Abstract
Increased expression of cyclooxygenase-2 (COX-2) is associated with development of several cancers. The product of COX-2, prostaglandin H2 (PGH2), can undergo spontaneous rearrangement and nonenzymatic ring cleavage to form the highly reactive levuglandin E2 (LGE2) or D2 (LGD2). Incubation with LGE2 causes DNA-protein crosslinking in cultured cells, suggesting that levuglandins can directly react with DNA. We report the identification by liquid chromatography-tandem mass spectrometry of a stable levuglandin-deoxycytidine (LG-dC) adduct that forms upon levuglandin reaction with DNA. We found that LGE2 reacted with deoxycytidine, deoxyadenosine, or deoxyguanosine in vitro to form covalent adducts with a dihydroxypyrrolidine structure, as deduced from selective ion fragmentation. For LG-deoxycytidine adducts, the initial dihydropyrrolidine structure converted to a pyrrole structure over time. Reaction of LG with DNA yielded a stable LG-dC adduct with a pyrrole structure. These results describe the first structure of levuglandinyl-DNA adducts and provide the tools with which to evaluate the potential for LG-DNA adduct formation in vivo.
The concept that cyclooxygenase-2 (COX-2) participates in the multi-step evolution of many cancers is supported by a concerted body of evidence (1-5). That evidence includes epidemiological observations, demonstration of COX-2 expression in relevant neoplasms, overproduction of PGE2 patients with lung and other cancers, and experiments employing pharmacologic and genetic inhibition of COX-2. The immediate product of the cyclooxygenase (COX) enzymes is the cyclic endoperoxide, prostaglandin H2 (PGH2) (6), which is metabolized enzymatically in a cell specific manner to the prostaglandins E2, D2, F2α, I2 and thromboxane A2. Approximately 20% of PGH2 undergoes non-enzymatic rearrangement to the γ-ketoaldehydes, levuglandin E2 (LGE2) and LGD2 (7, 8). The levuglandins are among the most highly reactive molecules in mammalian biology, reacting almost instantaneously (7, 9) by Paal-Knorr condensation to form covalent adducts with free amines such as the ε-amine of lysine residues of proteins. Development of mass-spectrometric methods to measure levuglandinyl-lysine adducts in cellular proteins (7, 10) has permitted demonstration of COX-dependent formation of these adducts in activated platelets, in the hippocampus in Alzheimer’s disease, and in neoplastic cells (11, 12)
The COXs are present in the endoplasmic reticulum, and the COX-2 isoform is particularly densely localized to the nuclear envelope (13). In that location, access of PGH2 to the nucleus would be enhanced. In the nuclear environment, there are no synthases to dispose of PGH2 enzymatically, thus directing PGH2 to non-enzymatic rearrangement and formation of levuglandins within the nucleus. This raises the possibility that LG could react with available amino groups in nucleotides, and indirect evidence suggesting this possibility was the demonstration that LG can cause DNA-protein crosslinks in cells (14).
We here characterize products of the reaction of LG with the nucleosides deoxycytidine, deoxyguanosine, and deoxyadenosine, and demonstrate that reaction of DNA with LG produces a stable LG-deoxycytidine adduct with a pyrrole structure.
EXPERIMENTAL PROCEDURES
Materials
Methanol and acetonitrile were from Fisher Scientific (Pittsburgh, PA) and were HPLC grade or higher. LGE2 and [13C3] LGE2 were synthesized by V. Amernath, Vanderbilt University as described (15). All other chemicals and reagents were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
Formation of LG adducts
A 1:1 mixture of [12C]/[13C3] LGE2 was incubated in 5-fold molar excess with 2’-deoxycytidine or other nucleoside for 2 h unless indicated. All incubations were done at 37 °C, in phosphate-buffered saline, pH 6.5, using DMSO as a vehicle for LGE2. For LG-DNA adducts, a 1:1 mixture of [12C]/[13C3] LGE2 was mixed in 2-fold molar excess with 200 μg salmon sperm DNA, in 10 mM MOPS buffer, pH 7. The mixture was allowed to react for 2 h at 37 °C. After incubation, DNA was digested to nucleosides over a period of 24 h by serial hydrolysis. DNase I (10U) was added along with its associated 10x buffer (New England Biolabs, Ipswich, MA), and allowed to digest for 2 h at 37 °C. Following this, 15.5 U nuclease P1 was added, along with ZnCl2 (1 mM final concentration), and the digestion allowed to proceed overnight at 37 °C. Finally, alkaline phosphatase (30U) was added in 50 μL MOPS buffer, pH 10, and incubated for 1 h at 37 °C. The resulting digestion was filtered through a Spin-X (Costar) column and analyzed by LC/MS.
Mass Spectrometry
Samples were analyzed by liquid chromatography tandem mass spectrometry (LC/MS/MS) on a TSQ Quantum triple quadrupole machine (Thermo Fisher Scientific, Waltham, MA) using electrospray ionization. Samples were separated on a reversed-phase C18 column (Phenomenex Prodigy 5 μ; 150 × 1 mm) using mobile phase A (deionized water containing 0.1% glacial acetic acid) and mobile phase B (acetonitrile), and eluted at a flow rate of 0.1 mL/min by a linear gradient of 5%-80% solvent B over 10 min. The mass spectrometer was operated in positive ion mode, and selected reaction monitoring (SRM) data collected in profile mode. Nitrogen was used for both the sheath and auxiliary gases, which were set to 31 and 25 (arbitrary units), respectively. Source collision energy was 5 V; all other collision energy as noted. Capillary temperature was 300°C and the electrospray needle was maintained at 4400 V. For full scans, using Quad 1, the mass-spectral resolution was set to a full width at half maximum (FWHM) of 0.7 μ, and scan time was 2 sec. Full scan data was collected in centroid mode.
RESULTS
Formation and structure of LG-nucleoside adducts
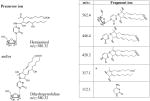
We examined whether LG has the capability of reacting with individual nucleosides. To better identify specific reaction products, we used a 1:1 mixture of [12C]/[13C3]-LG, which produces a characteristic ion doublet when analyzed by full scan mass spectrometry. The reaction of LG with deoxycytidine yielded a unique [12C] LG product at m/z = 580.4 together with a [13C3] LG product at m/z = 583.4 (Figure 2), which co-chromatographed with a lesser dehydration product (Figure 2). Fragmentation of the LG-dC adduct at m/z = 580 (Table 1) generated an ion fragment at m/z = 112.1, corresponding to the cytosine base, as well as an ion fragment at m/z = 317.1, corresponding to a previously described fragment ion of LGE2 (16). Larger fragment ions were consistent with the loss of deoxyribose at relatively low voltage, followed by a second dehydration product. We were able to assign two possible structures, equal in molecular weight, to the reaction product ion at m/z = 580 (Table 1). The first structure, a hemiaminal, is an intermediate formation of a Schiff base; the second, a dihydroxypyrrolidine, is a pentyl ring formation closed around the nitrogen of deoxycytidine. Both have been previously described as intermediates in the formation of the final pyrrole structure in the reaction of γ-ketoaldehydes with lysine (9).
Figure 2.
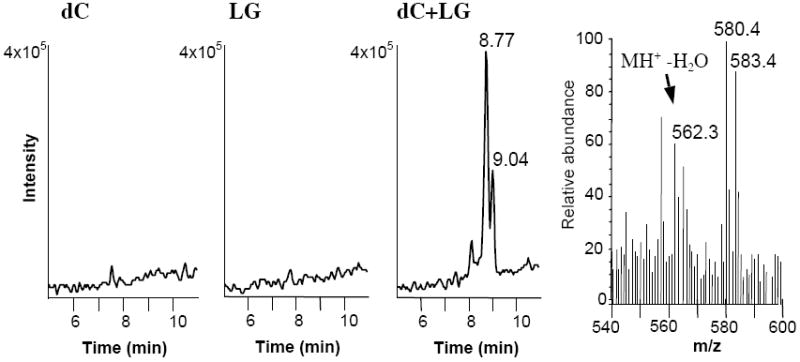
Unique ion formed in the reaction between dC and LG. A 50/50 mixture of [12C]- and [13C3] LGE2 was incubated with dC in a 5:1 ratio for 2 h before ESI-LC/MS analysis in full scan, positive ion mode. Equal amounts of LGE2 or dC alone in reaction buffer containing vehicle were used as controls. Shown are the current chromatograms for the ion at m/z = 580.3, as well as the ion spectrum for the peak at t = 8.77 minutes within the LG-dC reaction. The 580 and 583 ions correspond to [12C] LG and [13C3] LG adducts, respectively.
Table 1.
Structures of the products of fragmentation of the LG-dC ion at m/z = 580.4. LG was reacted with dC in a 5:1 ratio for 2 h at 37 °C, and the resulting mixture analyzed by ESI LC/MS/MS. Based on the product ions obtained, the precursor ion at m/z = 580.4 could be either a hemiaminal or an open ring, dihydroxypyrrolidine structure as shown. *, a previously reported LG product ion from (16).
 |
Fragmenting the LG-dC adduct at m/z = 580 predominantly yielded dehydration products, which prevented us from distinguishing between the dihydroxypyrrolidine and hemiaminal structures. Therefore, we fragmented the precursor ion corresponding to the LG-dC dehydration product (m/z = 562), which had an identical retention time of the major ion at m/z = 580, suggesting that the loss of a hydroxyl group occurred within the mass spectrometer. As expected, at increasing fragmenting voltages, there was a loss of deoxyribose, followed by several successive dehydrations (Table 2). Three fragment ions at m/z = 410.3, m/z = 299 and m/z = 162 are possible product of a dihydroxypyrrolidine structure but can not derive from an initial hemiaminal structure (Table 2), suggesting that the LG-dC reaction product of m/z = 580 is a dihydroxypyrrolidine. No fragment ions specific to a hemiaminal precursor ion were found.
Table 2.
Structures of the products of fragmentation of the dehydrated LG-dC ion at m/z = 562.3. Structures for the resulting fragment ions based on an initial dihydroxypyrrolidine structure are compared to a hemiaminal precursor ion. X = no corresponding structure found.
| m/z= | Dihydroxypyrrolidine | Hemiaminal |
|---|---|---|
| 446.3 |  |
 |
| 428.4 | 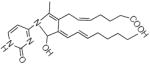 |
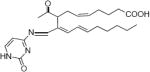 |
| 410.3 | 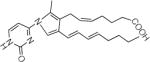 |
X |
| 299.5 | X | |
| 162.2 | 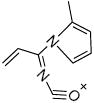 |
X |
| 112.3 | 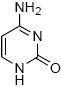 |
 |
Comparable adducts, differing only by the molecular weights of the respective nucleosides, were found after incubation of LGE2 with dA (m/z = 604.3) and dG (m/z = 620.3, Figure 3A). These unique ions, showing as a doublet when a mixture of [12C]/[13C3] LGE2 was used, were not seen in control incubations of individual nucleoside or LGE2 alone (data not shown). Because fragment ions of the LG-dA adduct at m/z = 604 and the LG-dG adduct at m/z = 620 were similar to those derived from the LG-dC dihydroxypyrrolidine adduct and corresponded to a loss of deoxyribose, individual bases, and shared LG fragments at m/z = 317 and m/z = 299 (data not shown), we conclude that these three nucleoside adducts have similar structures (Figure 3B). There was no individual preference of reaction in an equimolar mixture of nucleosides, and each adduct ion was detected in relatively equal abundance. Incubation of LG with thymidine, which lacks a free amino group, did not produce any unique reaction products (Figure 3A).
Figure 3.
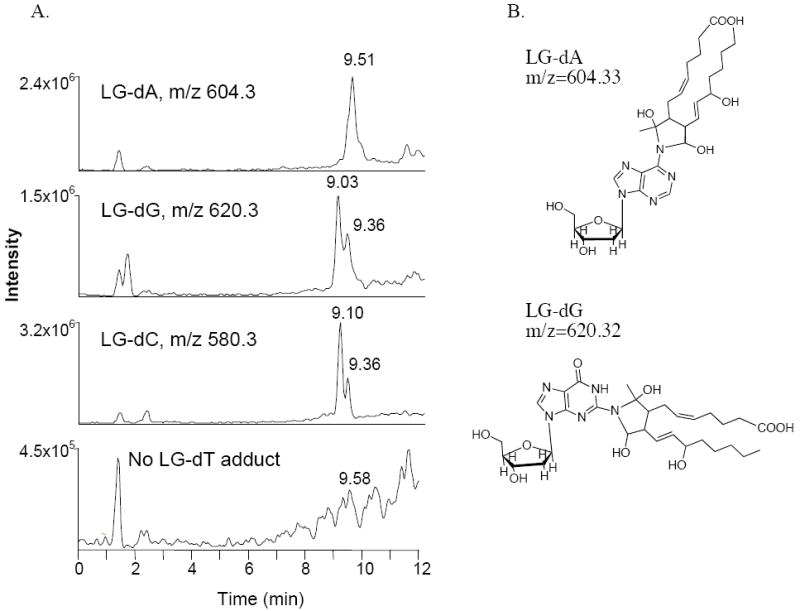
Unique ions formed after reaction of LG with an equimolar mix of nucleosides. A 50/50 mixture of [12C]- and [13C3] LGE2 was incubated at 37 °C with an equimolar nucleoside mix, with LG in a 3:1 ratio per individual nucleoside. After 2 h, the reaction and individual controls (not shown), were analyzed by ESI-LC/MS in full scan, positive ion mode. (A) The ion current chromatograms for the LG-dA, LG-dG, and LG-dC adducts within the LG-nucleoside reaction are shown, along with the chromatogram for the predicted ion at m/z = 595 of an LG-dT adduct. (B) The deduced dihydroxypyrimidine structures of the LG-dA and LG-dG adducts.
Formation of a stable LG-dC pyrrole structure
A unique ion at m/z = 544 was detected at longer incubation times in the LG-dC reaction. Fragmentation of the ion at m/z = 544 yielded a loss of deoxyribose at low energy, and fragment ions including a cytosine base at high energy. The molecular ion, as well as the fragment ions generated, correspond to a pyrrole LG-dC structure (Table 3). The major fragment ions at m/z = 428 and m/z = 176 were chosen for analysis by selected reaction monitoring (SRM), to increase the sensitivity of detection. To determine whether the m/z = 580 dihydroxypyrrolidine detected in the LG-dC reaction converts over time to a pyrrole structure of m/z = 544, we monitored both ions over a period of 48 hours using SRM. Over time, the ion at m/z = 580 disappeared in parallel with the appearance of the ion at m/z = 544 (Figure 4), suggesting that the dihydroxypyrrolidine is an intermediate of the final pyrrole structure. The proposed mechanism of LG-dC adduct formation and conversion to the pyrrole structure is shown in Figure 5.
Table 3.
Structure and fragment ions of the LG-dC ion at m/z = 544.3. LG was reacted in a 3:1 ration with dC for 48 h at 37 °C and the results analyzed by ESI LC/MS/MS. Based on the product ions seen, the precursor ion is most likely a pyrrole dC-LG structure, similar to the pyrrole-lysyl adduct seen in (19). The fragment ions at m/z = 428 and m/z = 176 were chosen for SRM analysis in later experiments.
| m/z= | Fragment ion | Precursor ion at m/z=544.3 |
|---|---|---|
| 428.2 | 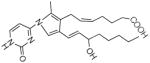 |
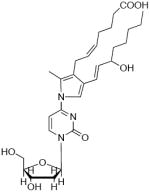 |
| 410.1 | 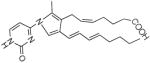 |
|
| 176.1 | 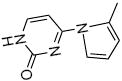 |
|
| 162.0 | 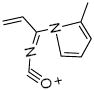 |
|
| 112.1 |  |
Figure 4.
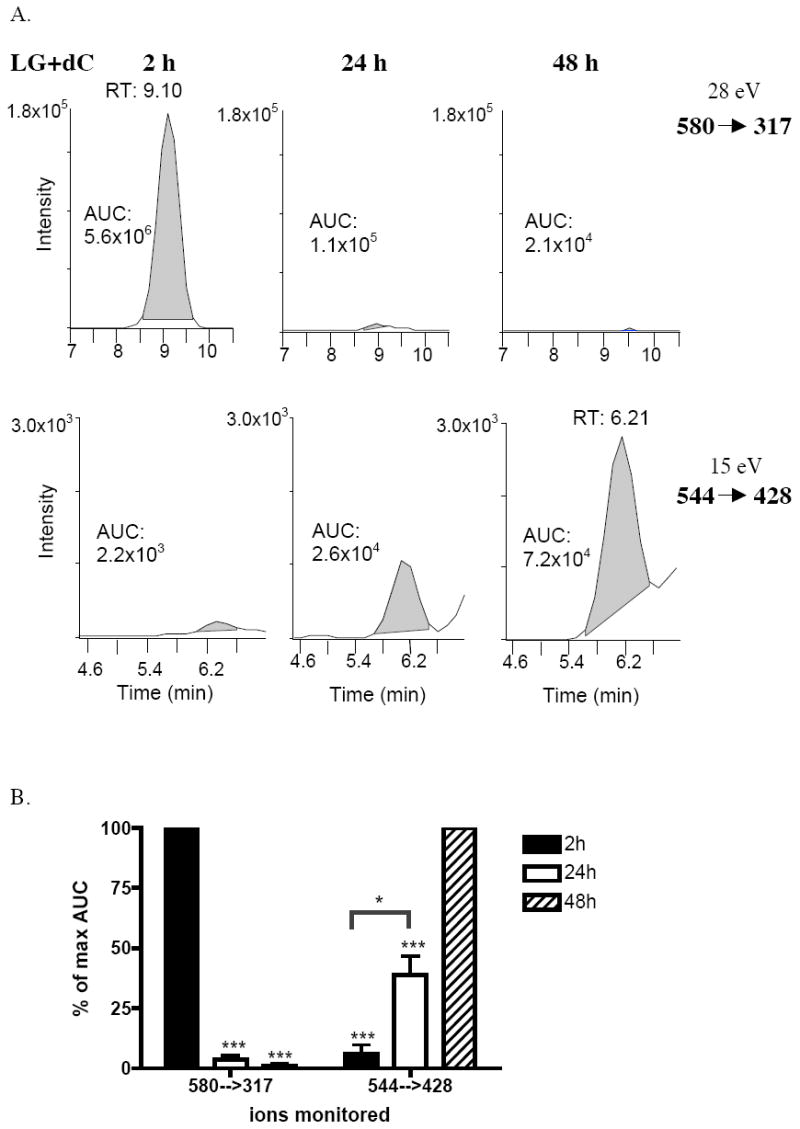
The ion at m/z = 580 disappears over time, while the ion at m/z = 544 appears. LGE2 was incubated in a 2:1 ratio with dC at pH = 7 and allowed to incubate for 2 h, 24 h, or 48 h at 37°C. Samples were immediately frozen following the time point and analyzed at a later date by ESI-LC/MS/MS, using SRM. The parent ions at m/z = 580 and m/z = 544 were analyzed in the same run. (A) Shown is the chromatograph of one fragment ion for each precursor ion from one experiment, along with the results of peak integrations. (B) The results of three separate experiments were expressed as a percent of the maximum integrated area from each experiment, corresponding to the 2 h time point for the ion at m/z = 580 and the 48 h time point for the ion at m/z = 544. These values are summarized by the mean (n = 3) and standard error. ***, p<0.001 from maximum AUC and *, p<0.05 by one-way ANOVA followed by Bonferroni’s multiple comparisons test.
Figure 5.
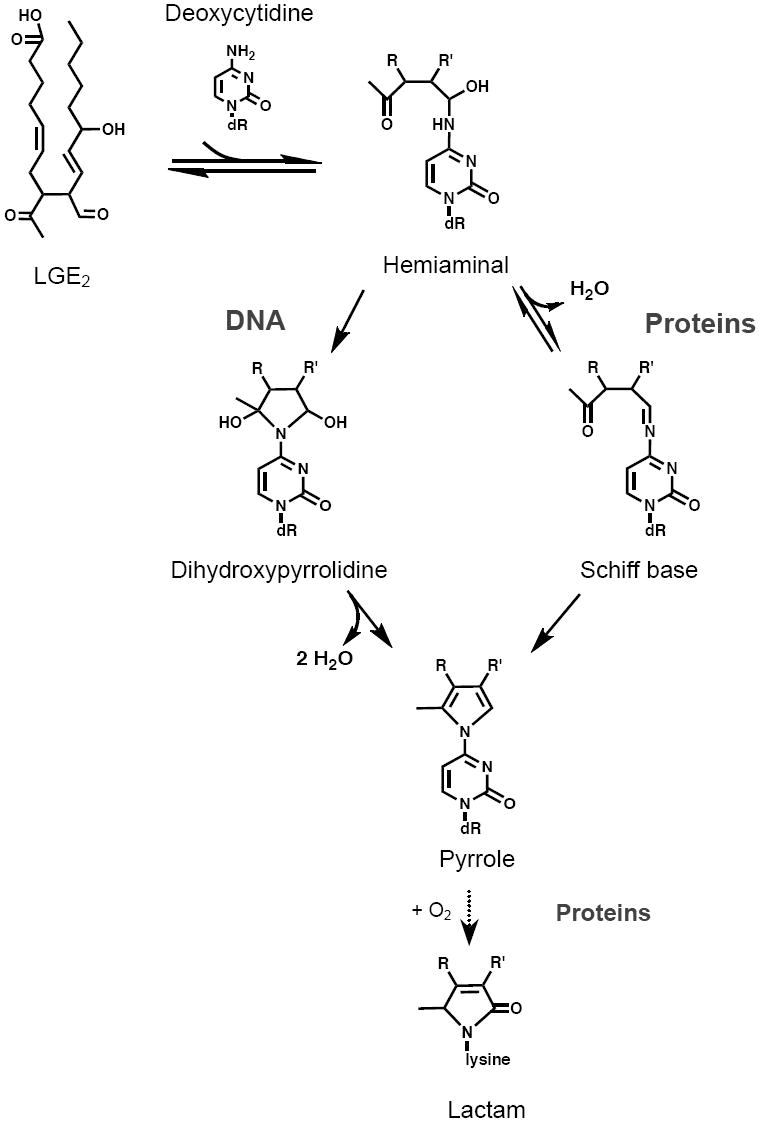
Proposed mechanism of LG reaction with dC. The final pyrrole structure proceeds from an intermediate dihydroxypyrrolidine as suggested in (36). In contrast, the LG-lysyl adduct in proteins proceeds via an intermediate Schiff base and the pyrrole is further oxidized into a terminal lactam structure (37).
Detection of LG-DNA adducts
To determine whether LG reacts with a base in double-stranded DNA, we incubated a mixture of [12C]/[13C3] LGE2 with commercial salmon sperm DNA. This was digested to nucleosides over 24 h and initially analyzed by LC/MS for the LG adducts previously identified through incubation with individual nucleosides. The unique reaction product identified (Figure 6A) corresponded by mass to the pyrrole LG-dC adduct (m/z = 544.3). This identity was confirmed through ion fragmentation, which yielded a cytosine base as well as fragment ions identical to those generated through extended reaction with individual deoxycytidine. SRM analysis of the LG-DNA adduct, using these fragment ions, yielded a similar chromatogram as analysis of the LG-dC pyrrole adduct (Figure 6B).
Figure 6.
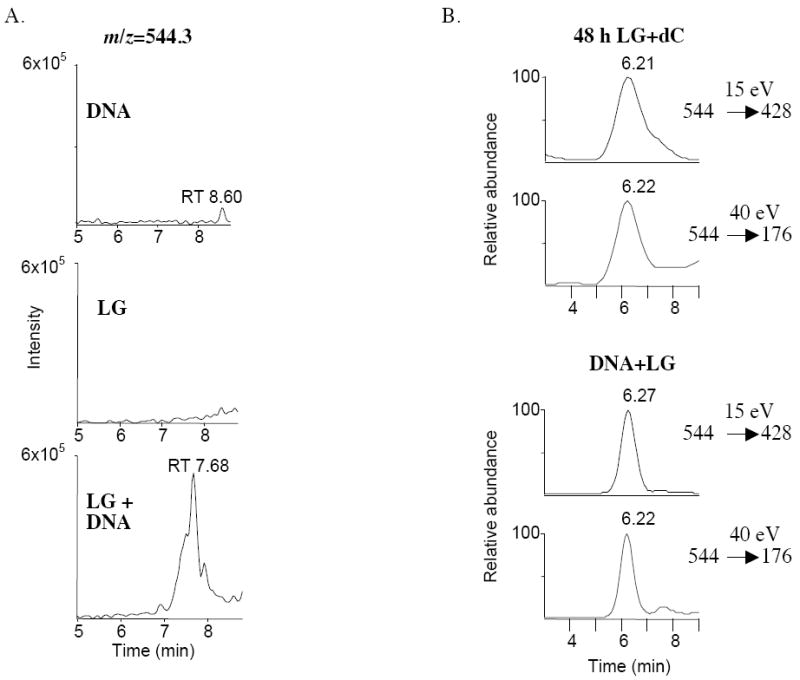
LG-dC formed in a reaction between LG and DNA. A mixture of [12C]- and [13C3]-LGE2 was incubated in a 2:1 ratio with 200 μg salmon sperm DNA at 37 °C for 2 h. Equal amounts of LGE2 or DNA alone in reaction buffer containing vehicle were used as controls. DNA was digested to nucleosides over a period of 24 hours using DNase I, nuclease P1, and alkaline phosphatase. (A) Samples were analyzed by ESI LC-MS in full scan positive ion mode. Shown are the chromatographs for the ion at m/z = 544.3. (B) The LG-DNA sample (bottom) was analyzed by SRM, monitoring the ion transition of m/z = 544 to fragments of m/z = 428 and 176, and the resulting chromatograph compared to identical SRM analysis of a 48 h LG-dC reaction (top).
DISCUSSION
Levuglandins, reactive byproducts of prostaglandin synthesis, have been shown to form covalent adducts with proteins (17, 18), and cross-link proteins and DNA (14). Here we characterize the structures of covalent adducts of LG with nucleosides, and demonstrate that LGE2 reacts with double-stranded DNA to form adducts of deoxycytidine (dC). Characterization of the LG-dC adduct of DNA now provides an experimental approach to determining whether the catalytic activity of COX-2 can lead to formation of this DNA adduct in cells.
We have found that LGE2 reacts with the free amino group of dC to form an immediate covalent adduct at m/z = 580 with a likely dihydroxypyrrolidine structure, according to specific fragment ions. Occurrence of an additional hemiaminal structure, of equal m/z, is also possible. The LG-dC dihydroxypyrrolidine at m/z = 580 is a probable intermediate of the pyrrole structure, as during extended incubation of LG with dC, the ion at m/z = 544 ion emerges while the ion at m/z = 580 declines over time. Due to the possibility of unequal ionization between the ions at m/z 580 and 544, at this time we are not able to determine the percentage of dihydroxypyrrolidine adduct that successfully converts to a stable end pyrrole.
A pyrrole structure, proceeding from a Schiff base, is a hypothesized intermediate in the formation of LG-lysyl lactam adducts (19). However, unlike LG-lysyl adducts (10), after reaction with dC there does not seem be oxidation to a final lactam structure. We were unable to detect any ion corresponding to an LG-dC lactam in either extended LG-dC reactions or within the LG-DNA reaction. This is likely due to the stabilizing effect of the pyrimidine ring. The stability of the LG-dC pyrrole structure is demonstrated by our ability to detect it after the lengthy enzymatic digestion necessary to reduce DNA to nucleosides. Whereas LG reacts with the individual nucleosides, dA and dG, an examination of the LG-DNA reaction spectra did not reveal any products corresponding to putative LG-dA or LG-dG pyrrole or lactam structures.
Adducts of DNA have the potential for producing genomic instability (20-22). Genomic lesions have been shown to result from lipid adducts of DNA. Lipid peroxidation produces aldehydic products that react covalently with DNA (23). The most-studied of these is 4-hydroxynonenal (HNE), which reacts with deoxyguanosine in DNA to form a mutagenic 1,N2-propano-2’-deoxyguanosine (HNE-dG) adduct (20, 24). This adduct is preferentially formed at the third base of codon 243 in the p53 gene, a frequent mutation spot in many human cancers (25). Another lipid peroxidation product that also can be generated as a byproduct of COX-2 (26), malondialdehyde, reacts with guanine residues to create a stable M1G adduct that has been shown to be mutagenic in a number of systems (27-29) and carcinogenic in rats (30). However, direct oxidation of DNA leading to base propenal formation is responsible for almost all of the M1G (31), making MDA derived from COX-2 an unlikely source of this adduct. 4-oxo-2(E)-nonenal (ONE) also forms following lipid peroxidation, COX-2 or lipoxygenase activity, and reacts with deoxyguanosine in DNA to form heptanone-etheno-deoxyguanosine adducts (32) that are increased in the small intestine of a mouse colorectal cancer model (33).
The evidence from investigations of these other lipid adducts of DNA engenders a hypothesis that LG-adducts of DNA also could cause genomic instability. The proximity of COX-2 to the nucleus, the absence of PGH2 metabolizing enzymes in the nucleus, and the fact that LG is far more reactive than HNE (9) together provide additional support for this hypothesis. Previous research on the involvement of COXs in cancer has provided evidence demonstrating that prostaglandins such as PGE2 lead to increased proliferation, protection from apoptosis, angiogenesis, suppression of the immune response, and enhanced metastasis (34). These effects result from the action of PGE2 on G-protein coupled receptors (GPCR) to initiate the relevant signaling pathways, which promote propagation of the malignant genotype and the dissemination of cancer cells, but do not directly explain the massive genomic instability derived from successive somatic mutations and chromosomal instability that occur during the evolution of the malignant phenotype. However, the combination of somatic mutations and chromosomal instability caused by levuglandin adducts of DNA together with prostaglandin-initiated mechanisms that promote clonal expansion and spread would synergize to contribute to the evolution of highly malignant neoplasms with poor prognosis. This severe malignant phenotype is associated with cancers of the colon, lung, breast, pancreas and other neoplasms that express COX-2 which account for more than half of the 560,000 cancer deaths in the U.S. annually (35).
The results presented here identify for the first time the structure of a stable covalent modification of DNA by levuglandin E2, a product of the cyclooxygenases and of lipid peroxidation. This provides a basis for investigating the biological significance of formation of LG-DNA adducts, and constitutes the basis for a novel hypothesis for the involvement of COXs and/or oxidative stress in carcinogenesis.
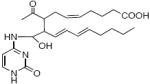
Figure 1.
Structure of levuglandin E2 and D2
Acknowledgments
The authors would like to acknowledge the helpful contribution of Dr. Irene Zagol-Ikapitte in mass spectrometry, as well as the Vanderbilt Mass Spectometry Core facility.
Abbreviations used
- COX
cyclooxygenase
- LG
levuglandin
- dC
2’-deoxycytidine
- dA
2’-deoxyadenosine
- dG
2’-deoxyguanosine
- HNE
4-hydroxynonenal
- ONE
4-oxo-2(E)-nonenal
- NSAIDS
non-steroidal anti-inflammatory drugs
- HPLC
high performance liquid chromatography
- LC/MS/MS
liquid chromatography tandem mass spectrometry
- SRM
selected reaction monitoring
References
- 1.Seyberth HW, Segre GV, Morgan JL, Sweetman BJ, Potts JT, Jr, Oates JA. Prostaglandins as mediators of hypercalcemia associated with certain types of cancer. N Engl J Med. 1975;293:1278–1283. doi: 10.1056/NEJM197512182932502. [DOI] [PubMed] [Google Scholar]
- 2.Murphey LJ, Williams MK, Sanchez SC, Byrne LM, Csiki I, Oates JA, Johnson DH, Morrow JD. Quantification of the major urinary metabolite of PGE2 by a liquid chromatographic/mass spectrometric assay: determination of cyclooxygenase-specific PGE2 synthesis in healthy humans and those with lung cancer. Anal biochem. 2004;334:266–275. doi: 10.1016/j.ab.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 3.Wang M-T, Honn KV, Nie D. Cyclooxygenases, prostanoids, and tumor progression. Cancer Metastasis Rev. 2007;26:525–534. doi: 10.1007/s10555-007-9096-5. [DOI] [PubMed] [Google Scholar]
- 4.Cha YI, Dubois RN. NSAIDs and cancer prevention: Targets downstream of COX-2. Annu Rev Med. 2007;58:239–252. doi: 10.1146/annurev.med.57.121304.131253. [DOI] [PubMed] [Google Scholar]
- 5.Muller-Decker K, Furstenberger G. The cyclooxygenase-2-mediated prostaglandin signaling is causally related to epithelial carcinogenesis. Mol Carcinog. 2007;46:705–710. doi: 10.1002/mc.20326. [DOI] [PubMed] [Google Scholar]
- 6.Hamberg M, Samuelsson B. Detection and isolation of an endoperoxide intermediate in prostaglandin biosynthesis. Proc Natl Acad Sci U S A. 1973;70:899–903. doi: 10.1073/pnas.70.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boutaud O, Brame CJ, Chaurand P, Li J, Rowlinson SW, Crews BC, Ji C, Marnett LJ, Caprioli RM, Roberts LJ, 2nd, Oates JA. Characterization of the lysyl adducts of prostaglandin H-synthases that are derived from oxygenation of arachidonic acid. Biochemistry. 2001;40:6948–6955. doi: 10.1021/bi002629k. [DOI] [PubMed] [Google Scholar]
- 8.Salomon RG, Miller DB, Zagorski MG, Coughlin DJ. Solvent-induced fragmentation of prostaglandin endoperoxides. New aldehyde products from PGH2 and a novel intramolecular 1,2-hydride shift during endoperoxide fragmentation in aqueous solution. J Am Chem Soc. 1984;106:6049–6060. [Google Scholar]
- 9.Davies SS, Amarnath V, Roberts LJ., 2nd Isoketals: highly reactive gamma-ketoaldehydes formed from the H2-isoprostane pathway. Chem Phys Lipids. 2004;128:85–99. doi: 10.1016/j.chemphyslip.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Brame CJ, Salomon RG, Morrow JD, Roberts LJ., 2nd Identification of extremely reactive gamma-ketoaldehydes (isolevuglandins) as products of the isoprostane pathway and characterization of their lysyl protein adducts. J Biol Chem. 1999;274:13139–13146. doi: 10.1074/jbc.274.19.13139. [DOI] [PubMed] [Google Scholar]
- 11.Boutaud O, Li J, Zagol I, Shipp EA, Davies SS, Roberts LJ, 2nd, Oates JA. Levuglandinyl adducts of proteins are formed via a prostaglandin H2 synthase-dependent pathway after platelet activation. J Biol Chem. 2003;278:16926–16928. doi: 10.1074/jbc.M300940200. [DOI] [PubMed] [Google Scholar]
- 12.Zagol-Ikapitte I, Masterson TS, Amarnath V, Montine TJ, Andreasson KI, Boutaud O, Oates JA. Prostaglandin H(2)-derived adducts of proteins correlate with Alzheimer’s disease severity. J Neurochem. 2005;94:1140–1145. doi: 10.1111/j.1471-4159.2005.03264.x. [DOI] [PubMed] [Google Scholar]
- 13.Morita I, Schindler M, Regier MK, Otto JC, Hori T, DeWitt DL, Smith WL. Different intracellular locations for prostaglandin endoperoxide H synthase-1 and -2. J Biol Chem. 1995;270:10902–10908. doi: 10.1074/jbc.270.18.10902. [DOI] [PubMed] [Google Scholar]
- 14.Murthi KK, Friedman LR, Oleinick NL, Salomon RG. Formation of DNA-protein cross-links in mammalian cells by levuglandin E2. Biochemistry. 1993;32:4090–4097. doi: 10.1021/bi00066a034. [DOI] [PubMed] [Google Scholar]
- 15.Amarnath V, Amarnath K, Masterson T, Davies SS, Roberts LJ. A simplified synthesis of the diastereomers of levuglandin E2. Synthetic Comm. 2005;35:397–408. [Google Scholar]
- 16.Zagol-Ikapitte I, Bernoud-Hubac N, Amarnath V, Roberts LJ, 2nd, Boutaud O, Oates JA. Characterization of bis(levuglandinyl) urea derivatives as products of the reaction between prostaglandin H2 and arginine. Biochemistry. 2004;43:5503–5510. doi: 10.1021/bi049842r. [DOI] [PubMed] [Google Scholar]
- 17.Boutaud O, Li J, Chaurand P, Brame CJ, Marnett LJ, Roberts LJ, Oates JA. Oxygenation of arachidonic acid by cyclooxygenases generates reactive intermediates that form adducts with proteins. Adv Exp Med Biol. 2001;500:133–137. doi: 10.1007/978-1-4615-0667-6_16. [DOI] [PubMed] [Google Scholar]
- 18.Salomon RG, Subbanagounder G, O’Neil J, Kaur K, Smith MA, Hoff HF, Perry G, Monnier VM. Levuglandin E2-protein adducts in human plasma and vasculature. Chem Res Toxicol. 1997;10:536–545. doi: 10.1021/tx960157y. [DOI] [PubMed] [Google Scholar]
- 19.Boutaud O, Brame CJ, Salomon RG, Roberts LJ, 2nd, Oates JA. Characterization of the lysyl adducts formed from prostaglandin H2 via the levuglandin pathway. Biochemistry. 1999;38:9389–9396. doi: 10.1021/bi990470+. [DOI] [PubMed] [Google Scholar]
- 20.Feng Z, Hu W, Amin S, Tang MS. Mutational spectrum and genotoxicity of the major lipid peroxidation product, trans-4-hydroxy-2-nonenal, induced DNA adducts in nucleotide excision repair-proficient and -deficient human cells. Biochemistry. 2003;42:7848–7854. doi: 10.1021/bi034431g. [DOI] [PubMed] [Google Scholar]
- 21.Breivik J, Guadernack G. Genomic instability, DNA methylation, and natural selection in colorectal carcinogenesis. Semin Cancer Biol. 1999;9:245–254. doi: 10.1006/scbi.1999.0123. [DOI] [PubMed] [Google Scholar]
- 22.Bardelli A, Cahill DP, Lederer G, Speicher MR, Kinzler KW, Vogelstein B, Lengauer C. Carcinogen-specific induction of genetic instability. Proc Natl Acad Sci U S A. 2001;98:5770–5775. doi: 10.1073/pnas.081082898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartsch H, Nair J. Oxidative stress and lipid peroxidation-derived DNA-lesions in inflammation driven carcinogenesis. Cancer Detect Prev. 2004;28:385–391. doi: 10.1016/j.cdp.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Winter CK, Segall HJ, Haddon WF. Formation of cyclic adducts of deoxyguanosine with the aldehydes trans-4-hydroxy-2-hexenal and trans-4-hydroxy-2-nonenal in vitro. Cancer Res. 1986;46:5682–5686. [PubMed] [Google Scholar]
- 25.Hu W, Feng Z, Eveleigh J, Iyer G, Pan J, Amin S, Chung FL, Tang MS. The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis. 2002;23:1981–1989. doi: 10.1093/carcin/23.11.1781. [DOI] [PubMed] [Google Scholar]
- 26.Plastaras JP, Guengerich FP, Nebert DW, Marnett LJ. Xenobiotic-metabolizing cytochromes P450 convert prostaglandin endoperoxide to hydroxyheptadecatrienoic acid and the mutagen, malondialdehyde. J Biol Chem. 2000;275:11784–11790. doi: 10.1074/jbc.275.16.11784. [DOI] [PubMed] [Google Scholar]
- 27.Benamira M, Johnson K, Chaudhary A, Bruner K, Tibbetts C, Marnett LJ. Induction of mutations by replication of malondialdehyde-modified M13 DNA in Escherichia coli: determination of the extent of DNA modification, genetic requirements for mutagenesis, and types of mutations induced. Carcinogenesis. 1995;16:93–99. doi: 10.1093/carcin/16.1.93. [DOI] [PubMed] [Google Scholar]
- 28.Marnett LJ. Lipid peroxidation-DNA damage by malondialdehyde. Mutat Res. 1999;424:83–95. doi: 10.1016/s0027-5107(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 29.Niedernhofer LJ, Daniels JS, Rouzer CA, Greene RE, Marnett LJ. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J Biol Chem. 2003;278:31426–31433. doi: 10.1074/jbc.M212549200. [DOI] [PubMed] [Google Scholar]
- 30.Spalding JW. NTP technical report on the toxicology and carcinogenesis studies of malonaldehyde sodium salt (3-hydroxy-2-propenal, sodium salt) in F344/N rats and B6C3F1 mice. NTP Tech Rep. 1988;331:1–182. [PubMed] [Google Scholar]
- 31.Zhou X, Taghizadeh K, Dedon PC. Chemical and biological evidence for base propenals as the major source of the endogenous M1dG adduct in cellular DNA. J Biol Chem. 2005;280:25377–25382. doi: 10.1074/jbc.M503079200. [DOI] [PubMed] [Google Scholar]
- 32.Lee SH, Williams MV, DuBois RN, Blair IA. Cyclooxygenase-2-mediated DNA damage. J Biol Chem. 2005;280:28337–28346. doi: 10.1074/jbc.M504178200. [DOI] [PubMed] [Google Scholar]
- 33.Williams MV, Lee SH, Pollack M, Blair IA. Endogenous lipid hydroperoxide-mediated DNA-adduct formation in Min mice. J Biol Chem. 2006;281:10127–10133. doi: 10.1074/jbc.M600178200. [DOI] [PubMed] [Google Scholar]
- 34.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, Mariotto A, Miller BA, Feuer EJ, Altekruse SF, Lewis DR, Clegg L, Eisner MP, Reichman M, Edwards BK, editors. SEER Cancer Statistics Review, 1975-2005. National Cancer Institute; 2008. [Google Scholar]
- 36.Bernoud-Hubac N, Davies SS, Boutaud O, Montine TJ, Roberts LJ. Formation of highly reactive gamma-ketoaldehydes (neuroketals) as products of the neuroprostane pathway. J Biol Chem. 2001;276:30964–30970. doi: 10.1074/jbc.M103768200. [DOI] [PubMed] [Google Scholar]
- 37.Salomon RG. Distinguishing levuglandins produced through the cyclooxygenase and isoprostane pathways. Chem Phys Lipids. 2005;134:1–20. doi: 10.1016/j.chemphyslip.2004.12.003. [DOI] [PubMed] [Google Scholar]


