Synopsis
Canine Leishmaniasis is a fatal zoonotic visceralizing disease usually associated with tropical areas. The etiologic agent is an obligate intracellular protozoan, Leishmania infantum. In 1999, an outbreak of a canine leishmaniasis was reported in a Foxhound kennel in New York, and since that report, several other outbreaks have occurred across the United States in additional Foxhound kennels. Because of the high mortality and transmissibility associated with these outbreaks, it is essential that clinicians be aware of this disease to permit its rapid recognition and institution of control measures. Cases with a travel history may suggest imported disease, these are mainly observed from Southern Europe (south of France, Spain, Italy). Breeds from these and other endemic areas may be at higher risk of infection with Leishmania due to vertical transmission. The purpose of this report is to discuss the clinical signs, epidemiology, diagnosis, control and treatment of canine leishmaniasis with focus on the aspects of this disease within North America.
Keywords: canine, Leishmania infantum, protozoa, emerging, treatment, diagnosis
Introduction to Canine Visceral leishmaniasis
Leishmania infantum, an obligate intracellular parasite, is the causative agent of visceral leishmaniasis (VL) in the Mediterranean Basin and more recently North America. Natural hosts include dogs and humans (1) and transmission is usually via a sand fly vector. Infected dogs are the primary reservoir for zoonotic visceral leishmaniasis in endemic regions (Figure 1A), and are the most significant risk factor predisposing humans to infection (2). Dogs have a wide range of clinical presentation due to infection with Le. infantum, ranging from asymptomatic to fatal visceralizing disease. Host factors which determine clinical outcome are poorly understood. When clinical signs in dogs occur, they include enlarged lymph nodes and hepato- and splenomegaly due to parasitic invasion of the reticulo-endothelial system of phagocytic lymphocytes (3). Visceral leishmaniasis symptoms often can persist in canine patients for several weeks to months before patients seek medical care and in the United States it may be even longer before a correct diagnosis is made. In the meanwhile these patients are at risk of death from bacterial co-infections, massive bleeding, severe anemia (3) or renal failure.
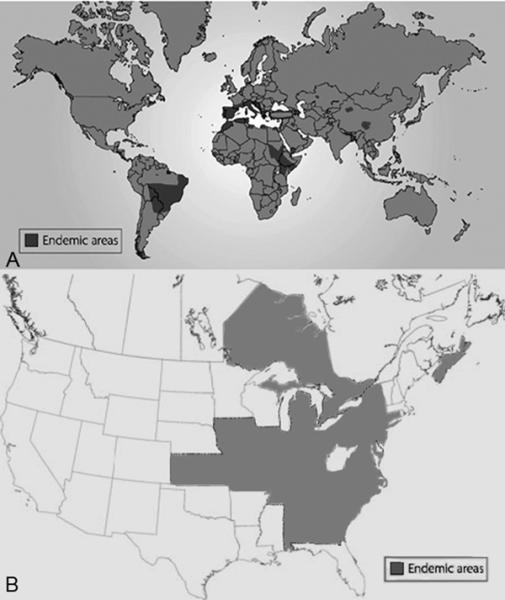
Figure 1. Prevalence of canine visceral Leishmaniasis in the World and United States.
A. Global seroprevalence of Canine VL. Adapted with permission from Chappuis F, Sundar S, Hailu A, et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5(11):873–82.. B. Seroprevalence of CVL in Foxhounds in North America. Adapted from Duprey ZH, Steurer FJ, Rooney JA, et al. Canine visceral leishmaniasis, United States and Canada, 2000–2003. Emerg Infect Dis. 2006 Mar;12(3):440–6.
Transmission of Leishmania infantum
In endemic areas, the primary means of transmission is vector-borne via the sand fly (Figure 2). Vector-borne transmission has not been shown in the U.S. to date (4, 5). Instead vertical transmission appears to be the primary means of transmission in U.S. dogs without a travel history to an endemic region (4). The frequency of vertical transmission in endemic areas is unknown due to the overwhelming likelihood of vector contact (6).
Figure 2. The classical Leishmania life cycle.
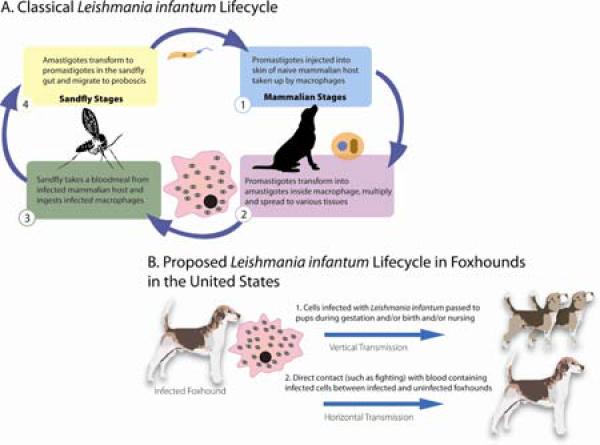
(A) requires both a sand fly and mammalian host. (B) A proposed Leishmania infantum life cycle in the United States Foxhound population with a prominent role for vertical transmission.
A potential sand fly vector of Le. infantum, Lutzomyia shannoni, is present within Southern and Southeastern United States (4). Lu. shannoni is known to bite dogs and other mammals and has been incriminated in the transmission of Le. brasiliensis in South and Central America (7). Anecdotal data indicate that U.S. species of Lu. shannoni can become infected with Le. infantum, but it is not known whether these flies permit Le. infantum development into infectious metacyclic infectious parasites. Vector feeding preferences can importantly influence disease transmission. In the U.S., Lu. shannoni has also been shown to feed on dogs (Rowton personal communication).
In many settings dogs have been shown to be a link between sylvatic and domestic cycles of visceral leishmaniasis. Dogs often cross forest-edge boundaries, thereby potentially bringing parasites to, or from, sylvatic systems and to and from other potential mammal hosts (such as foxes and opossums). In the U.S., due to frequent exchange of Foxhounds between kennels and these dogs' penchant for spending time in the woods, these dogs may be a primary focal point for transmission of Le. infantum to continue transmission to sand flies. Thus, if Lu. shannoni indeed prefers to feed on dogs in comparison to other mammals, infected dogs are more likely than other mammals to serve as a source of Le. infantum to an uninfected fly.
Epidemiology of Canine Visceral Leishmaniasis in the U.S.
A retrospective study performed by the Centers for Disease Control and Prevention, Division of Parasitic Diseases, employed sera samples that were collected between April 2000 and December 2003. Samples were taken from greater than 12,000 Foxhounds and other canids in the U.S., and an 8.9% seroprevalence was observed in Foxhounds but not other randomly selected domestic dogs or wild canids (4). Samples detected at 1:16 and 1:32 were considered suspect (4). This study initially had participation from almost all registered Foxhound kennels in the U.S., but after the first year participation greatly decreased, perhaps leading to a selection bias in further years of kennels with known clinical infection with Le. infantum.
Between years 2000 and 2001, even though the number of participating kennels decreased, the number of Leishmania seropositive samples increased, most likely indicating that there was increased infection/incidence of disease in these participating kennels. In studies of Foxhound kennels, we observe a similar 9.8% overall seropositivity/seroprevalence in our current cohort of 10 kennels and over 500 dogs, but among high risk kennels the seropositivity and presence of polysymptomatic disease is 13.5%. Infection in this cohort is greater than observed by serology as indicated by a 22.8% quantitative Polymerase Chain Reaction Assay (qPCR) positivity in the overall cohort. The percent qPCR positive dogs in high-risk kennels is 44.8%. Roughly 1/2 of the qPCR positive (infected) population was clinically asymptomatic (Petersen unpublished data). In dog breeds from endemic countries, a higher sero- or PCR prevalence is also seen as compared to the overall canine population. This includes dog breeds from Southern Europe, such as Corsicas, Italian Spinones and Neapolitan Mastiffs (Petersen and CDC serological unpublished data.)
Transmission of Visceral Leishmaniasis in the U.S.
Visceral Leishmaniasis is classically transmitted to a suitable mammalian host by the bite of an infected sand fly after which the promastigote form of the parasite is phagocytosed by macrophages (Figure 2) (1). Although endemic in many parts of the world, this disease has only recently been described in the U.S. (8). Previously, sporadic cases have been reported in the United States, in canine travelers returning to the U.S. from endemic areas (5). However, in 2000, a kennel in New York State reported four Foxhounds to be infected with Le. infantum (8). By 2005, 60 kennels in 22 states and two Canadian provinces had reported seropositive Foxhounds (9). Non-vector based mechanisms postulated for transmission of canine visceral leishmaniasis in the U.S. include vertical transmission (transplacental or transmammary) and horizontal transmission by direct contact with infected cells in blood (4, 5, 10) (Figure 2). Transmission has been documented via packed red blood cell transfusion from infected Foxhounds (11). It is not known how frequently vertical transmission occurs naturally in endemic areas, although studies which used collars or topical insecticides to prevent transmission do not see transmission reduced below 4% in dogs(2, 12). There are reports of congenital transmission of visceral leishmaniasis in humans and during experimental Le. infantum infection of beagles (9). In spite of a possible change in primary suspected route of transmission, clinical signs and microscopic lesions of visceral leishmaniasis of U.S. Foxhounds is equivalent to that seen in dogs infected in endemic areas through sand fly transmission (10). Whether vertical transmission itself is solely responsible for the focus of disease particularly in Foxhounds, Corsicas, Spinones and Neapolitan Mastiffs in the U.S. or whether there are genetic factors predisposing particular breeds to disease has not been well investigated. In endemic areas all breeds of dogs are affected.
The genotype of Le. Infantum isolated from Foxhounds in the U.S. is MON-1. The MON-1 genotype is isolated most frequently from dogs living in the Mediterranean basin suggesting that infected dogs may have originally been brought to the U.S. from this area. A Centers of Disease Control and Prevention investigation indicated that it was most likely that these infected hounds first originated from Southern France, were then imported into Great Brittan, and further brought to the U.S. (Schantz et al, unpublished data.)
Common clinical and pathologic findings with Visceral Leishmaniasis
Physical exam findings may include depression, loss of condition particularly decreased muscle mass over shoulders, hips, and spine, with a mildly distended abdomen, serosanguineous nasal discharge, dull hair coat, splenomegaly and generalized lymphadenopathy. About one third of cases have a fever. Other clinical signs may include diarrhea, vomiting, epistaxis, melena, dry brittle hair coat and long brittle nails. Although officially categorized as a form of visceral leishmaniasis, cutaneous lesions including bilaterally symmetrical non-pruritic alopecia, hyperkeratosis, excessive epidermal scale with thickening, depigmentation, and chapping of the muzzle and footpads, occur with some regularity. Abnormal clinical pathologic values often include decreased hematocrit, thrombocytopenia, and signs of renal failure including azotemia, increased blood urea nitrogen and creatinine, hyperphosphatemia, hypermagnesemia, and proteinuria. Signs of hepatic compromise are also common including elevated alkaline phosphatase (ALP), elevated alanine transferase (ALT), and hypercholesterolemia. Other common clinical chemistry abnormalities include hyperproteinemia observed with hypergammaglobulinemia and hypoalbuminemia.
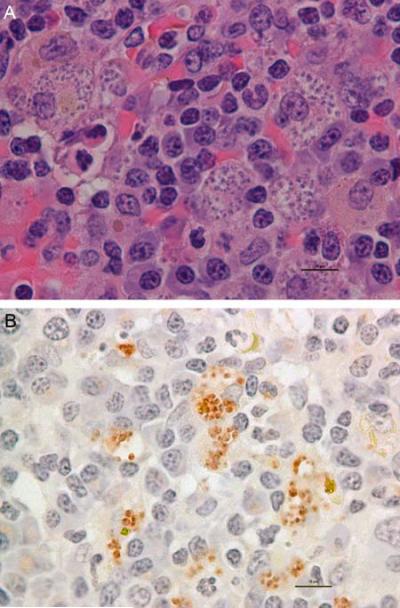
Gross pathological examination may find emaciation with minimal adipose tissue in body cavities and subcutaneous tissues. Many lymph nodes, including peripheral, mesenteric and mediastinal, are often moderately to markedly enlarged. The liver and spleen will also be diffusely enlarged. Kidneys may be moderately enlarged and diffusely pale. Impression smears obtained at necropsy from the spleen, popliteal lymph node, liver stained with Diff-Quick, often will reveal widely scattered macrophages with intracellular amastigotes consistent with Leishmania species. Cytologically within the liver, spleen, bone marrow and lymph nodes there will often also be amastigotes consistent with Leishmania species. These organisms are 1–3 μm in diameter, and have a round, deeply basophilic nucleus and a rod shaped kinetoplast (Figure 3A). These can be specifically identified as Leishmania by immunohistochemistry (Figure 3B.)
Figure 3. Photomicrograph of numerous Leishmania infantum amastigotes in a section of spleen from a U.S. Foxhound.
Notice multiple amastigotes within macrophages. A. 40X H&E stain, multiple amastigotes. B. Immunohistochemistry for Leishmania infantum amastigotes (red); bar = 20 μm.
Diagnosis of Visceral Leishmaniasis
In both humans and dogs, infection with Le. infantum frequently does not equate with clinical illness. The ratio of incident asymptomatic infection to incident clinical cases varies with location, vector and parasite. Ratios of 18:1 in Brazil and 50:1 in Spain have been observed in human populations (3) and is estimated to be 2:1 in high-risk U.S. Foxhounds. We suggest that a different means of transmission, as observed in U.S. Foxhounds, will also alter this ratio. At present, diagnosis and control of visceral leishmaniasis is difficult as both humans and dogs can be infected but seronegative for years (13). Various means of serology are the primary diagnostic tests used for surveillance of visceral leishmaniasis. For public health surveillance in the U.S. where this disease is not endemic in humans, testing is performed via an indirect fluorescent antibody assay (IFA) by the Centers for Disease Control and Prevention (CDC). IFA is sufficient for screening purposes, but is found to cross react with antibodies to the kinetoplastid Trypanosoma cruzi. T. cruzi infects dogs in the Southeastern U.S., thus further testing is required to determine parasite specificity unless clinical signs are much more consistent with one infection over the other; e.g. cardiomyopathy in the case of Chagas' disease. Other serologic tests are available in the U.S. for detection of canine leishmaniasis including a highly sensitive and specifc kELISA available through the Cornell University diagnostic laboratory and a K39-antigen based assay available through Heska. Positive serology in Foxhounds appears to more closely correlate with the appearance of clinical disease than incidence of infection. Reports have shown that qPCR performed by a well-regulated and stringently tested laboratory can be a more sensitive test for Le. infantum infection in dogs and can detect asymptomatic dogs and/or dogs that have yet to seroconvert (10). qPCR is available through Iowa State University and the CDC.
Immune alteration and pathogenesis of VL
Mammalian host responses which prevent progression to clinical VL has been shown to be dependent on promoting T helper-1 production-based immunity and parasiticidal activity within infected macrophages(3). A key immunological feature of late stage clinical VL in dogs is an inability to proliferate or to produce IFN-γ in response to Leishmania antigen, (14, and Petersen preliminary data). Pharmacologically-cured individuals are resistant to re-infection and mount antigen-specific IFN-γ responses in vitro, indicating that there is not an inherent defect in host CD4+ T cell responses of clinical patients once they have reached this stage. High levels of TNF-γ have been proposed to stimulate production of regulatory cytokines, specifically IL-10, as a homeostatic response to prevent further inflammation-mediated pathology. High leisonal IL-10 mRNA production is frequently found in human patients with VL (15, 16), and produced by polysymptomatic Foxhounds (Petersen preliminary data). IL-10 can be produced by many cell types including T cells, B cells and macrophages. One of the proposed mechanisms of IL-10 promotion of VL is by conditioning macrophages for parasite growth and survival vs. killing of intracellular parasites.
In our surveillance studies, we have observed repeated cases where Foxhounds do not show clinical signs of visceral leishmaniasis until there is secondary immunosuppression due to pregnancy, concomitant Lyme disease or other tick-borne illness (10). This clinical shift towards disease consistently appears upon a change from being seronegative to seropositive in these dogs. Further studies are required to determine the effects of immune alterations that lead to clinical disease in these dogs. Congenital infection secondary to vertical transmission may predispose to initial immune abnormalities, although by the time clinical signs of disease and seroconversion have appeared, evidence shows that CD4+ T cells from these dogs are able to respond normally to parasite antigen. In advanced disease it is not unusual to see immunosuppression including T-cell changes, in terms of both reduced CD4+ T cell proliferation in response to whole Le. infantum antigen or routine canine vaccines and decreased ability of these cells to produce IFN-γ in response to Le. infantum antigen.
Genetic factors related to Visceral Leishmaniasis disease susceptibility
Although several genetic polymorphisms, including alterations in TNF-γ and solute carrier family 11A1 (SLC11A1, formerly NRAMP1) allelic expression, have been indicated to predispose to disease (17, 18), causative factors of disease susceptibility in both humans and dogs, specifically those associated with heritability, remain elusive. Breed type has also been shown to alter the response to therapy, suggesting that canine breed-related genetic factors modulate disease progression and are therefore prognostically significant (19).
Numerous Foxhounds have tested positive for visceral leishmaniasis in the U.S. and infection appears to be endemic only within this breed here. If vertical transmission is indeed the primary route of transmission in these dogs, a particular genetic susceptibility is not absolutely necessary for widespread infection to occur in the Foxhound population. Both the observance of visceral leishmaniasis within specific families of Foxhounds and finding dogs that are Leishmania disease-resistant suggests that it is highly likely that particular genetic traits of dogs at least in part determine which dogs develop visceral leishmaniasis vs. remain clinically disease-free.
Treatment/Prognosis
Treatment of canine visceral leishmaniasis (CVL) is rarely curative. Prognosis for emaciated chronically infected animals is very poor and in these cases euthanasia should be considered. It is critical to advise the owner of potential zoonotic transmission of organisms from lesions to humans prior to maintaining a Leishmania-infected dog in their household, particularly if there are immunosupressed people sharing the household. The owner should be informed that the organism will never be completely eliminated (i.e. no “sterile cure”) and relapse occurs very frequently requiring retreatment. Treatment should be undertaken on an outpatient basis. Due to the chronic wasting that can occur with leishmaniasis, it is important to provide a good high-quality protein diet or a diet appropriate for renal insufficiency if this manifestation of leishmaniasis is present.
Due to difficulty obtaining certain drugs in the United States, treatment is recommended to begin with allopurinol (Zyloric®). This drug is efficacious and relatively non-toxic when used as a maintenance drug. Clinical remission is often achieved when used alone. Relapses are common when treatment ceases, complete cures are rare but survival occurs in 80% of cases over 4 years if renal insufficiency is not present when treatment is initiated. This drug is sometimes used in combination with pentavalent antimony (Glucantime), as drug resistance is seen for pentavalent antimony alone in endemic areas (France, Spain and Italy). Pentavalent antimonials are not licensed for use in the United States and can only be obtained via an investigational drug use protocol from the CDC (20, 21). The two main drugs in this class are 1) sodium stibogluconate (Pentostam®, Wellcome Foundation Ltd, U.K.), which requires daily injection and has severe side effects, and 2) meglutamine antimoniate (Glucantime ®, Pfizer/Merial, France), which has less side effects. Dosages have been listed (Table 1). Amphotericin B. in the lipid emulsion or liposomal form is relatively non-nephrotoxic and is effective against the organism, although it is not thought to be superior to allopurinol as it is still both more costly and more toxic. Renal insufficiency must be treated prior to giving antimonial drugs or amphotericin B as prognosis is dependent on renal function at the onset of treatment. Treatment efficacy is best monitored by clinical improvement and presence of organisms in biopsy or as measured by rigorously controlled qPCR. Relapses occur a few months to a year after therapy, so dogs should be rechecked at least every 2 months after the end of treatment. Prognosis for a cure is very guarded, but therapy does provide infected dogs improved quality of life.
Table 1.
First-line treatment options for canine visceral leishmaniasis.
| DRUG | DOSE (mg/kg) | ROUTE | INTERVAL (HOURS) | DURATION (MONTHS) |
|---|---|---|---|---|
| Allopurinol | 7 – 20 | PO | 8–12 | 3 – 24a |
| Amphotericin B – Fungizone® | 0.25 – 0.5b | IV | 48c | d |
| Meglumine antimoniatee – Glucantime® | 100 | IV, SC | 24 | 1 |
| Sodium stibogluconate – Pentostam® | 30 – 50 | IV, SC | 24 | 1 |
Or for rest of dog's life.
Reconstituted in 5% dextrose (do not reconstitute in electrolyte solutions which precipitate the drug) and dilute to administer; if normal renal function, dilute in 60 – 120 ml 5% dextrose given over 15 minutes; if renal compromise, dilute in 0.5 to 1 liter 5% dextrose given over 3 to 4 hrs to reduce further renal toxicity.
Or 3 times a week.
Administer until a total cumulative dose of 5 – 10 mg/kg is reached.
Not available in the U.S.
Second-line drugs, which require further clinical studies to understand their efficacy in dogs, include miltefosine (Impavido® or Miltex®) and paromomycin (Humantin ®). Paromymycin has been shown to have fewer side effects than other drugs in humans. Use of this drug has been primarily targeted to the cutaneous versions of Leishmania, less is known about its ability to remove organ-based infection. There is no effective vaccine against CVL available in the United States. A secreted parasite antigen-based vaccine has recently been licensed for use in dogs in Brazil. Sand fly vector control measures, including deltamethrin or permethrin-impregnated collars are useful to date to prevent disease (22). In many countries, due to the tie of canine infection to human disease, culling of dogs is still used as a means to prevent human disease (23, 24).
Summary
Canine of VL is endemic in the U.S. Foxhound population. Current evidence indicates that vertical transmission may be a primary route of transmission of the parasite in this population, although Lutzomyia species in the U.S. may be involved in transmission. Further study is necessary to determine the likelihood of vector-borne transmission in the U.S. There are two main diagnostic tools to characterize ongoing disease in this population; quantitative PCR to detect infection and IFA, ELISA or K39-based serology to indicate the onset or presence of clinical visceral leishmaniasis. Treatment options include allopurinol, glucantime and newer less-toxic formulations of amphotericin B, but none of these drugs lead to life-long sterile cure and recrudescence of infection is common. Due to lack of surveillance and imperfect diagnosis in the United States, this disease may be been present within at-risk canine populations prior to the more recently recognized “outbreaks” in Foxhounds.
Acknowledgments
Dr. Petersen is currently funded by AKC CHF grants 1159 and 1220 and NIH R21AI074711.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roberts LJ, Handman E, Foote SJ. Science, medicine, and the future: Leishmaniasis. Bmj. 2000;321(7264):801–4. doi: 10.1136/bmj.321.7264.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gavgani AS, Hodjati MH, Mohite H, et al. Effect of insecticide-impregnated dog collars on incidence of zoonotic visceral leishmaniasis in Iranian children: a matched-cluster randomised trial. Lancet. 2002;360(9330):374–9. doi: 10.1016/s0140-6736(02)09609-5. [DOI] [PubMed] [Google Scholar]
- 3.Chappuis F, Sundar S, Hailu A, et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5(11):873–82. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- 4.Duprey ZH, Steurer FJ, Rooney JA, et al. Canine visceral leishmaniasis, United States and Canada, 2000–2003. Emerg Infect Dis. 2006 Mar;12(3):440–6. doi: 10.3201/eid1203.050811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schantz PM, Steurer FJ, Duprey ZH, et al. Autochthonous visceral leishmaniasis in dogs in North America. J Am Vet Med Assoc. 2005 Apr 15;226(8):1316–22. doi: 10.2460/javma.2005.226.1316. [DOI] [PubMed] [Google Scholar]
- 6.Mancianti F, Gramiccia M, Gradoni L, et al. Studies on canine leishmaniasis control. 1. Evolution of infection of different clinical forms of canine leishmaniasis following antimonial treatment. Trans R Soc Trop Med Hyg. 1988;82(4):566–7. doi: 10.1016/0035-9203(88)90510-x. [DOI] [PubMed] [Google Scholar]
- 7.Travi BL, Ferro C, Cadena H, et al. Canine visceral leishmaniasis: dog infectivity to sand flies from non-endemic areas. Res Vet Sci. 2002;72(1):83–6. doi: 10.1053/rvsc.2001.0527. [DOI] [PubMed] [Google Scholar]
- 8.Gaskin AA, Schantz P, Jackson J, et al. Visceral leishmaniasis in a New York foxhound kennel. J Vet Intern Med. 2002;16(1):34–44. doi: 10.1892/0891-6640(2002)016<0034:vliany>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Rosypal AC, Troy GC, Duncan RB, et al. Utility of diagnostic tests used in diagnosis of infection in dogs experimentally inoculated with a North American isolate of Leishmania infantum infantum. J Vet Intern Med. 2005;19(6):802–9. doi: 10.1892/0891-6640(2005)19[802:uodtui]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Gibson-Corley KN, Hostetter JM, Hostetter SJ, et al. Disseminated Leishmania infantum infection in two sibling American Foxhound dogs from potential vertical transmission. Can Vet J. 2008;49:1005–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Owens SD, Oakley DA, Marryott K, et al. Transmission of visceral leishmaniasis through blood transfusions from infected English foxhounds to anemic dogs. J Am Vet Med Assoc. 2001;219(8):1076–83. doi: 10.2460/javma.2001.219.1076. [DOI] [PubMed] [Google Scholar]
- 12.Maroli M, Mizzon V, Siragusa C, et al. Evidence for an impact on the incidence of canine leishmaniasis by the mass use of deltamethrin-impregnated dog collars in southern Italy. Med Vet Entomol. 2001;15(4):358–63. doi: 10.1046/j.0269-283x.2001.00321.x. [DOI] [PubMed] [Google Scholar]
- 13.Quinnell RJ, Courtenay O, Garcez LM, et al. IgG subclass responses in a longitudinal study of canine visceral leishmaniasis. Vet Immunol Immunopathol. 2003;91(3–4):161–8. doi: 10.1016/s0165-2427(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 14.Sacks DL, Lal SL, Shrivastava SN, et al. An analysis of T cell responsiveness in Indian kala-azar. J Immunol. 1987 Feb 1;138(3):908–13. [PubMed] [Google Scholar]
- 15.Nylen S, Maurya R, Eidsmo L, et al. Splenic accumulation of IL-10 mRNA in T cells distinct from CD4+CD25+ (Foxp3) regulatory T cells in human visceral leishmaniasis. J Exp Med. 2007;204(4):805–17. doi: 10.1084/jem.20061141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nylen S, Sacks D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol. 2007;28(9):378–84. doi: 10.1016/j.it.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Blackwell JM, Mohamed HS, Ibrahim ME. Genetics and visceral leishmaniasis in the Sudan: seeking a link. Trends Parasitol. 2004;20(6):268–74. doi: 10.1016/j.pt.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Karplus TM, Jeronimo SM, Chang H, et al. Association between the tumor necrosis factor locus and the clinical outcome of Leishmania chagasi infection. Infect Immun. 2002;70(12):6919–25. doi: 10.1128/IAI.70.12.6919-6925.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Modiano JF, Breen M, Burnett RC, et al. Distinct B-cell and T-cell lymphoproliferative disease prevalence among dog breeds indicates heritable risk. Cancer Res. 2005;65(13):5654–61. doi: 10.1158/0008-5472.CAN-04-4613. [DOI] [PubMed] [Google Scholar]
- 20.Miro G, Cardoso L, Pennisi MG, Oliva G, Baneth G. Canine leishmaniosis--new concepts and insights on an expanding zoonosis: part two. Trends Parasitol. 2008;24(8):371–7. doi: 10.1016/j.pt.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19(1):111–26. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foglia Manzillo V, Oliva G, Pagano A, et al. Deltamethrin-impregnated collars for the control of canine leishmaniasis: Evaluation of the protective effect and influence on the clinical outcome of Leishmania infection in kennelled stray dogs. Vet Parasitol. 2006;142(1–2):142–5. doi: 10.1016/j.vetpar.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 23.Ashford DA, David JR, Freire M, et al. Studies on control of visceral leishmaniasis: impact of dog control on canine and human visceral leishmaniasis in Jacobina, Bahia, Brazil. Am J Trop Med Hyg. 1998;59(1):53–7. doi: 10.4269/ajtmh.1998.59.53. [DOI] [PubMed] [Google Scholar]
- 24.Moreira ED, Jr., Mendes de Souza VM, Sreenivasan M, et al. Assessment of an optimized dog-culling program in the dynamics of canine Leishmania transmission. Vet Parasitol. 2004;122(4):245–52. doi: 10.1016/j.vetpar.2004.05.019. [DOI] [PubMed] [Google Scholar]