Abstract
Nine dihydroartemisinin acetal dimers (6–14) with diversely functionalized linker units were synthesized and tested for in vitro antiprotozoal, anticancer and antimicrobial activity. Compounds 6, 7 and 11 [IC50: 3.0–6.7 nM (D6) and 4.2–5.9 nM (W2)] were appreciably more active than artemisinin (1) [IC50: 32.9 nM (D6) and 42.5 nM (W2)] against the chloroquine-sensitive (D6) and chloroquine-resistant (W2) strains of the malaria parasite, Plasmodium falciparum. Compounds 10, 13 and 14 displayed enhanced anticancer activity in a number of cell lines compared to the control drug, doxorubicin. The antifungal activity of 7 and 12 against Cryptococcus neoformans (IC50: 0.16 and 0.55 μM, respectively) was also higher compared to the control drug, amphotericin B. The antileishmanial and antibacterial activities were marginal. A number of dihydroartemisinin acetal monomers (15–17) and a trimer (18) were isolated as byproducts from the dimer synthesis and were also tested for biological activity.
Keywords: Artemisia annua, Artemisinin, Dihydroartemisinin, Acetal monomers, dimers and trimers, Antimalarial activity, Antileishmanial activity, Anticancer activity, Antifungal activity, Antibacterial activity
1. Introduction
Malaria, a devastating infectious disease caused by highly adaptable protozoan parasites of the genus Plasmodium, has impacted on humans for more than 4,000 years, causing illness and an estimated 1.5–2.5 million deaths each year.1 Malaria is endemic throughout the tropics, especially in sub-Saharan Africa and the developing world, threatening about 40% of the world’s population. Although four Plasmodium parasite species can infect humans, P. falciparum causes the majority of illnesses and deaths. Severe malaria, defined as acute malaria with major signs of organ dysfunction or high levels of parasitemia, predominantly affects children and pregnant women.2
Chemotherapy is still at the forefront in the fight against malaria due to the unavailability of effective vaccines.3 Numerous drugs have been developed for the treatment of uncomplicated malaria, e.g., mefloquine, primaquine, quinidine, proguanil, atovaquone and pyrimethamine. In areas where malaria is endemic, control is limited by drug resistance, cytotoxicity, cost and availability of new drugs. The persistence, and in some cases, resurgence, of malaria, especially in the developing world, is almost exclusively due to multiple drug resistance caused by monotherapy drug treatment with traditional antimalarials.4 Chloroquine resistance has become unequivocal in P. falciparum regions. The WHO recommends the use of combination therapies for the treatment of uncomplicated P. falciparum malaria to delay or prevent drug resistance.
The emergence of artemisinin (1), derived from the Chinese herb Qing Hao (Artemisia annua L., family: Asteraceae), as a fast-acting effective antimalarial agent resulted in the development of numerous derivatives as well as artemisinin-based combination therapies (ACTs) to prevent parasite resistance.5–6 The advantages of artemisinins over conventional antimalarial drugs include killing parasites more rapidly, toxicity at nanomolar concentrations, short fever clearance time (32 h versus 2–3 days for conventional antimalarials), activity against the sexual and asexual parasite stages, rapid action against all erythrocytic stages of parasite resulting in decreased transmission, limited resistance, safer and simpler oral and parenteral administration, no significant side effects and efficacy for uncomplicated and severe malaria. Artemisinins have, however, short half-lives (1–4 h), resulting in recrudescence of infecting parasites and recurrent illness within days to weeks when used as a monotherapy over a standard three day course.5–7 Artemisinin monotherapy was therefore banned by the WHO in 2006, while ACT was adopted as the suggested first-line treatment for uncomplicated P. falciparum malaria in all endemic areas. Intravenous artesunate (dihydroartemisinin hemisuccinate) became the treatment of choice for severe malaria, except for children in Africa.8 ACT in conjunction with a long acting agent is a highly efficacious first-line therapy used in most countries where malaria is endemic. Disadvantages of ACTs are higher cost and a shorter shelf-life compared to traditional antimalarial drugs.5,9 There is currently no ACT available in the United States.2a
The mechanism(s) of action of artemisinins have not been fully elucidated, with parasite specific and non-specific mechanisms hypothesized. The former includes interference with sarcoplasmic/endoplasmic reticulum Ca2+-ATPases (SERCA) through homolytic cleavage of the endoperoxide bond, forming free radicals, and subsequent alkylation of heme and Plasmodium-specific proteins. Non-specific mechanisms include iron mediated production of reactive species via a reductive scission or an open peroxide model.10
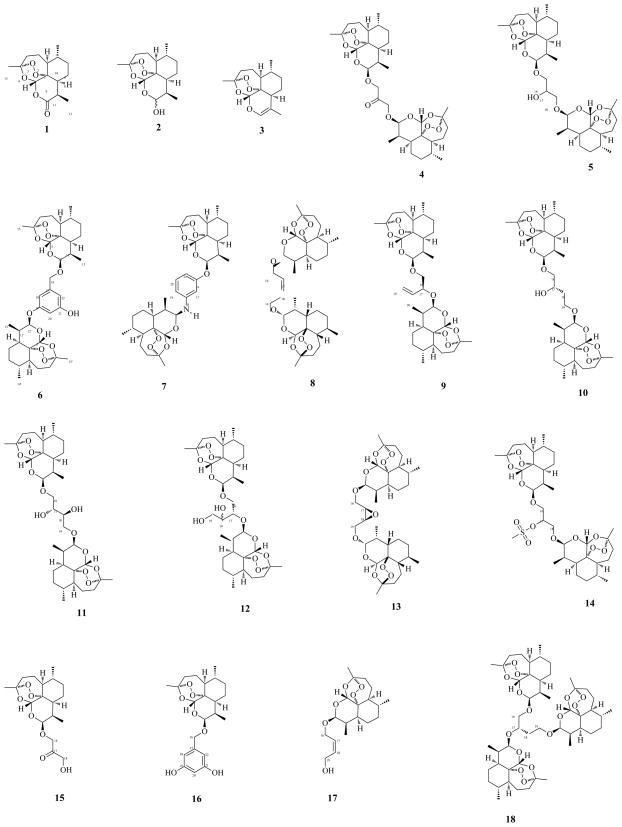
Recent reports of artemisinin-resistant malaria warrants the development of new artemisinin based drugs that can be used as ACTs.11 Artemisinin dimers are known to possess not only significant antimalarial activity, but also remarkable cytotoxicity against tumor cells, with inhibitory activity of artemisinins in the nano- to micromolar range.12 This manuscript reports the synthesis (Scheme 1, Table 1) of nine dihydroartemisinin acetal dimers (6–14) with diversely functionalized linker units (Fig. 1). A number of monomers (15–17) and a trimer (18) were isolated as byproducts from the dimer synthesis. The antimalarial, antileishmanial, anticancer (Tables 2–4), antifungal and antibacterial activities of the artemisinins are also presented.
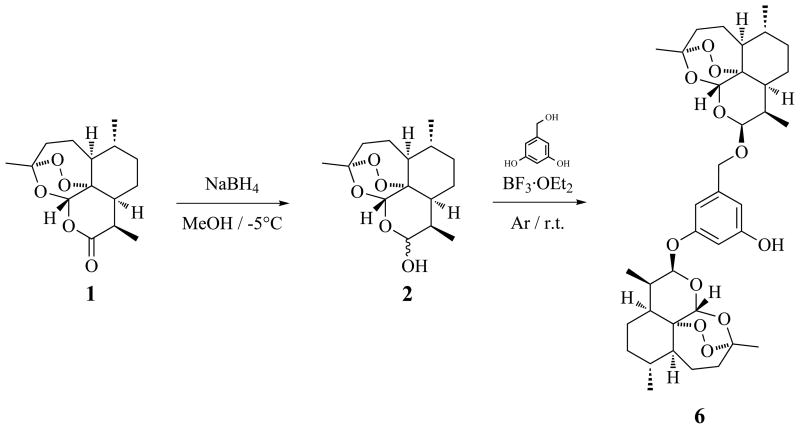
Scheme 1.
Synthesis of 6.
Table 1.
Reaction details for synthesis of 6–10
| Compound | DHA (2) |
Linker |
Ether |
BF3·OEt2 |
MSa |
Crudeb |
Product |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg | mmol | eq. | mg | mmol | eq. | mL | μL | g | mg | mg | % | |
| 6 | 360 | 1.27 | 2.11 | 84.0 | 0.60 | 1 | 120 | 400 | 1.7 | 330 | 28.0 | 6.9 |
| 7 | 510 | 1.79 | 18.82 | 10.4 | 0.10 | 1 | 75 | 700 | - | 410 | 44.0 | 71.9 |
| 8 | 402 | 1.41 | 2.22 | 56.2 | 0.64 | 1 | 75 | 220 | - | 335 | 88.0 | 22.2 |
| 9 | 480 | 1.69 | 3.10 | 48.0 | 0.54 | 1 | 50 | 410 | - | 416 | 135.0 | 39.9 |
| 10 | 540 | 1.90 | 2.08 | 97.0 | 0.91 | 1 | 60 | 450 | - | 575 | 55.0 | 9.4 |
MS: molecular sieve.
Crude: crude reaction mixture after workup.
Figure 1.
Artemisinin (1), dihydroartemisinin (DHA) (2), anhydrodihydroartemisinin (3), DHA acetal dimers 4–14, monomers 15–17 and trimer 18.
Table 2.
Antimalarial and cytotoxic activity of 1–18
|
Plasmodium falciparum |
Cytotoxicity |
||||
|---|---|---|---|---|---|
| Compound | D6 clone |
W2 clone |
Vero |
||
| IC50 (nM) | S.I. | IC50 (nM) | S.I. | TC50 (nM) | |
| 1 | 32.9 | > 51 | 42.5 | > 40 | > 1,686 |
| 2 | 14.8 | 95 | 14.1 | 100 | 1,407 |
| 3 | 15.8 | > 113 | 25.5 | > 70 | > 1,787 |
| 4 | 5.3 | 49 | 5.8 | 44 | 257 |
| 5 | 11.2 | 34 | 3.8 | 100 | 384 |
| 6 | 3.0 | > 2,380 | 4.2 | > 1,700 | > 7,075 |
| 7 | 5.8 | > 1,287 | 5.9 | > 1,253 | > 7,417 |
| 8 | 6.3 | 113 | 6.1 | 116 | 709 |
| 9 | 24.2 | 32 | 25.8 | 30 | 773 |
| 10 | 43.8 | > 170 | 45.4 | > 164 | > 7,452 |
| 11 | 6.7 | > 1,082 | 5.2 | > 1,400 | > 7,270 |
| 12 | 11.7 | 71 | 14.0 | 59 | 825 |
| 13 | 45.5 | > 164 | 47.1 | > 159 | > 7,475 |
| 14 | 11.8 | 542 | 11.8 | 542 | 6,403 |
| 15 | 7.9 | > 1,700 | 7.0 | > 1,904 | > 13,355 |
| 16 | 20.9 | > 560 | 20.7 | > 567 | > 11,711 |
| 17 | 6.2 | > 2,164 | 7.6 | > 1,763 | > 13,430 |
| 18 | 298.3 | 18 | 243.1 | 22 | 5,259 |
| chloroquinea | 41.6 | > 357.9 | 378.3 | > 39.3 | > 14,881 |
IC50: concentration causing 50% growth inhibition. TC50: concentration toxic to 50% of the cells. S.I.: selectivity index [TC50 (Vero)/IC50 (P. falciparum)].
Control.
Table 4.
Anticancer and cytotoxic activity of 4–18
| Cancer cell lines |
Noncancerous cell lines |
|||||
|---|---|---|---|---|---|---|
| Compound | SK-MEL | KB | BT-549 | SK-OV-3 | Vero | LLC-PK11 |
| IC50(μM) | TC50(μM) | |||||
| 4 | 0.56 | 0.24 | 0.24 | NA | > 8.03 | 0.10 |
| 5 | 8.00 | 0.26 | 0.48 | NA | 5.60 | 0.15 |
| 6 | NA | NA | NA | NA | NA | NA |
| 7 | NA | NA | NA | NA | NA | NA |
| 8 | NA | 0.32 | 1.53 | NA | 0.18 | NC |
| 9 | 12.93 | 0.27 | 2.58 | NA | 0.43 | > 16.11 |
| 10 | 0.24 | 0.16 | 0.08 | NA | > 1.57 | 0.10 |
| 11 | NA | NA | NA | NA | NA | NA |
| 12 | NA | NA | NA | NA | NA | NA |
| 13 | 0.22 | 0.47 | 0.30 | NA | > 1.57 | 0.68 |
| 14 | NA | 0.28 | NA | NA | 2.13 | NC |
| 15 | NA | NA | NA | NA | NA | 20.76 |
| 16 | NA | NA | NA | NA | NA | 2.46 |
| 17 | NA | NA | NA | NA | NA | NA |
| 18 | 0.24 | 0.11 | 0.06 | NA | > 1.10 | 0.07 |
| doxorubicina | 1.73 | 2.04 | 1.96 | 2.04 | NC | 1.29 |
| paclitaxela | 4.10 | 0.02 | 0.02 | 0.53 | 0.53 | 4.39 |
IC50: concentration causing 50% growth inhibition. TC50: concentration toxic to 50% of the cells. SK-MEL: human malignant melanoma. KB: human epidermal carcinoma. BT-549: human breast carcinoma (ductal). SK-OV-3: human ovary carcinoma. Vero: African monkey kidney fibroblast. LLC-PK11: pig kidney epithelial. NA: not active. NC: not cytotoxic.
Control.
2. Results and discussion
2.1. Chemistry
Artemisinin (1) was obtained commercially.13 Dihydroartemisinin (DHA) (2) was prepared by sodium borohydride (NaBH4) reduction of 1 (Scheme 1).14 Anhydrodihydroartemisinin (3)15 was isolated during the purification of 4.12b Dimers 4–5 were prepared and purified as previously described.12b,16 Dimers 6–10 (Fig. 1) were synthesized by reacting DHA (2) with 3,5-dihydroxybenzyl alcohol, 3-aminophenol, cis-2-butene-1,4-diol, (S)-(−)-1-butene-3,4-diol and (S)-(−)-1,2,4-butanetriol, respectively, under mild acidic conditions using borontrifluoride etherate (BF3·OEt2) as a catalyst in dry ether (Scheme 1, Table 1). The reaction mixtures were chromatographed to isolate 6–10, as well as monomers 15 (byproduct from 412b), 16 (byproduct from 6), 17 (byproduct from 817) and trimer 18 (byproduct from 10). The vicinal diols 11 and 12 were prepared by osmium tetroxide syn-hydroxylation of 8 and 9, respectively. Prilezhaev stereospecific cis-epoxidation of 8 with m-chloroperoxybenzoic acid (m-CPBA) yielded 13, while methanesulfonyl chloride sulfonation of 512b yielded 14.
2.2. Biological activity
2.2.1. Antimalarial activity
The in vitro antimalarial activity of 1–18 against chloroquine-sensitive (D6) and chloroquine-resistant (W2) clones of P. falciparum was evaluated based on the determination of plasmodial LDH activity.18 In order to determine the selectivity index (S.I.) of antimalarial activity, cytotoxicity of 1–18 was also determined against mammalian Vero cells (Table 2).19 S.I. is calculated as the ratio of TC50 cytotoxicity values and IC50 antimalarial activity values, and measures the therapeutic index of the compound under investigation to malaria parasites in comparison to its toxicity to the host cells (if there is any). A S.I. ≥ 10 is generally considered significant, indicating that antimalarial activity is not due to cytotoxicity.20 The synthesized analogs 6–17 exhibited strong in vitro antimalarial activities, with IC50 values in the nanomolar range (3.0–45.5 nM for D6 clone and 4.2–47.1 nM for W2 clone) (Table 2). Dimers 6, 7, 8 and 11, and monomers 15 and 17 displayed excellent activity against both clones (IC50 < 10 nM), while trimer 18 displayed weak activity compared to the monomers and dimers. The S.I. for all compounds was > 10, indicating that there is at least a 10 fold or higher difference in the effective dose for antimalarial activity and cytotoxicity; however, no significant difference was observed in the antimalarial efficacy towards D6 and W2 clones (Table 2). The activities of monomers 15 and 17 were comparable to their dimer analogs 4 and 8; however, monomer 16 was 5–6 times less active than the corresponding dimer 6. Also, dimer 8 was ca. 4 times more active than its structural isomer 9. Dihydroxylation of 8 and 9 yielded 11 and 12, respectively, with 8 and 11 having similar activity, while 12 was twice as active as 9. Dimer 5 was appreciably more active than its methylene homologue, dimer 10; however, the hydroxy-analog of 10, namely 11, had activity comparable to 5. These results, taken together with previously published data,12b indicate that increasing the chain length between two DHA units decreases antimalarial activity, while hydroxylation of the linker-chain increases the activity.
2.2.2. Antileishmanial activity
The three major forms of human leishmaniasis are normally differentiated as cutaneous, mucocutaneous and visceral, with the latter being potentially lethal. Leishmaniasis is caused by various species of the protozoan parasite Leishmania, which is transmitted by female sandflies.21 Artemisinin (1) has been shown to have promising antileishmanial activity that is mediated by induction of apoptosis and therefore warrants further study as a therapeutic option for the treatment of leishmaniasis.12b,22 Compounds 1–18 were tested against the protozoan parasite Leishmania donovani, using pentamidine and amphotericin B as drug controls (Table 3).18 The in vitro antileishmanial activity of 6, 10–13, 16 and 18 was in the low micromolar range (IC50: 2.5 6.4 μM; IC90: 9.4 47.1 μM), with 10 possessing excellent IC50 (2.5 μM) and IC90 (9.4 μM) values. Dimers 10 and 13, which showed relatively weak antimalarial activity (Table 2), were the most active compounds in the antileishmanial assay, with IC50 values comparable to the control drug, pentamidine.
Table 3.
Antileishmanial activity of 1–18
| Compound | IC50 (μM) | IC90 (μM) |
|---|---|---|
| 1 | 40.7 | NA |
| 2 | 87.9 | NA |
| 3 | 30.0 | NA |
| 4 | 7.4 | 38.5 |
| 5 | 12.8 | 47.9 |
| 6 | 5.9 | 32.7 |
| 7 | 24.9 | 62.3 |
| 8 | NA | NA |
| 9 | 20.9 | 62.8 |
| 10 | 2.5 | 9.4 |
| 11 | 6.0 | 22.9 |
| 12 | 6.4 | 29.7 |
| 13 | 3.9 | 47.1 |
| 14 | 22.8 | 55.5 |
| 15 | 25.8 | 112.2 |
| 16 | 5.7 | 24.6 |
| 17 | 42.3 | NA |
| 18 | 5.0 | 44.2 |
| pentamidinea | 2.8 | 6.4 |
| amphotericin Ba | 0.2 | 0.4 |
IC50: concentration causing 50% growth inhibition. IC90: concentration causing 90% growth inhibition.
NA: not active.
Control.
2.2.3. Anticancer activity
Artemisinins have shown promising anticancer properties in various cell lines and animal models, displaying activity against a variety of unrelated tumor cells lines, e.g., colon, breast, lung, leukemia and pancreatic cancer.23 Artemisinins cause decreased proliferation, apoptosis-induction, angiogenesis-inhibition and increased levels of oxidative stress to cancer cells.23 The selectivity of artemisinins towards cancer cells coupled with its non-cytotoxicity towards normal cells makes it an ideal candidate for targeted delivery via, e.g., the transferrin receptor mechanism, since artemisinins only become toxic after reacting with iron.23 The in vitro cytotoxicity of 4–18 was determined against four human solid tumor (SK-MEL, KB, BT-549 and SK-OV-3) and two noncancerous mammalian cell lines (Vero and LLC-PK11) (Table 4).18–19 The anti-cell proliferative activity of 8–10, 13–14 and 18 was significantly enhanced compared to the control drug, doxorubicin, with 14 showing selectivity towards epidermal carcinoma (KB). The observation that 10, 13 and 18 exhibited relatively weak antimalarial activity, strong antileishmanial activity and higher toxicity to all six cell lines indicates that the observed antileishmanial activity for these compounds could be related to their cytotoxic effect, while potential antimalarial activity of the other analogs is more specific.
The antitumor activity of 6 was evaluated against the National Cancer Institute’s 60 cultured human tumor cell lines (leukemia, non-small cell lung cancer, colon cancer, central nervous system cancer, melanoma, ovarian cancer, renal cancer, prostate cancer and breast cancer).24 The cytostatic activity of 6 was evaluated at five concentrations (100, 10, 1.0, 0.1 and 0.01 μM) via the sulforhodamine B (SRB) protein assay25 to estimate cell growth. Anticancer activity, inferred from dose-response curves, is expressed through GI50 (drug concentration resulting in a 50% reduction in the net protein increase), TGI (drug concentration of total growth inhibition) and LC50 (concentration of drug resulting in a 50% reduction in the measured protein at the end of the drug treatment as compared to that at the beginning) values for each cell line. The mean value for all tested cancer cell lines [MG_MID (mean graph midpoint)] was also calculated. Dimer 6 displayed MG_MID GI50, TGI and LC50 values of 24.5 nM, 2.82 μM and 15.1 μM, respectively.
2.2.4. Antifungal activity
The in vitro antifungal activity of 1–18 indicated selective activity against C. neoformans, with 7, 12 and 17 displaying enhanced activity compared to the control drug, amphotericin B, confirming previous reports (See Supplementary data).26
2.2.5. Antibacterial activity
The in vitro antibacterial assay of 1–18 showed almost complete inactivity to a panel of bacteria (See Supplementary data). A cursory electronic literature search did not reveal any reports of significant antibacterial activity for artemisinins.
3. Conclusion
The remarkable chemistry and biological activity of artemisinin (1) and its derivatives, especially towards malaria and cancerous tumors, have been demonstrated in numerous reports, including the current communication. However, the disconcerting accounts of parasite resistance to artemisinins indicate that this valuable natural product might follow the same fate as numerous other drugs, i.e., worldwide clinical resistance. Careful management of the current artemisinins and newly developed derivatives, such as the dimers reported herein, by health officials is critical in ensuring the longevity of these drugs in the global fight against disease. The excellent in vitro antimalarial and anticancer activity of the selected analogs presented in this study warrants further study as potential drug leads through in vivo assays and pharmacokinetic and pharmacodynamic analysis.
4. Experimental
4.1. General experimental conditions
All reactions were carried out in oven dried glassware. Diethyl ether (anhydrous) was dried over molecular sieves prior to use. All chemicals were purchased from Sigma-Aldrich or Acros Organics and used without further purification. Flash column chromatography was conducted with silica gel (particle size 230–400 mesh; SiliCycle). Analytical thin-layer chromatography (TLC) was performed with silica gel 60 F254 plates (250 μm thickness; SiliCycle) using hexanes/EtOAc mixtures as solvent systems. Visualization was accomplished by spraying with p-anisaldehyde spray reagent followed by heating with a hot-air gun. IR spectra were obtained using an AATI Mattson Genesis Series FTIR. Optical rotations were recorded at ambient temperature using a Rudolph Research Analytical Autopol IV automatic polarimeter. 1D and 2D NMR spectra were obtained on Varian AS400 and Bruker DRX 400 spectrometers at 400 MHz (1H) and 100 MHz (13C) using the solvent peak as internal standard. The spectra were recorded in CDCl3 and pyridine-d5. The following abbreviations are used for NMR multiplicities: singlet (s), broad singlet (br s), doublet (d), broad doublet (br d), doublet of doublet (dd), triplet (t), broad triplet (br t) and multiplet (m). Chemical shifts (δ) are reported in ppm and coupling constants (J) in Hertz. HRESIFTMS were obtained on an Agilent Series 1100 SL mass spectrometer.
4.2. Synthesis
See Supplementary data for IUPAC nomenclature of 1–18.
4.2.1. Artemisinin (1), DHA (2), anhydrodihydroartemisinin (3) and dimers 4–5
Artemisinin (1) was obtained commercially.13 DHA (2) was prepared by NaBH4 reduction of 1 (Scheme 1).14 Anhydrodihydroartemisinin (3) was isolated during the purification of 4.15 Dimers 4–5 were prepared and purified as previously described.12b,16
4.2.2. Dimers 6–10
To a solution of DHA (2) (360 mg, 1.27 mmol, 2.11 eq.) in dry ether (120 mL) and molecular sieves (1.7 g) was added 3,5-dihydroxybenzyl alcohol (84 mg, 0.60 mmol, 1 eq.) and BF3·OEt2 (400 μL) (Scheme 1, Table 1).12b The reaction mixture was stirred (2 h, r.t., Ar) and quenched with 2% aqueous NaHCO3, followed by addition and extraction with ether. The combined organic layer was washed with water, dried over anhydrous Na2SO4 and concentrated under reduced pressure to yield a crude product (330 mg) which was purified by column chromatography (hexanes/EtOAc, 1:0 to 1:1, stepwise) to yield 6 (28 mg, 6.9% yield).
Dimers 7–10 were similarly prepared as described for 6 using DHA (2) and 3-aminophenol, cis-2-butene-1,4-diol, (S)-(−)-1-butene-3,4-diol and (S)-(−)-1,2,4-butanetriol, respectively, as linkers (Table 1). Molecular sieves were, however, not utilized in these reactions.
4.2.3. Dimers 11–12
Dimer 8 (30 mg, 48.3 μmol, 1 eq.) was dissolved in dry pyridine (1 mL), osmium tetroxide (47 mg, 184.9 μmol, 3.83 eq.) added and the mixture stirred (105 min., r.t.). The reaction was subsequently quenched by addition of 10% aqueous Na2S2O5 and stirring (30 min), followed by addition and extraction with CH2Cl2. The organic layer was sequentially washed with 4% HCl and 2% NaHCO3, dried over anhydrous Na2SO4 and concentrated under high vacuum to yield 11 (30 mg, 94.8% yield). Dimer 9 (42 mg, 67.7 μmol, 1 eq.) was similarly oxidized (osmium tetroxide, 60 mg, 236.0 μmol, 3.49 eq.), yielding 12 (44 mg, 99.3 % yield).
4.2.4. Dimer 13
Dimer 8 (22 mg, 35.4 μmol, 1 eq.), dissolved in dry CH2Cl2 (2 mL), was epoxidized with m-CPBA acid (15 mg, 86.9 μmol, 2.45 eq.) (500 min., r.t.). The reaction mixture was quenched with 2% aqueous NaHSO3, followed by addition and extraction with CH2Cl2. The crude product was purified via preparative TLC (hexanes/EtOAc, 75:25) to afford 13 (15 mg, 66.5% yield).
4.2.5. Dimer 14
Dimer 5 (24 mg, 38.4 μmol, 1 eq.), dissolved in dry pyridine (2 mL), was sulfonated with methanesulfonyl chloride (31 mg, 270.6 μmol, 7.04 eq.) (180 min., 0–6°C, N2). The reaction mixture was quenched by the addition of water, followed by addition and extraction with CH2Cl2. The organic layer was sequentially washed with 4% HCl and distilled water, dried over anhydrous Na2SO4 and concentrated under high vacuum to yield 14 (21 mg, 77.8% yield).
4.2.6. Monomers 15–17 and trimer 18
Monomers 15 (80 mg), 16 (99 mg) and 17 (46 mg), and trimer 18 (155 mg) were isolated during the purification of 4,12b,16 6, 8 and 10, respectively.
4.3. Spectroscopic and physical data for 6–18
NMR spectroscopic data are given in Table 5 (1H) and Table 6 (13C), and HRESIFTMS data are given in Table 7.
Table 5.
1H NMR data for 5 and 6–18 (δH in ppm, J values in Hz)
| Position | 5a | 6a | 7a | 8a | 9a | 10a | 11a | 12a | 13a | 14b | 15a | 16b | 17a | 18a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5/5′ | 5.35 (s) | 5.45 (s) 5.40 (s) |
5.49 (s) | 5.40 (s) | 5.36 (s) 5.41 (s) |
5.37 (s) 5.43 (s) |
5.8 (s) 5.9 (s) |
5.44 (s) 5.43 (s) |
5.39 (s) | 5.65 (s) 5.72 (s) |
5.42 (s) | 5.69 (s) | 5.35 (s) | 5.33 (s) 5.40 (s) |
| 2.64 (m) | ||||||||||||||
| 11/11′ | 2.61 (m) | 2.76 (m) | 2.78 (m) | 2.54 (br s) | 2.58 (m) | 2.60 (m) | 2.65 (m) | 2.66 (m) | 2.62 (br s) | 2.78 (br s) | 2.70 (m) | 2.81 (br m) | 2.54 (br s) | 2.62 (m) |
| 12/12′ | 4.773 (d, 3.8) 4.766 (d, 3.8) |
4.87 (d, 3.6) 5.42 (d, 3.2) |
5.45 (d, 4.0) 6.48 (d, 1.2) |
4.80 (d, 3.2) | 4.74 (d, 2.8) 4.84 (d, 3.6) |
4.78 (d, 3.2) 4.76 (d, 3.2) |
4.80 (d, 3.6) 4.82 (d, 3.6) |
4.85 (d, 3.2) 4.94 (d, 3.2) |
4.77 (d, 3.2) 4.84 (d, 3.2) |
4.92 (d, 2.8) 4.98 (d, 3.2) |
4.83 (d, 3.6) | 5.06 (br s) | 4.80 (d, 3.6) | 4.72 (d, 3.2) 4.79 (d, 2.8) 4.81 (d, 3.2) |
| 13/13′ | 0.88 (d,7.4) | 0.97 (d, 7.6) 0.99 (d, 7.2) |
0.99 (d, 7.2) | 0.90 (d, 7.6) | 0.86 (d, 7.2) 0.83 (d, 7.2) |
0.88 (d, 7.2) | 0.89 (d, 7.2) | 0.93 (d, 7.2) | 0.91 (d, 7.2) | 0.90 (d, 7.2) | 0.94 (d, 6.0) | 0.87 (d, 7.2) | 0.85 (d, 7.6) | 0.86 (d, 7.2) 0.90 (d, 7.6) |
| 14/14′ | 0.91 (d, 6.3) | 0.93 (d, 6.0) | 0.95 (d, 6.0) | 0.94 (d, 6.4) | 0.92 (d, 6.4) | 0.91 (d, 6.0) | 0.93 (d, 6.8) | 0.95 (d, 6.4) | 0.92 (d, 6.4) | 0.85 (d, 6.8) | 0.99 (d, 6.4) | 0.80 (d, 6.0) | 0.89 (d, 6.4) | 0.925 (d, 6.4) 0.932 (d, 6.0) |
| 15/15′ | 1.38 (s) | 1.41 (s) 1.44 (s) |
1.43 (s) | 1.43 (s) | 1.39 (s) | 1.38 (s) | 1.40 (s) | 1.40 (s) 1.43 (s) |
1.40 (s) | 1.48 (s) | 1.43 (s) | 1.51 (s) | 1.37 (s) | 1.36 (s) 1.39 (s) 1.40 (s) |
| 16 | 3.47 (dd, 10.3, 4.3) 3.84 (m) |
4.40 (d, 12.4) 4.74 (d, 12.4) |
- | 4.01 (d, 10.8) 4.26 (d, 10.8) |
3.43 (m) 3.89 (m) |
3.48 (m) 3.68 (m) |
4.01 (dd, 10.8, 3.2) 3.91 (m) |
3.80 (m) | 3.96 (dd, 3.2, 2.8) 3.57 (m) |
3.76 (dd, 5.6, 10.8) 4.37 (dd, 3.6, 10.8) |
4.23 (d, 17.6) 4.42 (s) |
4.62 (d, 12.0) 5.04 (d, 13.6) |
5.59 (m) 5.75 (m) |
3.71 (dd, 3.6, 6.8) 3.86 (m) |
| 17 | 3.84 (m) | - | 6.48 (d, 1.2) | 5.59 (br s) | 4.33 (m) | 3.88 | 3.73 (m) | 3.80 (m) | 3.19 (m) | 5.30 (m) | - | - | 5.59 (m) | 3.86 (m) |
| 18 | 3.40 (dd, 9.8, 5.4) 3.84 (m) |
6.45 (s) 6.56 (s) |
- | 5.59 (br s) | 5.61 (m) | 1.72 (m) | 3.73 (m) | 3.80 (m) | 3.19 (m) | 3.80 (dd, 4.4, 11.2) 4.37 (dd, 3.6, 10.8) |
4.53 (d, 17.2) 4.42 (s) |
6.90 (s) | 5.75 (m) | 1.73 (m) 1.86 (m) |
| 19 | - | - | 6.32 (d, 7.6) | 4.01 (d, 10.8) 4.26 (d, 10.8) |
5.23 (m) | 3.52 (m) 3.93 (m) |
3.66 (dd, 11.2, 3.6) 3.56 (m) |
4.14 (br d, 8.8) 3.66 (m) |
3.83 (m) 3.57 (m) |
- | - | - | 5.59 (m) 5.75 (m) |
3.44 (m) 3.86 (m) |
| 20 | - | 6.57 (s) | 7.03 (d, 8.0) | - | - | - | - | - | - | - | - | 6.96 (s) | - | - |
| 21 | - | - | 6.50 (br d) | - | - | - | - | - | - | - | - | - | - | - |
| 22 | - | 6.45 (s) 6.56 (s) |
- | - | - | - | - | - | - | - | - | 6.90 (s) | - | - |
CDCl3.
pyridine-d5.
Table 6.
13C NMR data for 5 and 6–18 (δC in ppm)
| Position | 5a | 6a | 7a | 8a | 9a | 10a | 11a | 12a | 13a | 14b | 15a | 16b | 17a | 18a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/1′ | 52.9 | 52.6 | 52.8 | 52.7 | 52.8 | 52.7/52.8 | 52.7 | 52.65/52.71 | 52.7/52.8 | 53.00/53.03 | 52.4 | 53.0 | 52.6 | 52.8 |
| 2/2′ | 25.0 | 24.4/24.5/24.67/24.71 | 24.7/24.9 | 24.6/24.8 | 24.5/24.7/24.9 | 24.69/24.85/24.87 | 24.8 | 24.8/24.9 | 24.7/24.9 | 25.1/25.3 | 24.4/24.6 | 25.11/25.38 | 24.6/24.8 | 24.6/24.7/24.8/24.90/24.91 |
| 3/3′ | 36.9 | 36.4/36.5 | 36.6 | 36.6 | 36.62/36.64 | 36.59/36.63 | 36.6 | 36.6 | 36.6 | 37.1 | 36.3 | 37.1 | 36.5 | 36.7 |
| 4/4′ | 104.5 | 104.2/104.3 | 104.4 | 104.2 | 104.3 | 104.27/104.32 | 104.4 | 104.3/104.5 | 104.3/104.4 | 104.0 | 104.3 | 104.5 | 104.5 | 104.17/104.27/104.30 |
| 5/5′ | 88.3 | 88.1/88.3 | 88.5 | 88.1 | 88.27/88.30 | 88.07/88.12 | 88.1/88.2 | 88.3/88.2 | 88.1/88.2 | 88.5/88.6 | 88.1 | 88.5 | 88.1 | 88.11/88.15/88.17 |
| 6/6′ | 81.4 | 81.0/81.2 | 81.3 | 81.3 | 81.3/81.4 | 81.1/81.2 | 81.1/81.2 | 81.2 | 81.3 | 80.2/81.4 | 80.9 | 81.5 | 81.3 | 81.2/81.3 |
| 7/7′ | 44.8 | 44.42/44.44 | 44.7 | 44.6 | 44.59/44.64 | 44.5/44.6 | 44.4/44.5 | 44.5 | 44.55/44.56 | 44.9/45.0 | 44.1 | 45.1 | 44.5 | 44.64/44.69/44.74 |
| 8/8′ | 25.0 | 24.4/24.5/24.67/24.71 | 24.7/24.9 | 24.6/24.8 | 24.5/24.7/24.9 | 24.69/24.85/24.87 | 24.8 | 24.8/24.9 | 24.7/24.9 | 25.1/25.3 | 24.4/24.6 | 25.11/25.38 | 24.6/24.8 | 24.6/24.7/24.8/24.87/24.91 |
| 9/9′ | 35.0 | 34.66/34.69 | 34.9 | 34.8 | 34.9 | 33.9/34.2 | 34.8 | 34.8 | 34.8 | 35.1 | 34.5 | 35.0 | 34.7 | 34.8/34.9 |
| 10/10′ | 37.8 | 37.41/37.46 | 37.6 | 37.5 | 37.56/37.64 | 37.66/37.67 | 37.7 | 37.6/37.7 | 37.6/37.7 | 37.5/37.7 | 37.5 | 37.6 | 37.6 | 37.65/37.69 |
| 11/11′ | 31.3 | 31.0 | 31.3 | 31.0 | 30.9/31.2 | 31.1 | 31.1 | 31.0/31.4 | 31.0/31.1 | 31.56/31.64 | 30.7 | 31.7 | 31.0 | 31.1/31.2/31.6 |
| 12/12′ | 103.2/103.1 | 100.6/101.4 | 100.6/103.8 | 101.7 | 98.9/103.0 | 102.4/102.6 | 102.8/103.0 | 103.2/103.3 | 102.4/103.0 | 102.5/103.2 | 102.7 | 101.7 | 101.6 | 102.3/102.6/103.1 |
| 13/13′ | 13.5 | 13.0/13.2 | 13.2 | 13.2 | 13.2/13.3 | 13.19/13.21 | 13.3 | 13.2/13.5 | 13.2 | 13.39/13.45 | 13.0 | 13.6 | 13.14 | 13.2/13.4/13.7 |
| 14/14′ | 20.8 | 20.36/20.39 | 20.6 | 20.6 | 20.58/20.62 | 20.50/20.53 | 20.6 | 20.5/20.6 | 20.5 | 20.77/20.81 | 20.3 | 20.7 | 20.54 | 20.49/20.53 |
| 15/15′ | 26.5 | 26.0/26.2 | 26.3 | 26.3 | 26.4 | 26.29/26.34 | 26.3 | 26.4 | 26.3 | 26.3/26.5 | 26.0 | 26.5 | 26.2 | 26.4 |
| 16 | 70.2 | 69.4 | 158.9 | 64.1 | 71.8 | 73.3 | 70.6 | 63.7 | 67.1 | 67.3/67.8 | 71.5 | 70.4 | 58.3 | 71.7 |
| 17 | 70.1 | 141.2 | 103.8 | 129.2 | 76.4 | 68.7 | 71.4 | 78.5 | 54.4 | 80.2 | 207.8 | 142.0 | 132.7 | 75.6 |
| 18 | 70.3 | 108.0/108.2 | 148.0 | 129.2 | 135.9 | 33.9 | 71.4 | 71.6 | 55.1 | 67.3/67.8 | 66.7 | 106.6 | 128.0 | 33.0 |
| 19 | - | 156.9/158.5 | 109.2 | 64.1 | 119.3 | 65.7 | 70.2 | 69.5 | 66.5 | - | - | 160.8 | 63.6 | 64.6 |
| 20 | - | 103.3 | 130.0 | - | - | - | - | - | - | - | - | 103.4 | - | - |
| 21 | - | 156.9/158.5 | 107.2 | - | - | - | - | - | - | - | - | 160.8 | - | - |
| 22 | - | 108.0/108.2 | - | - | - | - | - | - | - | - | - | 106.6 | - | - |
CDCl3.
pyridine-d5.
Table 7.
HRESIFTMS (direct injection, positive mode) data for 6–18
| Compound | Molecular formula | [M+Na]+ |
[M+K]+ |
||||
|---|---|---|---|---|---|---|---|
| Exact | Exp | Error | Exact | Exp | Error | ||
| 6 | C37H52O11 | 695.3408 | 695.3284 | 17.8 | - | - | - |
| 7 | C36H51NO9 | 664.3462 | 664.3330 | 19.9 | 680.3201 | 680.3117 | 12.3 |
| 8 | C34H52O10 | 643.3458 | 643.3391 | 10.4 | 659.3197 | 659.3090 | 16.2 |
| 9 | C34H52O10 | 643.3458 | 643.3369 | 13.8 | 659.3197 | 659.3101 | 14.6 |
| 10 | C34H54O11 | 661.3564 | 661.3624 | 9.1 | - | - | - |
| 11 | C34H54O12 | 677.3513 | 677.3413 | 14.8 | 693.3252 | 693.3115 | 19.8 |
| 12 | C34H54O12 | 677.3513 | 677.3416 | 14.3 | 693.3252 | 693.3172 | 11.5 |
| 13 | C34H52O11 | 659.3408 | 659.3630 | 33.7 | - | - | - |
| 14 | C34H54O13S | 725.3183 | 725.3140 | 5.9 | 741.2922 | 741.2840 | 11.1 |
| 15a | C18H28O7 | 355.1757 | 355.1762 | 1.5 | - | - | - |
| 16 | C22H30O7 | 429.1890 | 429.1888 | 0.5 | 445.1629 | 445.1613 | 3.6 |
| 17 | C19H30O6 | 377.1940 | 377.1913 | 7.2 | 393.1679 | 393.1680 | 0.3 |
| 18 | C49H76O15 | 927.5082 | 927.5106 | 2.6 | 943.4821 | 943.5021 | 21.2 |
Exp: experimental. Error: error in ppm.
[M−H]− data.
4.3.1. Dimer 6
White solid; Rf 0.28 (hexanes/EtOAc, 1:1); [α]D +181.5 (c 0.13, MeOH); IR (neat) νmax: 3393 (OH) cm−1.
4.3.2. Dimer 7
Yellowish solid; Rf 0.43 (hexanes/EtOAc, 7:3); [α]D +176.0 (c 0.10, MeOH); IR (neat) νmax: 3447 (NH), 3376 (NH) cm−1.
4.3.3. Dimer 8
White solid; Rf 0.59 (hexanes/EtOAc, 7:3); [α]D +160.0 (c 0.10, MeOH).
4.3.4. Dimer 9
White solid; Rf 0.67 (hexanes/EtOAc, 7:3); [α]D +131.8 (c 0.10, MeOH).
4.3.5. Dimer 10
White solid; Rf 0.29 (hexanes/EtOAc, 7:3); [α]D +134.8 (c 0.095, MeOH); IR (neat) νmax: 3503 (OH) cm−1.
4.3.6. Dimer 11
White solid; Rf 0.53 (hexanes/EtOAc, 3:7); [α]D +147.8 (c 0.10, MeOH).
4.3.7. Dimer 12
White solid; Rf 0.12 (hexanes/EtOAc, 3:7); [α]D +135.8 (c 0.10, MeOH).
4.3.8. Dimer 13
White solid; Rf 0.51 (hexanes/EtOAc, 75:25); [α]D +141.1 (c 0.095, MeOH).
4.3.9. Dimer 14
Yellowish solid; Rf 0.40 (hexanes/EtOAc, 7:3); [α]D +104.0 (c 0.10, MeOH); IR (neat) νmax: 1361 (SO2Me) cm−1.
4.3.10. Monomer 15
White solid; Rf 0.47 (hexanes/EtOAc, 1:1); [α]D +105.5 (c 0.165, MeOH); IR (neat) νmax: 3462 (OH), 1733 (C=O) cm−1.
4.3.11. Monomer 16
White solid; Rf 0.06 (hexanes/EtOAc, 1:1); [α]D +134.2 (c 0.155, MeOH); IR (neat) νmax: 3385 (OH) cm−1.
4.3.12. Monomer 17
Oil; Rf 0.18 (hexanes/EtOAc, 7:3); [α]D +125.8 (c 0.10, MeOH); IR (neat) νmax: 3482 (OH) cm−1.
4.3.13. Trimer 18
White solid; Rf 0.41 (hexanes/EtOAc, 7:3); [α]D +157.3 (c 0.15, MeOH).
4.4. Biological assays
4.4.1. In vitro antimalarial assay
The assay is based on the determination of plasmodial LDH activity.18 A suspension of red blood cells infected with D6 or W2 strains of P. falciparum [200 μL, 2% parasitemia and 2% hematocrit in RPMI 1640 medium supplemented with 10% human serum and Amikacin (60 μg/mL)] was added to a 96-well plate containing 10 μL of serially diluted test samples. The plate was placed in a modular incubation chamber flushed with N2/O2/CO2 (90:5:5) and incubated (37°C, 72 h). Parasitic LDH activity was determined by using Malstat™ reagent (Flow Inc., Portland, OR).27 The incubation mixture (20 μL) was mixed with the Malstat™ reagent (100 μL) and incubated (30 min). Nitro blue tetrazolium (NBT)/phenazine ethosulfate (PES) (20 μL, 1:1) (Sigma, St. Louis, MO) was added and the plate incubated in the dark (60 min). The reaction was stopped by the addition of 5% acetic acid (100 μL) and the plate was read at 650 nm on an EL340 BioKinetics Reader (Bio-Tek Instruments, Winooski, VT). IC50 values were computed from the dose-response curves by plotting percent growth versus test concentration. Artemisinin (1) and chloroquine were included in each assay as drug controls.
4.4.2. In vitro antileishmanial assay
The in vitro antileishmanial activity was evaluated against a culture of L. donovani promastigotes grown in RPMI 1640 medium supplemented with 10% GIBCO fetal calf serum at 26°C.18 A three-day-old culture was diluted to 5 × 105 promastigotes/mL. Drug dilutions (50–3.1 μg/mL) were prepared directly in cell suspension in a 96-well plate, followed by incubation (26°C, 48 h). Growth of leishmanial promastigotes was determined by the Alamar Blue assay (BioSource International, Camarillo, CA).28 Standard fluorescence was measured by a Fluostar Galaxy plate reader (excitation wavelength, 544 nm; emission wavelength, 590 nm). Pentamidine and amphotericin B were used as the drug controls. Percent growth was calculated and plotted against the tested concentrations in order to determine the IC50 and IC90 values.
4.4.3. Anticancer assay
The in vitro cytotoxicity was determined against a panel of cancer (SK-MEL, KB, BT-549 and SK-OV-3) and noncancerous cell lines (Vero and LLC-PK11) (American Type Culture Collection, Rockville, MD). The assay was performed in 96-well tissue culture-treated microplates.18–19 Cells (25,000 cells/well) were seeded in the wells of the plate and incubated (24 h), followed by addition of the samples and further incubation (48 h). The number of viable cells was determined using Neutral Red.29 IC50 values were determined from logarithmic graphs of growth inhibition versus concentration. Doxorubicin was used as a positive control drug, while DMSO was used as the negative (vehicle) control.
4.4.4. Antimicrobial assay
All organisms were obtained from the American Type Culture Collection (Manassas, VA) [fungi: Candida albicans (ATCC 90028), C. glabrata (ATCC 90030), C. krusei (ATCC 6258), Cryptococcus neoformans (ATCC 90113) and Aspergillus fumigatus (ATCC 90906); bacteria: Staphylococcus aureus (ATCC 29213), methicillin-resistant S. aureus (MRSa) (ATCC 43300), Escherichia coli (ATCC 35218), Pseudomonas aeruginosa (ATCC 27853) and Mycobacterium intracellulare (ATCC 23068)]. Susceptibility testing was performed for all organisms, except M. intracellulare, using modified versions of the CLSI/NCCLS methods.30 M. intracellulare susceptibility was tested using a modified Franzblau-method.31 Samples dissolved in DMSO were serially diluted in DMSO/saline (20%/0.9%) and transferred in duplicate to 96-well flat bottom microplates. Microbial inocula were prepared by correcting the OD630 of microbe suspensions in incubation broth to afford final target inocula. Controls [fungi: amphotericin B; bacteria: ciprofloxacin (ICN Biomedicals, OH)] were included in each assay. All organisms were read at 630 or 544(ex)/590(em) nm (M. intracellulare and A. fumigatus) prior to and after incubation. Percent growth was plotted versus test concentration to afford the IC50. Minimum fungicidal or bactericidal concentrations were determined by removing 5 μL from each clear well, transferring to agar, and incubating until growth was seen. The MFC/MBC is defined as the lowest test concentration that kills the organism (allows no growth on agar).
Supplementary Material
Acknowledgments
The authors sincerely thank Mr. John M. Trott, Ms. Marsha A. Wright, Rajnish Sahu, Dr. Bharathi Avula and Dr. Paulo Carvalho for their assistance in biological and analytical analysis. The NCI’s Developmental and Therapeutics Program is acknowledged for the anticancer screening of these compounds. This work was supported in part by the United States Department of Agriculture, Agricultural Research Service Specific Cooperative Agreement No. 58-6408-2-0009, and by the National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS, Grant No. AI 27094.
Footnotes
Supplementary data: Supplementary data associated with this article can be found, in the online version, at doi:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Pierce SK, Miller LH. J Immunol. 2009;182:5171. doi: 10.4049/jimmunol.0804153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Rosenthal PJ. N Engl J Med. 2008;358:1829. doi: 10.1056/NEJMct0709050. [DOI] [PubMed] [Google Scholar]; (b) White NJ. Science. 2008;320:330. doi: 10.1126/science.1155165. [DOI] [PubMed] [Google Scholar]
- 3.(a) Genton B. Expert Rev Vaccines. 2008;7:597. doi: 10.1586/14760584.7.5.597. [DOI] [PubMed] [Google Scholar]; (b) Vekemans J, Ballou WR. Expert Rev Vaccines. 2008;7:223. doi: 10.1586/14760584.7.2.223. [DOI] [PubMed] [Google Scholar]
- 4.(a) Turschner S, Efferth T. Mini-Rev Med Chem. 2009;9:206. doi: 10.2174/138955709787316074. [DOI] [PubMed] [Google Scholar]; (b) Edwards G, Biagini GA. Br J Clin Pharmacol. 2006;61:690. doi: 10.1111/j.1365-2125.2006.02674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Aweeka FT, German PI. Clin Pharmacokin. 2008;47:91. doi: 10.2165/00003088-200847020-00002. [DOI] [PubMed] [Google Scholar]; (b) Plowe CV. Clin Infect Dis. 2007;44:1075. doi: 10.1086/512743. [DOI] [PubMed] [Google Scholar]; (c) Kindermans J-M, Pilloy J, Olliaro P, Gomes M. Malar J. 2007:6. doi: 10.1186/1475-2875-6-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Efferth T. Planta Med. 2007;73:299. doi: 10.1055/s-2007-967138. [DOI] [PubMed] [Google Scholar]; (b) Namdeo AG, Mahadik KR, Kadam SS. Phcog Mag. 2006;2:106. [Google Scholar]
- 7.(a) Gomes M, Ribeiro I, Warsame M, Karunajeewa H, Petzold M. BMC Infect Dis. 2008;8:1. doi: 10.1186/1471-2334-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Rydén A-M, Kayser O. Chemistry, Biosynthesis and Biological Activity of Artemisinin and Related Natural Peroxides. In: Gupta RR, Khan MTH, editors. Bioactive Heterocycles III (Topics in Heterocyclic Chemistry) Vol. 9. Springer-Verlag; Berlin, Heidelberg: 2007. pp. 1–31. [Google Scholar]; (c) Sharma VP. Current Science. 2006;90:1323. [Google Scholar]; (d) Pasvol G. Br Med Bull. 2005;75–76:29. doi: 10.1093/bmb/ldh059. [DOI] [PubMed] [Google Scholar]
- 8.Global Malaria Programme. World Health Organization; 2006. Guidelines for the treatment of malaria. ( www.who.int/malaria/docs/TreatmentGuidelines2006.pdf) [Google Scholar]
- 9.Li Q, Weina PJ, Milhous WK. Curr Drug Ther. 2007;2:210. [Google Scholar]
- 10.(a) Turschner S, Efferth T. Mini-Rev Med Chem. 2009;9:206. doi: 10.2174/138955709787316074. [DOI] [PubMed] [Google Scholar]; (b) Lelievre J, Berry A, Benoit-Vical F. Curr Opin Investig Drugs. 2007;8:117. [PubMed] [Google Scholar]; (c) Golenser J, Waknine JH, Krugliak M, Hunt NH, Grau GE. Int J Parasitol. 2006;36:1427. doi: 10.1016/j.ijpara.2006.07.011. [DOI] [PubMed] [Google Scholar]; (c) Stockwin LH, Han B, Yu SX, Hollingshead MG, ElSohly MA, Gul W, Slade D, Galal AM, Newton DL. Int J Cancer. 2009;125:1266. doi: 10.1002/ijc.24496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Maude RJ, Pontavornpinyo W, Saralamba S, Aguas R, Yeung S, Dondorp AM, Day NPJ, White NJ, White LJ. Malar J. 2009:8. doi: 10.1186/1475-2875-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. N Engl J Med. 2008;359:2619. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 12.(a) Chadwick J, Mercer AE, Park BK, Cosstick R, O’Neill PM. Bioorg Med Chem. 2009;17:1325. doi: 10.1016/j.bmc.2008.12.017. [DOI] [PubMed] [Google Scholar]; (b) Galal AM, Gul W, Slade D, Ross SA, Feng S, Hollingshead MG, Alley MC, Kaur G, ElSohly MA. Bioorg Med Chem. 2009;17:741. doi: 10.1016/j.bmc.2008.11.050. [DOI] [PubMed] [Google Scholar]; (c) Posner GH, D’Angelo J, O’Neill PM, Mercer A. Expert Opin Ther Pat. 2006;16:1665. [Google Scholar]
- 13.Holley Pharmaceutical Company, Inc., 1440 N. Harbor Blvd., # 900, Fullerton, CA 92835, USA (www.holleypharma.com).
- 14.Lin AJ, Klayman DL, Milhous WK. J Med Chem. 1987;30:2147. doi: 10.1021/jm00394a037. [DOI] [PubMed] [Google Scholar]
- 15.Lin AJ, Lee M, Klayman DL. J Med Chem. 1989;32:1249. doi: 10.1021/jm00126a017. [DOI] [PubMed] [Google Scholar]
- 16.ElSohly, M.A.; Gul, W. WO2006002105A1, US20080275106A1, 2006.
- 17.Grellepois F, Crousse B, Bonnet-Delpon D, Begue JP. Org Lett. 2005;7:5219. doi: 10.1021/ol052056+. [DOI] [PubMed] [Google Scholar]
- 18.(a) Jain M, Khan SI, Tekwani BL, Jacob MR, Singh S, Singh PP, Jain R. Bioorg Med Chem. 2005;13:4458. doi: 10.1016/j.bmc.2005.04.034. [DOI] [PubMed] [Google Scholar]; (b) Bharate SB, Khan SI, Tekwani BL, Jacob M, Khan IA, Singh IP. Bioorg Med Chem. 2008;16:1328. doi: 10.1016/j.bmc.2007.10.055. [DOI] [PubMed] [Google Scholar]
- 19.Mustafa J, Khan SI, Ma G, Walker LA, Khan IA. Lipids. 2004;39:167. doi: 10.1007/s11745-004-1215-5. [DOI] [PubMed] [Google Scholar]
- 20.Vonthron-Senecheau C, Weniger B, Ouattara M, Bi Fezan T, Kamenan A, Lobstein A, Brun R, Anton RJ. Ethnopharmacol. 2003;87:221. doi: 10.1016/s0378-8741(03)00144-2. [DOI] [PubMed] [Google Scholar]
- 21.(a) Singh IP, Lal UR, Bodiwala HS, Mahajan RC, Bhutani KK. Recent Prog Med Plants. 2006;13:115. [Google Scholar]; (b) Maltezou HC. Recent Patents on Anti-Infective Drug Discovery. 2008;3:192. doi: 10.2174/157489108786242341. [DOI] [PubMed] [Google Scholar]
- 22.(a) Sen R, Bandyopadhyay S, Dutta A, Mandal G, Ganguly S, Saha P, Chatterjee MJ. Med Microbiol. 2007;56:1213. doi: 10.1099/jmm.0.47364-0. [DOI] [PubMed] [Google Scholar]; (b) Menon RB, Kannoth MM, Tekwani BL, Gut J, Rosenthal PJ, Avery MA. Comb Chem High Throughput Screening. 2006;9:729. doi: 10.2174/138620706779026051. [DOI] [PubMed] [Google Scholar]
- 23.Krishna S, Bustamante L, Haynes RK, Staines HM. Trends Pharmacol Sci. 2008;29:520. doi: 10.1016/j.tips.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.(a) Wang H, Klinginsmith J, Dong X, Lee AC, Guha R, Wu Y, Crippen GM, Wild DJ. Journal of Chemical Information and Modeling. 2007;47:2063. doi: 10.1021/ci700141x. [DOI] [PubMed] [Google Scholar]; (b) Huang R, Wallqvist A, Covell DG. Journal of Medicinal Chemistry. 2006;49:1964. doi: 10.1021/jm051029m. [DOI] [PubMed] [Google Scholar]; (c) Boyd MR, Paull KD. Drug Development Research. 1995;34:91. [Google Scholar]
- 25.Vichai V, Kirtikara K. Nature Protocols. 2006;1:1112. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 26.(a) Galal AM, Ross SA, Jacob M, ElSohly MA. J Nat Prod. 2005;68:1274. doi: 10.1021/np050074u. [DOI] [PubMed] [Google Scholar]; (b) Lee S. Mini-Rev Med Chem. 2007;7:411. doi: 10.2174/138955707780363837. [DOI] [PubMed] [Google Scholar]
- 27.(a) Makler MT, Hinrichs DJ. Am J Trop Med Hyg. 1993;48:205. doi: 10.4269/ajtmh.1993.48.205. [DOI] [PubMed] [Google Scholar]; (b) Nkhoma S, Molyneux M, Ward S. Am J Trop Med Hyg. 2007;76:1107. [PubMed] [Google Scholar]
- 28.(a) Ma G, Khan SI, Jacob MR, Tekwani BL, Li Z, Pasco DS, Walker LA, Khan IA. Antimicrob Agents Chemother. 2004;48:4450. doi: 10.1128/AAC.48.11.4450-4452.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mikus J, Steverding D. Parasitol Int. 2000;48:265. doi: 10.1016/s1383-5769(99)00020-3. [DOI] [PubMed] [Google Scholar]; (c) Hamid R, Rotshteyn Y, Rabadi L, Parikh R, Bullock P. Toxicol in Vitro. 2004;18:703. doi: 10.1016/j.tiv.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Borenfreund E, Babich H, Martin-Alguacil N. In Vitro Cell Dev Biol: J Tissue Culture Assoc. 1990;26:1030. doi: 10.1007/BF02624436. [DOI] [PubMed] [Google Scholar]
- 30.(a) National Committee for Clinical Laboratory Standards (NCCLS) (Wayne, Pa.): Reference method for broth dilution antifungal susceptibility testing of yeasts; Approved Standard – Second Edition. Document M27-A2, 2002, 22. (b) NCCLS: Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved Standard – Seventh Edition. Document M7-A7, 2006, 26. (c) NCCLS: Susceptibility testing of mycobacteria, nocardia, and other aerobic actinomycetes; Tentative Standard – Approved Standard. Document M24-A, 2003, 23. (d) NCCLS: Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; Approved Standard. Document M38-A, 2002, 22.
- 31.Franzblau SG, Witzig RS, McLaughlin JC, Torres P, Madico G, Hernandez A, Degnan MT, Cook MB, Quenzer VK, Ferguson RM, Gilman RH. J Clin Microbiol. 1998;36:362. doi: 10.1128/jcm.36.2.362-366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.