zonta et al. 2003).
Pericytes are another cell population found in close association with endothelial cells. Pericytes are cells of microvessels including capillaries, venules, and arterioles that wrap around the endothelial cells. They provide structural support to the microvasculature (Lindahl et al. 1997, Ballabh et al. 2004). Although the function of pericytes in vivo was unclear for a long time, we know now that they are required for vessel maturation (Lindahl et al. 1997). Several factors such as Tie2/angiopoietin-1 (Suri et al. 1996), platelet-derived growth factor B (PDGF-B) (Lindahl et al. 1997), and adhesion molecules such as N-cadherin (Gerhardt et al. 2000) have been identified as necessary for proper endothelial cell-pericyte interactions and may play a role in vessel maturation.
STRUCTURAL ATTRIBUTES OF THE BLOOD-BRAIN BARRIER
Occluding junctions, which form the morphological basis of the BBB, vary in nature. They may exist as classical TJs, in which the adjacent cell membranes fuse, partially or completely, or exist as SJs in which the cell membranes remain separated by distinct intercellular clefts straddled by columns or septal ribbons, between which a charged matrix substance can be found. Restrictive linker junctions that form the basis of the barrier in cephalopod CNS are characterized by cross-striations or columns that are responsible for decelerating the entry of exogenous molecules. SJs, which occur between glial cells in invertebrates, exhibit ladder-like electron-dense structures (Lane 1991). For reviews on the anatomical organization and function of SJs, the readers should refer to the following reviews: Tepass & Hartenstein (1994), Tepass et al. (2001), Banerjee et al. (2006b).
Tight Junctions and the Blood-Brain Barrier
TJs form at apical regions of endothelial cells and are the primary molecular basis for the highly impermeable properties of the BBB. These junctions have been investigated morphologically by freeze fracture and ultrathin sectioning and physiologically by measurements of paracellular permeability and electrical resistance. In conventional ultrathin sections, the TJs form pentalaminar layers that result from the fusion of the external leaflets of the adjacent cell membranes. Depending on the orientation of the section, the TJs appear mostly as a chain of “kissing points” or as a domain of an occluded intercellular cleft of variable length (Brightman & Reese 1969, Farquhar & Palade 1963). In contrast with ultrathin sections, the freeze-fracture technique allows the investigation of the microarchitecture in the plane of the membrane. Freeze-fracture ultrastructural analyses have revealed that TJs form anastomosing strands along the external face of endothelial plasma membranes (Martin-Padura et al. 1998, Schneeberger & Lynch 2004) that fuse at kissing points to eliminate intracellular space and restrict paracellular flux. Freeze-fracture data on TJs clearly show that when particles occur at the external fracture face (E-face), they are arranged in chains; whereas although they occur at the protoplasmic fracture face (P-face), they are frequently formed as smooth continuous cylindrical profiles. In freeze-fracture replicas, the BBB TJs of mammalian species are characterized by a high percentage of P-face association compared with that observed in endothelial cells of peripheral blood vessels. The altered particle distribution in brain microvessel TJs may be indicative of a strong TJ-cytoskeleton interaction. Recent studies suggest that interactions between TJs and the actin cytoskeleton may play a modulatory role in the permeability of the BBB (Lai et al. 2005).
The TJ is an intricate complex of several transmembrane proteins including junctional adhesion molecules (Martin-Padura et al. 1998), occludin (Furuse et al. 1993) and claudins (Furuse et al. 1998, Morita et al. 1999, Furuse & Tsukita 2006), and cytoplasmic proteins, such as zonula occludens (ZO) (Itoh et al. 1999, Willott et al. 1993) and cingulin (Citi et al. 1989). Some of these components are directly linked to the actin cytoskeleton. For example, the cytoplasmic protein (ZO-1) links membrane proteins (occludin) to actin cytoskeleton to maintain the endothelium’s structural and functional integrity (Fanning et al. 1998). Table 2 provides a detailed description of many junction-associated proteins that play a role in blood-barrier formation/function in Drosophila and vertebrates. The expression and subcellular localization of TJ proteins are modulated by several intrinsic signaling pathways, including those involving calcium, phosphorylation, and G proteins (see below).
Table 2.
Proteins that establish the blood barriers in Drosophila and vertebrates
| Protein | Expressed in | Type of junction | Type of barrier | References |
|---|---|---|---|---|
| Drosophila | ||||
| Nrx IV | Glia | SJs | BBB/BNB | Banerjee et al. 2006a, Baumgartner et al. 1996 |
| Nrg | Neuron, glia | SJs | BBB/BNB | Banerjee et al. 2006a, Schwabe et al. 2005 |
| Cont | Glia | SJs | BNB | Banerjee et al. 2006a |
| Gliotactin | Glia | SJs | BNB | Auld et al. 1995 |
| Moody | Glia | SJs | BBB | Bainton et al. 2005, Schwabe et al. 2005 |
| Lachesin | Subset of neurons, glia | SJs | BBB | Strigini et al. 2006 |
| Loco | Glia | SJs | BBB | Schwabe et al. 2005 |
| Claudins | Glia | SJs | ? | Behr et al. 2003, Wu et al. 2004 |
| Vertebrates | ||||
| Claudins | EC | TJs | BBB | Schneeberger & Lynch 2004 |
| Occuldins | EC | TJs | BBB | Schneeberger & Lynch 2004 |
| JAMs | EC | TJs | BBB | Martin-Padura et al. 1998 |
| ZOs | EC | TJs | BBB | Schneeberger & Lynch 2004 |
| SSeCKS | EC | BBB | Lee et al. 2003 | |
| PDGFR-B | Pericyte | BBB | Lindahl et al. 1997 | |
| Tie2 | EC | BBB | Suri et al. 1996 | |
| αVβ8 integrin | Neuron, glia | BBB | McCarty et al. 2005 | |
The dynamics of synthesis, regulatory sensitivity to extrinsic factors, and the morphological appearance of TJs differ between endothelial and epithelial cells. However, the molecular constitution of TJs in endothelial cells is quite similar to that found in epithelial cells (for review, see Anderson & Van Itallie 1995, Mitic & Anderson 1998). Detailed description of the structure and composition of TJs at the BBB is beyond the scope of this review. Several recent reviews have addressed this topic in greater detail (Huber et al. 2001a, Wolburg & Lippoldt 2002, Abbott et al. 2006).
SIGNALING PATHWAYS AND THE BLOOD-BRAIN BARRIER
Mechanisms of signal transduction at the TJs or SJs are not completely understood. Signal transduction processes associated with TJs involve signals transduced from the cell interior toward TJ to guide their assembly and regulate paracellular permeability and signals transmitted from TJ to the cell interior to modulate gene expression, cell proliferation, and differentiation (Matter & Balda 2003). Researchers have implicated multiple signaling pathways and proteins in the regulation of TJ assembly: These include calcium, protein kinase A, protein kinase C, G proteins, calmodulin, cAMP, and phospholipase C (Balda et al. 1991, Matter & Balda 2003). Calcium acts both intracellularly and extracellularly to regulate TJ activity, and several of the molecules modulating BBB permeability seem to act by altering intracellular calcium (Ballabh et al. 2004). Intracellular calcium plays a role in increasing transendothelial resistance and restoring the TJ assembly (Stevenson & Begg 1994). Increasing the extracellular calcium triggers a series of molecular events, which increases resistance across the membrane and decreases its permeability (Stevenson & Begg 1994). These events are mediated through heterotrimeric G proteins and protein kinase C signaling pathways (Ballabh et al. 2004).
Phosphorylation is a major regulatory mechanism of both transmembrane and accessory proteins at the TJs (Staddon et al. 1995, Sakakibara et al. 1997). For example, serine phosphorylation regulates the subcellular localization of occludin (Andreeva et al. 2001), and both serine and threonine phosphorylation of occludin are highly correlated with the TJ reassembly following disruption (Tsukamoto & Nigam 1999). Other signaling pathways that have been implicated in TJ assembly regulation or that play a modulatory role at the TJs include the PAR3-PAR6-APKC pathway and the evolutionarily conserved signaling complex related to the Drosophila Stardust-Disc lost-Crumbs complex, equivalent to the mammalian Pals1-PATJ-Crumbs complex (Matter & Balda 2003). Whether there is a crosstalk between these various signaling pathways to orchestrate the organization and assembly of the TJs remains to be established.
CONTRIBUTION OF IN VITRO MODELS IN THE STUDY OF THE BLOOD-BRAIN BARRIER
The in vitro cell culture system has been exploited for a detailed study of the BBB induction. These studies aimed to mimic the in vivo situation by establishing a monolayer of pure cerebral capillary endothelial cells, which could then be cocultured with astrocytes and tested for morphological, biochemical, and functional alterations. However, in vitro experiments concerning BBB induction have been judged critically because the results may not always reflect the in vivo environment in which multiple cell types are involved (Holash et al. 1993).
Some of the earlier in vitro studies were done using cultured cerebral capillary endothelial cells after coculture with glial cells. Several reports support the idea that cocultured astrocytes or glioma cells may be able to reinduce BBB properties in cultured endothelial cells (DeBault & Cancilla 1980, Beck et al. 1986, Lobrinus et al. 1992). Of special interest is the question of whether the high electrical resistance and the complex TJs are among the properties of endothelial cells that can be reinduced and maintained in culture by astrocytes or other cell factors. Some evidence indicates an astrocyte-mediated induction of TJs in cell culture (Arthur et al. 1987). Various in vitro assays using cultured endothelial cells or cocultures of endothelial and glial cells have been developed to study BBB permeability (Rubin 1991, Rubin et al. 1991).
The ability of most epithelia to form TJs and develop other barrier properties appears to be constitutive; thus epithelial cells can reproduce most features of the in situ barrier when grown on porous filters in vitro. In contrast, endothelial cells grown in vitro show less intrinsic ability to form barrier layers because they require elements of a basal lamina to adhere and form monolayers (Nobles & Abbott 1996).
DISEASE PATHOLOGY AND CLINICAL RELEVANCE
During a pathological insult, the BBB is capable of modulating the cytoarchitecture to yield an increase in permeability while retaining structural integrity. Up to a point, this allows the BBB to protect the brain and maintain homeostasis; however, under extreme conditions or prolonged insult, TJs in the BBB dissociate from one another with subsequent edema formation, decreased neuronal function, and brain damage. TJs are dynamic structures, and their molecular components are subject to changes in expression, sub-cellular localization, posttranslational modification, and protein-protein interactions under both physiological and pathophysiological conditions (Huber et al. 2001a, Hawkins & Davis 2005). Although the loss of BBB function is an etiologic component of many neurological diseases, an intact BBB poses serious problems by restricting the delivery of certain therapeutic substances to the brain. A great majority of drugs do not cross the brain capillary wall, which forms the BBB in vivo. Only a small class of drugs that are of a low molecular mass of <400–500 Da with high lipid solubility are capable of crossing the BBB (Pardridge 2003). However, most of the serious neurobiological disorders including Alzheimer’s disease, brain cancer, HIV infection, and stroke do not respond to conventional lipid-soluble low molecular mass therapeutics (Pardridge 2002). Thus BBB drug targeting forms a critical and intense area of investigation (for reviews see Pardridge 2002, 2003).
Neurobiological Diseases Associated with the Blood-Brain Barrier Defects
Substantial progress has been made to understand the pathophysiology and mechanisms involved in the attenuation of BBB permeability. In many diseases that affect the brain, the cerebral endothelium plays an active part in the disease process; the BBB becoming disrupted or modified in such a way that vascular permeability dramatically increases. Various molecules can pass the endothelium in several ways, including intercellular routes, vesicular transport, or direct transcellular penetration through damaged endothelium due to aberrant TJs. Neurobiological diseases involving BBB breakdown and dysfunction include stroke, neoplasia, neurodegenerative diseases (e.g., Parkinson’s disease, Alzheimer’s disease), epilepsy, infections, or inflammatory processes [meningitis, multiple sclerosis (MS), HIV], trauma etc. (Abbott et al. 2006, Rubin & Staddon 1999). The effects of a disease on BBB function secondarily affect the cerebral blood flow, further influencing transport across the BBB. Besides the effects of increased vascular permeability on the brain parenchyma, a more important question is whether in certain neuropathological conditions, the BBB disturbance constitutes the main pathogenic factor itself, which then triggers a sequence of events molding the final pathological state.
Structural Alterations and Barrier Permeability: Contribution to the Pathogenesis of Diseases
Failure of the BBB is a critical event in the development and progression of several neurological diseases (Hawkins & Davis 2005). In some cases, increased BBB permeability is a consequence of the pathology, such as with ischemic stroke and traumatic brain injury (Morganti-Kossmann et al. 2002). Although the relationship between BBB breakdown and pathology is not clear in several cerebrovascular abnormalities (Wardlaw et al. 2003), the contribution of specific BBB TJ alterations to neurological conditions is a rapidly growing area of investigation.
Inflammatory mediators are known modulators of BBB permeablility (Abbott 2000), and compromised BBB TJs are a hallmark of neuroinflammatory disease states (Petty & Lo 2002). BBB disruption is well established as an early event in the progression of MS; MRI studies indicate a compromised barrier preceding clinical symptoms (de Vries & Dijkstra 2004). MS lesions are associated with loss of occludin and ZO-1 in the microvasculature (Bolton et al. 1998). Inflammation occurring in the periphery also has profound effects on BBB permeability and TJ proteins (Huber et al. 2001b). These changes in permeability are associated with decreased expression of occludin and increased expression of ZO-1 (Huber et al. 2001b). Additionally, ZO-1 is found to be associated less with actin and more with ZO-2, suggesting a disruption between TJ complex and the cytoskeleton interaction (Huber et al. 2002). Some studies suggest that BBB TJs may be subject to modulation via centrally mediated responses to nociceptive stimuli (Sanchez-del-Rio & Reuter 2004).
Blood barriers of both CNS and PNS are sensitive to any structural alterations of the junctions. CNS disorders, such as cerebral ischemia, which is a complex insult that involves blood flow loss and depletion of oxygen and essential nutrients, are associated with increased microvascular permeability and/or disruption of BBB TJs (Kempski 2001). In Alzheimer’s disease, beta-amyloid deposition leads to degeneration of the microvascular basement membrane and alterations in BBB permeability (Berzin et al. 2000). BBB can also be compromised by drugs of abuse, such as cocaine and nicotine (Hawkins et al. 2002). In the PNS, breakdown followed by recovery of the BNB after crush injury to the rat sciatic nerve are closely associated with changes in the expression of TJ proteins, such as claudin-1, claudin-5, and occludin (Hirakawa et al. 2003), which in turn affect the permeability of the BNB.
IMPORTANCE OF BARRIERS ACROSS SPECIES
The BBB plays a crucial role in maintaining brain homeostasis by keeping brain extracellular fluid within a precise physiological range, independent of fluctuations within the blood, to maintain optimal conditions for neuronal functions. The BBB formed by brain capillary endothelial cells or the invertebrate glial cells has specific transport systems that facilitate the uptake of important nutrients. These cells also have active pumps that help regulate the concentrations of ions and metabolites in the brain’s interstitial fluid or the hemolymph. Enzymes present in the endothelial or glial cells metabolize neurotransmitters, drugs, and toxins before they can enter the brain and disrupt its function. Many of these properties are likely under strict regulation either by neurotransmitters and hormones released in the brain or by those present in systemic circulation. The BBB, once thought to be a static, rigid wall between the CNS and the periphery, is actually a dynamic, complex structure capable of rapid modulation, even under adverse conditions (Huber et al. 2001). Thus the barriers evolved not only to maintain an ionic environment suitable for neuronal impulse generation and propagation but also to restrict substances that could damage the neuronal cells.
CONCLUSIONS AND FUTURE DIRECTIONS
Upon close scrutiny, the actual knowledge about BBB induction is rather sparse, and many questions still remain unanswered. Despite the considerable progress made in the field of BBB research since the earliest injection studies, researchers still need a major breakthrough in addressing critical questions concerning the inductive mechanisms leading to BBB formation, the timing of BBB formation, and the role of neuron-glial interactions in BBB formation and function. Most elusive are the cellular and molecular mechanisms underlying the various disorders resulting from dysfunctional BBB.
The BBB is a severe impediment in the battle to treat CNS diseases. Physiologically altered brain endothelial cells, smooth muscle cells, pericytes, astrocytes, and neurons are important targets for controlling the arterial and brain capillary disorder in various disease pathologies. These cell types should be studied mechanistically as a neurovascular unit. The current knowledge of the neurovascular unit and its utilization for development of desperately needed therapeutic agents is modest, and clearly more research is needed to understand and exploit this modular unit. A more thorough understanding of how cells of the neurovascular unit collectively contribute to BBB formation and function will aid in improving BBB permeability and in designing and delivering drugs across the BBB for treatment of CNS diseases. A search for new genes and disease-associated pathways that regulate vascular cell differentiation and/or cause predisposition for neurovascular disintegration needs to be undertaken.
A number of puzzles concerning the functional property of the BBB are yet to be resolved: How do TJ molecules assemble? How do they interact with several mediators, neurotransmitters, and medications? How are these junctions altered in disease conditions? Over the coming years, emerging information on the mechanism of BBB disruption may help to formulate strategies to protect BBB and to prevent and treat BBB-related pathologies. Further consideration of protein-protein interactions and signaling pathways involved in TJ modulation is still needed, especially in response to pathological insults. Understanding how the BBB and its transport mechanisms are altered in these conditions will provide important information about disease etiology and lead to new therapeutic approaches.
Acknowledgments
Our apologies to authors whose work could not be cited or discussed here because of space constraints. Research in our laboratory is supported by the grants from the National Institute of General Medical Sciences (GM63074) and the National Institute of Neurological Disorders and Stroke (NS050356) of the National Institutes of Health and by funds from the State of North Carolina.
- BBB
blood-brain barrier
- TJ
tight junction
- PNS
peripheral nervous system
- BNB
blood-nerve barrier
- SJ
septate junction
- BEB
blood-eye barrier
- ZO
zonula occludens
Contributor Information
Swati Banerjee, Email: swati_banerjee@med.unc.edu.
Manzoor A. Bhat, Email: manzoor_bhat@med.unc.edu.
LITERATURE CITED
- Abbott NJ. Comparative physiology of the blood-brain barrier. In: Bradbury MWB, editor. Physiology and Pharmacology of the Blood-Brain-Barrier. Berlin: Springer-Verlag; 1992. pp. 371–96. [Google Scholar]
- Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol. 2000;20:131–47. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002;200:629–38. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol. 2005;25:5–23. doi: 10.1007/s10571-004-1374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ, Bundgaard M, Cserr HF. Tightness of the blood-brain barrier and evidence for brain interstitial fluid flow in the cuttlefish, Sepia officinalis. J Physiol. 1985;368:213–26. doi: 10.1113/jphysiol.1985.sp015854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ, Bundgaard M, Cserr HF. Comparative physiology of the blood-brain barrier. In: Sukling AJ, Rumsby MG, Bradbury MWB, editors. The Blood-Brain Barrier in Health and Disease. Chichester: Ellis Horwood; 1986a. pp. 52–72. [Google Scholar]
- Abbott NJ, Lane NJ, Bundgaard M. The blood-brain interface in invertebrates. Ann NY Acad Sci. 1986b;481:20–42. doi: 10.1111/j.1749-6632.1986.tb27136.x. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Revest PA, Romero IA. Astrocyte-endothelial interaction: physiology and pathology. Neuropathol Appl Neurobiol. 1992;18:424–33. doi: 10.1111/j.1365-2990.1992.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Allt G, Lawrenson JG. Pericytes: cell biology and pathology. Cells Tissues Organs. 2001;169:1–11. doi: 10.1159/000047855. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Nedergaard M. Astrocyte-mediated control of cerebral microcirculation. Trends Neurosci. 2003;26:340–44. doi: 10.1016/S0166-2236(03)00141-3. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol Gastrointest Liver Physiol. 1995;269:G467–75. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- Andreeva AY, Krause E, Muller EC, Blasig IE, Utepbergenov DI. Protein kinase C regulates the phosphorylation and cellular localization of occludin. J Biol Chem. 2001;276:38480–86. doi: 10.1074/jbc.M104923200. [DOI] [PubMed] [Google Scholar]
- Araque A, Li N, Doyle RT, Haydon PG. SNARE protein-dependent glutamate release from astrocytes. J Neurosci. 2000;20:666–73. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur FE, Shivers RR, Bowman PD. Astrocyte-mediated induction of tight junctions in brain capillary endothelium: an efficient in vitro model. Brain Res. 1987;433:155–59. doi: 10.1016/0165-3806(87)90075-7. [DOI] [PubMed] [Google Scholar]
- Auld VJ, Fetter RD, Broadie K, Goodman CS. Gliotactin, a novel transmembrane protein on peripheral glia, is required to form the blood-nerve barrier in Drosophila. Cell. 1995;81:757–67. doi: 10.1016/0092-8674(95)90537-5. [DOI] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LT, Schwabe T, DeSalvo M, Gaul U, Heberlein U. moody encodes two GPCRs that regulate cocaine behaviors and blood-brain barrier permeability in Drosophila. Cell. 2005;123:145–56. doi: 10.1016/j.cell.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Balda MS, Gonzalez-Mariscal L, Contreras RG, Macias-Silva M, Torres-Marquez ME, et al. Assembly and sealing of tight junctions: possible participation of G-proteins, phospholipase C, protein kinase C and calmodulin. J Membr Biol. 1991;122:193–202. doi: 10.1007/BF01871420. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: Structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Pillai AM, Paik R, Li J, Bhat MA. Axonal ensheathment and septate junction formation in the peripheral nervous system of. Drosophila J Neurosci. 2006a;26:3319–29. doi: 10.1523/JNEUROSCI.5383-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Sousa AD, Bhat MA. Organization and function of septate junctions: an evolutionary perspective. Cell Biochem Biophys. 2006b;46:65–78. doi: 10.1385/CBB:46:1:65. [DOI] [PubMed] [Google Scholar]
- Baumgartner S, Littleton JT, Broadie K, Bhat MA, Harbecke R, et al. A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell. 1996;87:1059–68. doi: 10.1016/s0092-8674(00)81800-0. [DOI] [PubMed] [Google Scholar]
- Beck DW, Roberts RL, Olson JJ. Glial cells influence membrane-associated enzyme activity at the blood-brain barrier. Brain Res. 1986;381:131–37. doi: 10.1016/0006-8993(86)90700-6. [DOI] [PubMed] [Google Scholar]
- Behr M, Riedel D, Schuh R. The claudin-like megatrachea is essential in septate junctions for the epithelial barrier function in Drosophila. Dev Cell. 2003;5:611–20. doi: 10.1016/s1534-5807(03)00275-2. [DOI] [PubMed] [Google Scholar]
- Bellen HJ, Lu Y, Beckstead R, Bhat MA. Neurexin IV, caspr and paranodin–novel members of the neurexin family: encounters of axons and glia. Trends Neurosci. 1998;21:444–49. doi: 10.1016/s0166-2236(98)01267-3. [DOI] [PubMed] [Google Scholar]
- Bellen HJ, Schulze KL. Invertebrate Glia. In: Lazzarini RA, editor. Myelin Biology and Disorders. Vol. 1. London: Elsevier Acad; 2003. pp. 199–222. [Google Scholar]
- Berzin TM, Zipser BD, Rafii MS, Kuo-Leblanc V, Yancopoulos GD, et al. Agrin and microvascular damage in Alzheimer’s disease. Neurobiol Aging. 2000;21:349–55. doi: 10.1016/s0197-4580(00)00121-4. [DOI] [PubMed] [Google Scholar]
- Bhat MA. Molecular organization of axo-glial junctions. Curr Opin Neurobiol. 2003;13:552–59. doi: 10.1016/j.conb.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, et al. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–83. doi: 10.1016/s0896-6273(01)00294-x. [DOI] [PubMed] [Google Scholar]
- Bolton SJ, Anthony DC, Perry VH. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood-brain barrier breakdown in vivo. Neuroscience. 1998;86:1245–57. doi: 10.1016/s0306-4522(98)00058-x. [DOI] [PubMed] [Google Scholar]
- Bradbury M. The Concept of a Blood-Brain Barrier. New York: Wiley; 1979. [Google Scholar]
- Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40:648–77. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SD, Hilgers SL, Garment MB. Blood-eye barrier of the developing Drosophila melanogaster. Int J Insect Morphol Embryol. 1998;27:241–47. [Google Scholar]
- Carlson SD, Hilgers SL, Juang JL. Ultrastructure and blood-nerve barrier of chordotonal organs in the Drosophila embryo. J Neurocytol. 1997;26:377–88. doi: 10.1023/a:1018564904170. [DOI] [PubMed] [Google Scholar]
- Carlson SD, Juang JL, Hilgers SL, Garment MB. Blood barriers of the insect. Annu Rev Entomol. 2000;45:151–74. doi: 10.1146/annurev.ento.45.1.151. [DOI] [PubMed] [Google Scholar]
- Carlson SD, Saint Marie RL. Structure and function of Insect Glia. Annu Rev Entomol. 1990;35:597–621. [Google Scholar]
- Chi C, Carlson SD. Membrane specializations in the first optic neuropil of the housefly, Musca domestica L. II. Junctions between glial cells. J Neurocytol. 1980;9:451–69. doi: 10.1007/BF01204836. [DOI] [PubMed] [Google Scholar]
- Chi C, Carlson SD. The perineurium of the adult housefly: ultrastructure and permeability to lanthanum. Cell Tissue Res. 1981;217:373–86. doi: 10.1007/BF00233587. [DOI] [PubMed] [Google Scholar]
- Citi S, Sabanay H, Kendrick-Jones J, Geiger B. Cingulin: characterization and localization. J Cell Sci. 1989;93(Pt 1):107–22. doi: 10.1242/jcs.93.1.107. [DOI] [PubMed] [Google Scholar]
- Davies DC. Blood-brain barrier breakdown in septic encephalopathy and brain tumours. J Anat. 2002;200:639–46. doi: 10.1046/j.1469-7580.2002.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBault LE, Cancilla PA. γ-Glutamyl transpeptidase in isolated brain endothelial cells: induction by glial cells in vitro. Science. 1980;207:653–55. doi: 10.1126/science.6101511. [DOI] [PubMed] [Google Scholar]
- Dehouck MP, Meresse S, Delorme P, Fruchart JC, Cecchelli R. An easier, reproducible, and mass-production method to study the blood-brain barrier in vitro. J Neurochem. 1990;54:1798–801. doi: 10.1111/j.1471-4159.1990.tb01236.x. [DOI] [PubMed] [Google Scholar]
- de Vries HE, Dijkstra DD. Mononuclear phagocytes at the blood-brain barrier in multiple sclerosis. In: Sharma HS, Westman J, editors. Blood-Spinal Cord and Brain Barriers in Health and Disease. San Diego: Elsevier; 2004. pp. 409–17. [Google Scholar]
- Dumstrei K, Wang F, Nassif C, Hartenstein V. Early development of the Drosophila brain: V. Pattern of postembryonic neuronal lineages expressing DE-cadherin. J Comp Neurol. 2003;455:451–62. doi: 10.1002/cne.10484. [DOI] [PubMed] [Google Scholar]
- Duport S, Robert F, Muller D, Grau G, Parisi L, Stoppini L. An in vitro blood-brain barrier model: cocultures between endothelial cells and organotypic brain slice cultures. Proc Natl Acad Sci USA. 1998;95:1840–45. doi: 10.1073/pnas.95.4.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich P. Eine Farbenanalytische Studie. Berlin: Hirschwald; 1885. Das sauerstuffbedürfnis des organismus. [Google Scholar]
- Faivre-Sarrailh C, Banerjee S, Li J, Hortsch M, Laval M, Bhat MA. Drosophila contactin, a homolog of vertebrate contactin, is required for septate junction organization and paracellular barrier function. Development. 2004;131:4931–42. doi: 10.1242/dev.01372. [DOI] [PubMed] [Google Scholar]
- Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–53. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Stevens-Graham B. New insights into neuron-glia communication. Science. 2002;298:556–62. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MR, Delrow J, Kim J, Johnson E, Doe CQ. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron. 2003;38:567–80. doi: 10.1016/s0896-6273(03)00289-7. [DOI] [PubMed] [Google Scholar]
- Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–50. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–88. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16:181–88. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Wolburg H, Redies C. N-cadherin mediates pericytic-endothelial interaction during brain angiogenesis in the chicken. Dev Dyn. 2000;218:472–79. doi: 10.1002/1097-0177(200007)218:3<472::AID-DVDY1008>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Giaume C, McCarthy KD. Control of gap-junctional communication in astrocytic networks. Trends Neurosci. 1996;19:319–25. doi: 10.1016/0166-2236(96)10046-1. [DOI] [PubMed] [Google Scholar]
- Goldmann EE. Vitalfarbung am zentralnervensystem. Abh Konigl Preuss Akad Wiss. 1913;1:1–60. [Google Scholar]
- Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H. Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat Neurosci. 1999;2:139–43. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- Haseloff RF, Blasig IE, Bauer HC, Bauer H. In search of the astrocytic factor(s) modulating blood-brain barrier functions in brain capillary endothelial cells in vitro. Cell Mol Neurobiol. 2005;25:25–39. doi: 10.1007/s10571-004-1375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–85. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Brown RC, Davis TP. Smoking and ischemic stroke: a role for nicotine? Trends Pharmacol Sci. 2002;23:78–82. doi: 10.1016/s0165-6147(02)01893-x. [DOI] [PubMed] [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–93. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Hirakawa H, Okajima S, Nagaoka T, Takamatsu T, Oyamada M. Loss and recovery of the blood-nerve barrier in the rat sciatic nerve after crush injury are associated with expression of intercellular junctional proteins. Exp Cell Res. 2003;284:196–210. doi: 10.1016/s0014-4827(02)00035-6. [DOI] [PubMed] [Google Scholar]
- Holash JA, Noden DM, Stewart PA. Re-evaluating the role of astrocytes in blood-brain barrier induction. Dev Dyn. 1993;197:14–25. doi: 10.1002/aja.1001970103. [DOI] [PubMed] [Google Scholar]
- Hoyle G. High blood potassium in insects in relation to nerve conduction. Nature. 1952;169:281–82. doi: 10.1038/169281a0. [DOI] [PubMed] [Google Scholar]
- Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001a;24:719–25. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- Huber JD, Hau VS, Borg L, Campos CR, Egleton RD, Davis TP. Blood-brain barrier tight junctions are altered during a 72-h exposure to λ-carrageenan-induced inflammatory pain. Am J Physiol Heart Circ Physiol. 2002;283:H1531–37. doi: 10.1152/ajpheart.00027.2002. [DOI] [PubMed] [Google Scholar]
- Huber JD, Witt KA, Hom S, Egleton RD, Mark KS, Davis TP. Inflammatory pain alters blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol. 2001b;280:H1241–48. doi: 10.1152/ajpheart.2001.280.3.H1241. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–60. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Ito K, Urban J, Technau GM. Distribution, classification, and development of Drosophila glial cells in the late embryonic and early larval ventral nerve cord. Roux’s Arch Dev Biol. 1995;204:284–307. doi: 10.1007/BF02179499. [DOI] [PubMed] [Google Scholar]
- Itoh M, Morita K, Tsukita S. Characterization of ZO-2 as a MAGUK family member associated with tight as well as adherens junctions with a binding affinity to occludin and α catenin. J Biol Chem. 1999;274:5981–86. doi: 10.1074/jbc.274.9.5981. [DOI] [PubMed] [Google Scholar]
- Jacobs JR. The midline glia of Drosophila: a molecular genetic model for the developmental functions of glia. Prog Neurobiol. 2000;62:475–508. doi: 10.1016/s0301-0082(00)00016-2. [DOI] [PubMed] [Google Scholar]
- Juang JL, Carlson SD. Fine structure and blood-brain barrier properties of the central nervous system of a dipteran larva. J Comp Neurol. 1992;324:343–52. doi: 10.1002/cne.903240305. [DOI] [PubMed] [Google Scholar]
- Kacem K, Lacombe P, Seylaz J, Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia. 1998;23:1–10. [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–92. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Kempski O. Cerebral edema. Semin Nephrol. 2001;21:303–7. doi: 10.1053/snep.2001.21665. [DOI] [PubMed] [Google Scholar]
- Kirchhoff F, Dringen R, Giaume C. Pathways of neuron-astrocyte interactions and their possible role in neuroprotection. Eur Arch Psychiatry Clin Neurosci. 2001;251:159–69. doi: 10.1007/s004060170036. [DOI] [PubMed] [Google Scholar]
- Klambt C, Goodman CS. The diversity and pattern of glia during axon pathway formation in the Drosophila embryo. Glia. 1991;4:205–13. doi: 10.1002/glia.440040212. [DOI] [PubMed] [Google Scholar]
- Kretzschmar D, Poeck B, Roth H, Ernst R, Keller A, et al. Defective pigment granule biogenesis and aberrant behavior caused by mutations in the Drosophila AP-3β adaptin gene ruby. Genetics. 2000;155:213–23. doi: 10.1093/genetics/155.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar D, Pflugfelder GO. Glia in development, function, and neurodegeneration of the adult insect brain. Brain Res Bull. 2002;57:121–31. doi: 10.1016/s0361-9230(01)00643-8. [DOI] [PubMed] [Google Scholar]
- Lai CH, Kuo KH, Leo JM. Critical role of actin in modulating BBB permeability. Brain Res Brain Res Rev. 2005;50:7–13. doi: 10.1016/j.brainresrev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Lane NJ. Invertebrate neuroglia: junctional structure and development. J Exp Biol. 1981;95:7–33. [Google Scholar]
- Lane NJ. Novel arthropod cell junctions with restrictive intercellular “linkers”. J Neurocytol. 1989;18:661–69. doi: 10.1007/BF01187085. [DOI] [PubMed] [Google Scholar]
- Lane NJ, Swales LS. Intercellular junctions and the development of the blood-brain barrier in Manduca sexta. Brain Res. 1979;168:22745. doi: 10.1016/0006-8993(79)90166-5. [DOI] [PubMed] [Google Scholar]
- Lane NJ. Morphology of glial blood-brain barriers. Ann NY Acad Sci. 1991;633:348–62. doi: 10.1111/j.1749-6632.1991.tb15626.x. [DOI] [PubMed] [Google Scholar]
- Lee SW, Kim WJ, Choi YK, Song HS, Son MJ, et al. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med. 2003;9:900–6. doi: 10.1038/nm889. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–45. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Lobrinus JA, Juillerat-Jeanneret L, Darekar P, Schlosshauer B, Janzer RC. Induction of the blood-brain barrier specific HT7 and neurothelin epitopes in endothelial cells of the chick chorioallantoic vessels by a soluble factor derived from astrocytes. Brain Res Dev Brain Res. 1992;70:207–11. doi: 10.1016/0165-3806(92)90199-7. [DOI] [PubMed] [Google Scholar]
- Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–27. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol. 2003;4:225–36. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- McCarty JH. Cell biology of the neurovascular unit: implications for drug delivery across the blood-brain barrier. Assay Drug Dev Technol. 2005;3:89–95. doi: 10.1089/adt.2005.3.89. [DOI] [PubMed] [Google Scholar]
- McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, et al. Selective ablation of alphav integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132:165–76. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu Rev Physiol. 1998;60:121–42. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T. Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr Opin Crit Care. 2002;8:101–5. doi: 10.1097/00075198-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Morita K, Sasaki H, Fujimoto K, Furuse M, Tsukita S. Claudin-11/OSP-based tight junctions of myelin sheaths in brain and Sertoli cells in testis. J Cell Biol. 1999;145:579–88. doi: 10.1083/jcb.145.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–99. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–30. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Newman EA. New roles for astrocytes: regulation of synaptic transmission. Trends Neurosci. 2003;26:536–42. doi: 10.1016/S0166-2236(03)00237-6. [DOI] [PubMed] [Google Scholar]
- Nobles M, Abbott NJ. Adhesion and growth of brain microvascular endothelial cells on treated glass. Endothelium. 1996;4:297–307. [Google Scholar]
- Pardridge WM. Drug and gene delivery to the brain: the vascular route. Neuron. 2002;36:555–58. doi: 10.1016/s0896-6273(02)01054-1. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Blood-brain barrier drug targeting: the future of brain drug development. Mol Interv. 2003;3:90–105. 51. doi: 10.1124/mi.3.2.90. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Pellegri G, Bittar PG, Charnay Y, Bouras C, et al. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci. 1998;20:291–99. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]
- Pentreath VW, Kai-Kai MA. Significance of the potassium signal from neurones to glial cells. Nature. 1982;295:59–61. doi: 10.1038/295059a0. [DOI] [PubMed] [Google Scholar]
- Pereanu W, Shy D, Hartenstein V. Morphogenesis and proliferation of the larval brain glia in Drosophila. Dev Biol. 2005;283:191–203. doi: 10.1016/j.ydbio.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Petty MA, Lo EH. Junctional complexes of the blood-brain barrier: permeability changes in neuroinflammation. Prog Neurobiol. 2002;68:311–23. doi: 10.1016/s0301-0082(02)00128-4. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW, Barres BA. Synaptic efficacy enhanced by glial cells in vitro. Science. 1997;277:1684–87. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- Radojcic T, Pentreath VW. Invertebrate glia. Prog Neurobiol. 1979;12:115–79. doi: 10.1016/0301-0082(79)90002-9. [DOI] [PubMed] [Google Scholar]
- Ramsauer M, Krause D, Dermietzel R. Angiogenesis of the blood-brain barrier in vitro and the function of cerebral pericytes. FASEB J. 2002;16:1274–76. doi: 10.1096/fj.01-0814fje. [DOI] [PubMed] [Google Scholar]
- Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34:207–17. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios JC, Rubin M, St Martin M, Downey RT, Einheber S, et al. Paranodal interactions regulate expression of sodium channel subtypes and provide a diffusion barrier for the node of Ranvier. J Neurosci. 2003;23:7001–11. doi: 10.1523/JNEUROSCI.23-18-07001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W, Wolburg H. Development of the blood-brain barrier. Trends Neurosci. 1990;13:174–78. doi: 10.1016/0166-2236(90)90043-a. [DOI] [PubMed] [Google Scholar]
- Rouach N, Glowinski J, Giaume C. Activity-dependent neuronal control of gap-junctional communication in astrocytes. J Cell Biol. 2000;149:1513–26. doi: 10.1083/jcb.149.7.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LL. The blood-brain barrier in and out of cell culture. Curr Opin Neurobiol. 1991;1:360–63. doi: 10.1016/0959-4388(91)90053-a. [DOI] [PubMed] [Google Scholar]
- Rubin LL, Hall DE, Porter S, Barbu K, Cannon C, et al. A cell culture model of the blood-brain barrier. J Cell Biol. 1991;115:1725–35. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LL, Staddon JM. The cell biology of the blood-brain barrier. Annu Rev Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Carlson SD. Glial membrane specializations and the compartmentalization of the lamina ganglionaris of the housefly compound eye. J Neurocytol. 1983;12:243–75. doi: 10.1007/BF01148464. [DOI] [PubMed] [Google Scholar]
- Sakakibara A, Furuse M, Saitou M, Ando-Akatsuka Y, Tsukita S. Possible involvement of phosphorylation of occludin in tight junction formation. J Cell Biol. 1997;137:1393–401. doi: 10.1083/jcb.137.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer JL. Polarized domains of myelinated axons. Neuron. 2003;40:297–318. doi: 10.1016/s0896-6273(03)00628-7. [DOI] [PubMed] [Google Scholar]
- Sanchez-del-Rio M, Reuter U. Migraine aura: new information on underlying mechanisms. Curr Opin Neurol. 2004;17:289–93. doi: 10.1097/00019052-200406000-00009. [DOI] [PubMed] [Google Scholar]
- Schinkel AH. P-Glycoprotein, a gatekeeper in the blood-brain barrier. Adv Drug Deliv Rev. 1999;36:179–94. doi: 10.1016/s0169-409x(98)00085-4. [DOI] [PubMed] [Google Scholar]
- Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–28. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- Schwabe T, Bainton RJ, Fetter RD, Heberlein U, Gaul U. GPCR signaling is required for blood-brain barrier formation in drosophila. Cell. 2005;123:133–44. doi: 10.1016/j.cell.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Shaw SR. Early visual processing in insects. J Exp Biol. 1984;112:225–51. doi: 10.1242/jeb.112.1.225. [DOI] [PubMed] [Google Scholar]
- Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–62. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staddon JM, Rubin LL. Cell adhesion, cell junctions and the blood-brain barrier. Curr Opin Neurobiol. 1996;6:622–27. doi: 10.1016/s0959-4388(96)80094-8. [DOI] [PubMed] [Google Scholar]
- Staddon JM, Smales C, Schulze C, Esch FS, Rubin LL. p120, a p120-related protein (p100), and the cadherin/catenin complex. J Cell Biol. 1995;130:369–81. doi: 10.1083/jcb.130.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson BR, Begg DA. Concentration-dependent effects of cytochalasin D on tight junctions and actin filaments in MDCK epithelial cells. J Cell Sci. 1994;107(Pt 3):367–75. doi: 10.1242/jcs.107.3.367. [DOI] [PubMed] [Google Scholar]
- Strazielle N, Khuth ST, Ghersi-Egea JF. Detoxification systems, passive and specific transport for drugs at the blood-CSF barrier in normal and pathological situations. Adv Drug Deliv Rev. 2004;56:1717–40. doi: 10.1016/j.addr.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Strigini M, Cantera R, Morin X, Bastiani MJ, Bate M, Karagogeos D. The IgLON protein Lachesin is required for the blood-brain barrier in Drosophila. Mol Cell Neurosci. 2006;32:91–101. doi: 10.1016/j.mcn.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–80. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Swales LS, Lane NJ. Insect intercellular junctions: rapid freezing by jet propane. J Cell Sci. 1983;62:223–36. doi: 10.1242/jcs.62.1.223. [DOI] [PubMed] [Google Scholar]
- Swales LS, Lane NJ. Embryonic development of glial cells and their junctions in the locust central nervous system. J Neurosci. 1985;5:117–27. doi: 10.1523/JNEUROSCI.05-01-00117.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–94. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U, Hartenstein V. The development of cellular junctions in the Drosophila embryo. Dev Biol. 1994;161:563–96. doi: 10.1006/dbio.1994.1054. [DOI] [PubMed] [Google Scholar]
- Tepass U, Tanentzapf G, Ward R, Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet. 2001;35:747–84. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- Tsukamoto T, Nigam SK. Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am J Physiol Renal Physiol. 1999;276:F737–50. doi: 10.1152/ajprenal.1999.276.5.F737. [DOI] [PubMed] [Google Scholar]
- Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci. 1999;19:6897–906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Kettenmann H. Calcium signaling in glial cells. Trends Neurosci. 1996;19:346–52. doi: 10.1016/0166-2236(96)10048-5. [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, Sandercock PA, Dennis MS, Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 2003;34:806–12. doi: 10.1161/01.STR.0000058480.77236.B3. [DOI] [PubMed] [Google Scholar]
- Weller RO, Kida S, Zhang ET. Pathways of fluid drainage from the brain—morphological aspects and immunological significance in rat and man. Brain Pathol. 1992;2:277–84. doi: 10.1111/j.1750-3639.1992.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Willott E, Balda MS, Fanning AS, Jameson B, Van Itallie C, Anderson JM. The tight junction protein ZO-1 is homologous to the Drosophila discs-large tumor suppressor protein of septate junctions. Proc Natl Acad Sci USA. 1993;90:7834–38. doi: 10.1073/pnas.90.16.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–37. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- Wolff T, Ready DF. Pattern formation in the Drosophila retina. In: Bate M, Martinez-Arias A, editors. The Development of Drosophila melanogaster. Vol. 2. Plainview, NY: Cold Spring Harbor Lab. Press; 1993. pp. 1277–325. [Google Scholar]
- Wu VM, Schulte J, Hirschi A, Tepass U, Beitel GJ. Sinuous is a Drosophila claudin required for septate junction organization and epithelial tube size control. J Cell Biol. 2004;164:313–23. doi: 10.1083/jcb.200309134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenker D, Begley D, Bratzke H, Rubsamen-Waigmann H, von Briesen H. Human blood-derived macrophages enhance barrier function of cultured primary bovine and human brain capillary endothelial cells. J Physiol. 2003;551:1023–32. doi: 10.1113/jphysiol.2003.045880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]


