Abstract
Due to the risk of central nervous system infection, relatively high weight-based echinocandin dosages may be required for successful treatment of invasive candidiasis and candidemia in young infants. This open-label study assessed safety and pharmacokinetics of micafungin in 13 young infants (> 48 hours of age and < 120 days of life) with suspected candidemia or invasive candidiasis. Infants weighing ≥ 1,000 g and < 1,000 g received 7 and 10 mg/kg/day, respectively, for a minimum of 4 to 5 days. Mean baseline weight and gestational age were 2101 g and 688 g, and 30 weeks and 25 weeks, in the 7 and 10 mg/kg/day groups, respectively. Median pharmacokinetic values for the 7 and 10 mg/kg/day groups, respectively, were: AUC0–24, 258.1 and 291.2 μg•h/ml; Clss/wt, 0.45 and 0.57 ml/min/kg; Cmax, 23.3 and 24.9 μg/ml; and Vdss/wt, 341.4 and 542.8 ml/kg. No deaths or discontinuations from treatment occurred. These data suggest that micafungin dosages of 7 and 10 mg/kg/day were well tolerated and provided exposure that was demonstrated in animal model to be adequate for central nervous system coverage.
Keywords: antifungal, Candida, dosing, neonates, infants
INTRODUCTION
Disseminated candidiasis and hematogenous Candida meningoencephalitis (HCME) are leading causes of morbidity and mortality in premature infants (1–4). The incidence of candidemia in extremely low birth weight (ELBW) infants (i.e., infants < 1,000 g birth weight) ranges from 2 to 18%, with an attendant attributable mortality of 20 to 30% (3–5). Severe morbidities such as end-organ damage and neurodevelopmental impairment in ELBW infants can occur as a result of the capacity of Candida spp. to invade virtually all tissues, including the retina, brain, heart, lung, liver, spleen, and joints (1, 2).
Micafungin is a once-daily, echinocandin antifungal agent with notable in vitro activity against all clinically relevant Candida spp. (6–15). It has high specificity for Candida spp. through potent inhibition of 1,3-β-D-glucan synthase, a key enzyme in fungal cell wall synthesis (16). The pharmacokinetics (PK), safety, and efficacy of micafungin have been described in a wide range of patients, including adults and children (17–22). Micafungin is approved in the United States for adult patients and in Europe for adults and children (including neonates) for treatment of invasive candidiasis (23, 24).
An initial study in premature infants assessed the PK of single micafungin dosages of 0.75 mg/kg, 1.5 mg/kg, and 3.0 mg/kg (25). However, results from experimental models of HCME and pediatric bridging studies of micafungin therapy suggest that higher weight-based dosages are required for successful therapy in young infants (20, 26, 27). Based on an experimental model of HCME in rabbits, which appears to be a faithful mimic of human infection, an area under the concentration-time curve (AUC0–24) ≥ 166.5 μg•h/ml is considered necessary to penetrate the central nervous system (CNS) and treat HCME (26). While the in vivo-to-human bridging study demonstrated the serum pharmacokinetics of micafungin in rabbits and premature neonates are similar (28), the use of a rabbit model to identify an appropriate dosage for neonates requires an assumption that the penetration of micafungin into the various compartments of the central nervous system is comparable.
The half-life of micafungin is shorter, and weight-adjusted clearance is higher, in premature infants and younger children (age 2 to 8 years) than in older children and adults (20, 25). Although the PK properties of micafungin have been shown to be very similar across diverse populations, (28) relatively little is known about the PK characteristics of micafungin in young infants, and while the PK characteristics of an elevated dosage of micafungin (15 mg/kg) have been reported (22), the optimal dosage to achieve sufficient systemic exposure to provide adequate CNS coverage has yet to be determined. The purpose of the present study was to assess the safety, tolerability, and PK profile of repeated dosages of micafungin in young infants with suspected candidemia or invasive candidiasis.
The dosages selected for this study— 7 mg/kg/day and 10 mg/kg/day— were chosen to achieve a plasma AUC0–24 of ≥ 166.5 μg•h/ml in young infants after considering similar plasma systemic exposure (AUC0–24, 170 μg•h/ml) effected a 90% reduction in fungal burden in the cerebrum and cerebellum of rabbits with experimental HCME (26). A previous dose-ranging PK study, in which infants weighing > 1,000 g received a single dose of micafungin 0.75, 1.5, or 3.0 mg/kg, demonstrated that the micafungin clearance rate was 39 ml/h/kg (25). Using these PK data as well as those derived from a previous Phase I study in adult patients, and applying corrections based on micafungin binding to serum albumin, differences in serum albumin levels between adults and infants, and higher micafungin clearance in young infants, a dose of 7 mg/kg was recommended for infants weighing ≥ 1,000 g, and 10 mg/kg for those weighing < 1,000 g.
RESULTS
Patients
Thirteen study subjects were enrolled from 5 study sites in the US. All 13 infants received ≥ 4 days of micafungin treatment (seven infants received micafungin 7 mg/kg/day and six received 10 mg/kg/day). Mean baseline weight and gestational age were 2,101 g and 688 g, and 30 weeks and 25 weeks in the 7 mg/kg/day and 10 mg/kg/day groups, respectively (Table 1). Nearly all infants (12 of 13) had a gestational age of < 34 weeks; one infant had a gestational age of 40 weeks. One infant was 119 days old and weighed 4.5 kg, whereas the other 12 were ≥ 63 days old and weighed < 3.6 kg.
Table 1.
Patient demographics
| Micafungin treatment group |
|||
|---|---|---|---|
| Characteristic | 7 mg/kg per day ≥ 1,000 g (n=7) | 10 mg/kg per day < 1,000 g (n=6) | Overall (n=13) |
| Sex, n (%) | |||
| Male | 2 (28.6) | 4 (66.7) | 6 (46.2) |
| Female | 5 (71.4) | 2 (33.3) | 7 (53.8) |
| Race, n (%) | |||
| White | 4 (57.1) | 5 (83.3) | 9 (69.2) |
| Black or African-American | 3 (42.9) | 1 (16.7) | 4 (30.8) |
| Ethnicity, n (%) | |||
| Non-Hispanic | 4 (57.1) | 1 (16.7) | 5 (38.5) |
| Hispanic | 3 (42.9) | 5 (83.3) | 8 (61.5) |
| Age, days | |||
| Mean (SD) | 44.1 (37.4) | 7.5 (5.5) | 27.2 (32.7) |
| Median (range) | 33.0 (13–119) | 6.5 (3–18) | 18.0 (3–119) |
| Gestational age, weeks | |||
| Mean (SD) | 29.6 (5.5) | 24.7 (0.8) | 27.3 (4.7) |
| Median (range) | 28.0 (25–40) | 24.5 (24–26) | 25.0 (24–40) |
| Weight, g | |||
| Mean (SD) | 2,101 (1,360) | 687.7 (106.7) | 1,449 (1,211) |
| Median (range) | 1,430 (1,210–4,500) | 670 (540–850) | 1,210 (540–4,500) |
All infants had ≥ 1 other medical condition, and > 60% had sepsis (not otherwise specified), metabolic acidosis, or thrombocytopenia at baseline. Most patients had common conditions associated with premature birth at baseline, including anemia (92.3%), neonatal respiratory distress syndrome (92.3%), hypotension (84.6%), feeding problems (69.2%), neonatal hyperbilirubinemia (69.2%), and patent ductus arteriosus (61.5%). All infants received concomitant antibacterial agents prior to and during the study.
Both the duration and extent of micafungin exposure among the safety and PK analysis sets at the respective dose levels were similar. One patient in the 7 mg/kg/day group inadvertently received a dosage of 10 mg/kg/day and was therefore excluded from the PK analysis set. Infants who received 7 mg/kg/day had a median duration of micafungin therapy similar to those who received 10 mg/kg/day (5 days vs. 4 days). When dose was controlled by body weight, the median cumulative dosage in the 7 mg/kg/day group was similar to that of the 10 mg/kg/day group, although the former group had a slightly lower median value for the mean daily treatment dose. One infant in the 7 mg/kg/day group received micafungin for 33 days because of confirmed rather than suspected candidiasis at study entry, which was successfully treated, whereas all others received no more than six doses.
Pharmacokinetic profile of micafungin
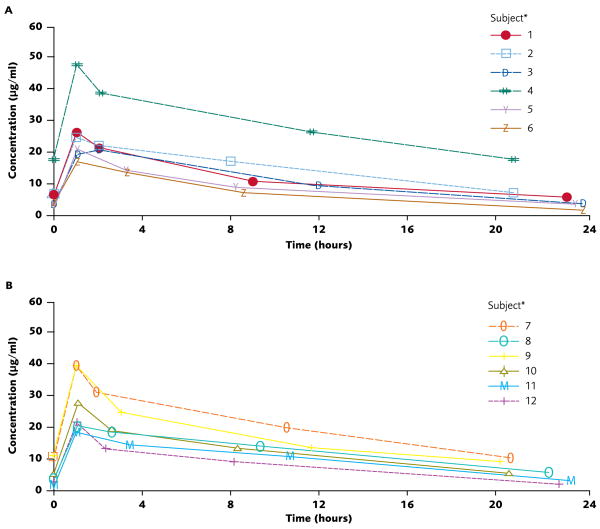
Plasma micafungin concentrations peaked immediately after the 1-hour infusion before declining over the next 23 hours. Inter-individual plasma concentration-time profiles for micafungin at each dose level were comparable (Figure 1). Systemic exposure to micafungin 7 mg/kg/day in one infant was greater than that observed in the six others of this group (subject 4 in Figure 1). This infant was atypical: age 119 days and weight 4.5 kg at the time of enrollment; and had a substantially higher AUC (643.2 μg•h/ml) than was observed for the other, smaller infants in the 7 mg/kg/day-dosage group.
Figure 1.
Individual plasma micafungin concentrations vs. time on day 4 of a repeated dosage regimen: 7 mg/kg/day in infants weighing ≥ 1,000 g (Panel A) and 10 mg/kg/day in infants weighing < 1,000 g (Panel B).
The plasma PK parameters of patients in the two micafungin-dosage groups are summarized in Table 2. All but one infant (in the 7 mg/kg/day group and with an AUC0–24 of 162.6 μg•h/ml) achieved the target systemic exposure of AUC0–24 ≥ 166.5 μg•h/ml. Median total body clearance adjusted for weight was 21% lower in infants weighing ≥ 1,000 g than in infants weighing < 1,000 g. The median micafungin elimination half-life was similar in both groups and Vdss adjusted for weight was higher in the infants weighing < 1,000 g.
Table 2.
Weight-stratified pharmacokinetic parameters of intravenous micafungin on day 4 of a repeated dosage regimen in young infants
| Parameter | Micafungin treatment group |
||
|---|---|---|---|
| 7 mg/kg per day Body weight ≥ 1,000 g | 10 mg/kg per day Body weight < 1,000 g | Overall | |
| Safety analysis set | n=6* | n=6 | n=12 |
| Cmax, μg/ml | |||
| Mean (SD) | 26.6 (11.0) | 28.1 (9.2) | 27.3 (9.7) |
| Median (range) | 23.3 (17.4–48.1) | 24.9 (19.2–40.0) | 23.4 (17.4–48.1) |
| Tmax, h | |||
| Mean (SD) | 1.2 (0.4) | 1.1 (0.04) | 1.2 (0.3) |
| Median (range) | 1.1 (1.0–2.1) | 1.1 (1.0–1.1) | 1.1 (1.0–2.1) |
| t1/2, h† | |||
| Mean (SD) | 11.4 (3.5) | 10.6 (3.2) | 11.0 (3.2) |
| Median (range) | 11.3 (6.9–15.4) | 10.4 (7.7–16.4) | 10.6 (6.9–16.4) |
| AUC0–24, μg•h/ml | |||
| Mean (SD) | 307.6 (173.7) | 308.0 (100.6) | 307.8 (135.4) |
| Median (range) | 258.1 (162.6–643.2) | 291.2 (185.3–460.5) | 275.2 (162.6–643.2) |
| CLss, ml/min | |||
| Mean (SD) | 0.9 (0.6) | 0.4 (0.1) | 0.6 (0.5) |
| Median (range) | 0.7 (0.5–2.1) | 0.4 (0.2–0.5) | 0.5 (0.2–2.1) |
| CLss/wt, ml/min/kg | |||
| Mean (SD) | 0.4 (0.2) | 0.6 (0.2) | 0.5 (0.2) |
| Median (range) | 0.4 (0.2–0.6) | 0.6 (0.4–0.8) | 0.5 (0.2–0.8) |
| Vdss, ml | |||
| Mean (SD) | 862.0 (665.7) | 351.6 (81.7) | 606.8 (524.9) |
| Median (range) | 553.9 (401.0–2,120.5) | 371.2 (227.5–463.6) | 426.3 (227.5–2,120.5) |
| Vdss/wt, ml/kg | |||
| Mean (SD) | 390.7 (128.3) | 509.5 (76.6) | 450.1 (118.3) |
| Median (range) | 341.4 (242.5–589.0) | 542.8 (357.7–559.6) | 503.2 (242.5–589.0) |
Excludes one patient in the micafungin 7 mg/kg/day group who was inadvertently given 10 mg/kg/day.
Calculated using the following two serial timepoints: 8–12 hours and 20–24 hours after infusion start.
Safety and tolerability
Consistent with the multimorbid underlying conditions of patients in this population, AEs irrespective of causality were experienced by 12/13 (92.3%). Serious AEs were experienced by four infants (30.8%); two in each treatment group. The most common AEs, which were reported in two infants each, were pulmonary edema, patent ductus arteriosus, vomiting, increased serum creatinine, renal impairment, respiratory distress, skin disorder, and vascular skin condition. Only three patients experienced AEs (increased alkaline phosphatase, infusion site phlebitis, hypokalemia, and temperature elevation) that were considered by the investigators to be possibly or probably related to micafungin. The increase in plasma alkaline phosphatase detected in one infant was classed as a serious AE. This patient was enrolled at 19 days of age with a confirmed Candida infection, a baseline weight of 3.6 kg, and a gestational age of 40 weeks. Onset of the AE was study day 17.
Changes from baseline to post-treatment in serum chemistry parameters were generally similar for patients treated with micafungin 7 mg/kg/kg and 10 mg/kg/day and changes in aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were minimal. Furthermore, there was no evidence of renal toxicity or significant changes in hematology parameters. No deaths occurred in this study and no patients withdrew prematurely from the study because of an AE.
DISCUSSION
This study evaluated the safety and PK of repeat, high-dose micafungin in two weight cohorts of critically ill infants. Given that candidemia is common, often persistent, and often fatal in the preterm infant (2, 4), an in-depth characterization of the safety and PK of micafungin is imperative in this population to avoid unnecessary adverse effects or suboptimal efficacy. It is also important to consider the need to define a dose level of micafungin sufficient to achieve adequate CNS exposure in young infants, given the propensity for Candida to invade the CNS in neonatal candidiasis. Initial studies in premature infants assessed dosages of 0.75 mg/kg, 1.5 mg/kg, and 3.0 mg/kg (25) and a subsequent study assessed an elevated dosage of 15 mg/kg (22). Further study was needed to define the optimal dose in this vulnerable patient population at risk for CNS invasion.
In this study, micafungin doses of up to 10 mg/kg were well tolerated and all infants completed the scheduled treatment duration of ≥ 4 days; furthermore, the weight-based dose regimens used in this study provided target systemic exposures that correspond to levels adequate to provide CNS penetration, as suggested by laboratory animal data (26). Following IV infusion, plasma micafungin concentrations over the 24-hour dosing interval showed only modest inter-individual variation among infants of each cohort. The differences in systemic exposure (based on Cmax and AUC0–24) between the groups were minimal; and all infants, with the exception of one infant in the 7 mg/kg/day group, attained the target plasma micafungin AUC0–24 of 166.5 μg•h/mL. This infant had lower baseline and on-treatment levels of serum albumin (17 g/l and 22 g/l, respectively) than the median group values (28 g/l and 30 g/l, respectively). Given that micafungin is highly protein bound, the relatively low serum albumin levels in this infant may have resulted in higher micafungin clearance, thus explaining the failure to attain the target systemic micafungin exposure of AUC0–24 ≥ 166.5 μg•h/mL. Micafungin AUC0–24 for this infant was 162.6 μg•h/ml whereas the median AUC0–24 in this study was 275.2 μg•hr/ml, and the highest observed AUC0–24 was 643.2 μg•hr/ml. In adults, a 150-mg dose of micafungin results in an average AUC of 166.7 μg•hr/ml with values of < 600 μg•hr/ml being well tolerated (17, 29). AUC0–24 data from the present study in preterm infants suggest 10 mg/kg micafungin dose in young infants provides a similar systemic exposure to a dose of approximately 3 to 4 mg/kg in adults (23, 29). Such relatively high micafungin plasma concentrations are imperative in young infants with candidemia due to the frequency of occurrence of meningoencephalitis (1).
As a function of body weight, micafungin clearance was higher in infants weighing < 1,000 g compared with infants weighing ≥ 1,000 g. These results are consistent with the fact that micafungin clearance in premature infants differs from clearance rates previously reported in adults, older children, and full-term infants (20, 25, 30). Although micafungin PK in infants, older children, and adolescents are dose-proportional and largely consistent with those observed in adult populations, (20, 25, 30) a reciprocal relationship has been detected between age and micafungin clearance (20). Analysis of Phase I data from a study of febrile neutropenic pediatric patients indicated that in children 2–8 years of age, micafungin clearance was 1.5–2.0 times higher than in children ≥ 9 years of age (20). In addition, findings from a single-dose, open-label study of premature infants weighing > 1,000 g who received micafungin 0.75–3 mg/kg demonstrated that mean micafungin clearance in this cohort was higher than that in older children and adults (25).
There were no instances of allergic or histamine-like reactions, infusion-related toxicity, nephrotoxicity or hepatotoxicity, and no deaths. While most of the critically ill infants in this study experienced AEs, few were considered to be related to micafungin. Moreover, one patient who was 119 days of age and weighed 4.5 kg at the time of the study, did not experience any AEs considered by the investigators to be related to micafungin therapy despite a relatively high micafungin systemic exposure (AUC=643.2 μg•h/ml). These findings are supported by safety data from a Phase I study of febrile neutropenic pediatric patients who received micafungin at dosages that provided similar levels of systemic exposure to those observed in the study described here (20).
In conclusion, the results of this study show that a relatively high dose of micafungin in preterm, critically ill infants is well tolerated and provides systemic exposure that, based on estimates from animal model experiments, likely provides coverage of HCME. Further study is required to ascertain the comparative efficacy of micafungin in this population, as well as the optimal length of therapy. Nevertheless, given the paucity of safety, PK, dosing, and efficacy information available to physicians for currently marketed antifungal agents, the data presented herein support further development of micafungin in the neonatal infant population as a viable treatment option in the management of invasive fungal infections.
METHODS
Study design and patient selection
This was a multicenter, open-label, repeat-dose study in 5 centers in the USA to determine the tolerability and PK profile of intravenous (IV) micafungin in young infants, age > 48 hours and up to day of life (DOL) 120, with suspected candidemia or invasive candidiasis. All patients had venous access for micafungin administration and were able to provide appropriate cultures (blood with or without urine/cerebrospinal fluid) at the time of study entry. Exclusion criteria were as follows: history of hypersensitivity to an echinocandin; exposure to an echinocandin in the month prior to the study; any condition that in the opinion of the investigator would preclude the patient from participation; and a life expectancy of < 96 hours.
Each study site received written approval from their respective Institutional Review Board. This study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki, International Conference on Harmonization Guidelines, Good Clinical Practice, and their applicable laws and regulations. All study participants were enrolled after obtaining written informed consent from the parent or legal guardian.
Treatment regimens
Infants weighing ≥ 1,000 g received micafungin 7 mg/kg/day, while those weighing < 1,000 g received micafungin 10 mg/kg/day, via a 1-hour IV infusion through central venous access, or a midline peripherally inserted catheter, using an appropriate infusion (syringe) pump to maintain a constant infusion rate. A dose of micafungin was administered every 24 hours for 4 to 5 days. Micafungin was not mixed with other drugs and a separate line was used for administration of concomitant medications. If the serial PK samples were not obtained relative to the day 4 dose, the infant was able to receive a final dose of micafungin on day 5, and PK samples obtained relative to that dose. In addition, if an infant had a documented fungal infection, micafungin therapy with study drug supply was permitted beyond day 4 or 5 at the discretion of the investigator. If micafungin therapy was continued, additional doses were not to be administered until after the final sample for pharmacokinetic analysis was drawn. If another echinocandin was used, it was not to be initiated until after the completion of the last dose of micafungin. Appropriate blood, urine, or cerebrospinal fluid cultures were obtained as part of patient care. A dose increase or dose reduction was not permitted and if a dose change was required, the infant was withdrawn from the study after all post-treatment procedures were performed within 72 hours of administration of the last dose of study drug.
Analysis populations
The safety analysis set in this study included all infants who received ≥ 1 dose of micafungin. The PK analysis set consisted of all patients from the safety analysis set for whom sufficient plasma micafungin concentration data were available to facilitate derivation of ≥ 1 PK parameter.
Sample collection and assay methods
In order to minimize the amount of blood sampling, a limited PK sampling procedure was implemented so that no more than 500 μl of blood was obtained from each infant. Serial blood samples (in 50–100 μl aliquots) were collected relative to the day 4 infusion as follows: within 1 hour prior to start of infusion; within 3 minutes of the end of infusion (1 hour); and at 2–4, 8–12, and 20–24 hours after start of infusion. Of note, blood was collected from a site different from where micafungin was infused.
Plasma micafungin concentrations were measured using validated liquid chromatography with tandem mass spectrometric detection (assays performed by Covance Laboratories, Inc., Madison, Wisconsin, USA). The lower limit of quantitation for micafungin in plasma was 0.05 μg/ml and the validation range was 0.05–25.0 μg/ml. Samples above the limit of quantitation were diluted and retested.
Pharmacokinetics and statistical analysis
Model-independent methods were used to determine micafungin PK parameters using the computer program WinNonlin Version 5.0.1 (Pharsight). The maximum plasma concentration (Cmax) and the time to Cmax (Tmax) were the observed values. AUC0–24 was calculated using the log-linear method. Clearance at steady state (CLss) was expressed as the ratio of the dose to AUC0–24,and the volume of distribution at steady state (Vdss) calculated as the product of CLss and mean residence time. Data from all patients who provided blood samples were included in the summaries of the PK profiles. The data were stratified by weight (≥ 1,000 g or < 1,000 g) and also averaged across the populations. Descriptive statistics were determined for all PK data.
Safety
Safety was evaluated by conducting physical examinations and clinical laboratory tests (hematology and serum chemistry), as well as assessing vital signs, at screening (within 72 hours before the first dose) and at specified time points throughout the study until 72 hours after the last dose of micafungin. The study investigators assessed adverse events (AEs) for severity and relationship to the study drug. Treatment-emergent AEs were defined as an AE occurring at any time throughout the treatment period and up to 72 hours after the last dose of micafungin. The Medical Dictionary for Regulatory Activities (MedDRA v. 5.0) was used to summarize AEs (31). Treatment-emergent AEs were those considered by the study investigators to be possibly or probably related to study drug.
Acknowledgments
This study was supported in part by the National Institute of Child Health and Human Development Pediatric Pharmacology Research Unit (grant 1U10-HD45962-06 to DK Benjamin and grant 1U10-HD046000-06 to PJ Sánchez). Further funding for this study was provided by Astellas Pharma Inc., Deerfield, IL. Medical writing and editorial support was provided by Paul Hassan, Ph.D. of Envision Pharma Ltd. and was funded by Astellas Pharma Global Development Inc. Study site coordinators, including Luz Muniz, M.D. of the University of Texas Southwestern Medical Center are thanked for their valuable contributions.
Footnotes
This study was presented, in part, at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 25 to 28 October 2008, abstract A-012, and at the Pediatric Academic Societies’ Annual Meeting, Baltimore, MD, 2 to 5 May 2009, abstract 751040.
CONFLICT OF INTEREST
Authors declare the following potential conflicts of interest:
DK Benjamin Jr. receives support from the United States Government for his work in pediatric and neonatal clinical pharmacology (1R01HD057956-02, 1R01FD003519-01, 1U10-HD45962-06, 1K24HD058735-01, and Government Contract HHSN267200700051C), the non-profit organization Thrasher Research Foundation for his work in neonatal candidiasis (http://www.thrasherresearch.org), and from industry for neonatal and pediatric drug development (http://www.dcri.duke.edu/research/coi.jsp). Dr. PB Smith has received grant support from Astellas Pharma Global Development Inc. A Arrieta has received grant support and consultancy fees from Astellas Pharma Global Development Inc. PJ Sanchez has received grant support from Astellas Pharma Inc. LJ Arnold, LL Kovanda, T Sawamoto, and DN Buell are employees of Astellas Pharma Global Development Inc.
L Castro, WW Hope, TJ Walsh, and D Kaufman do not declare any potential conflicts of interest.
References
- 1.Benjamin DK, Jr, Poole C, Steinbach WJ, Rowen JL, Walsh TJ. Neonatal candidemia and end-organ damage: a critical appraisal of the literature using meta-analytic techniques. Pediatrics. 2003;112:634–40. doi: 10.1542/peds.112.3.634. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin DK, Jr, et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006;117:84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Donowitz LG. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med. 2001;345:1660–6. doi: 10.1056/NEJMoa010494. [DOI] [PubMed] [Google Scholar]
- 4.Stoll BJ, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–91. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin DK, Jr, DeLong ER, Steinbach WJ, Cotton CM, Walsh TJ. R.H. Empirical therapy for neonatal candidemia in very low birth weight infants. Pediatrics. 2003;112:543–7. doi: 10.1542/peds.112.3.543. [DOI] [PubMed] [Google Scholar]
- 6.Ernst EJ, Roling EE, Petzold CR, Keele DJ, Klepser ME. In vitro activity of micafungin (FK-463) against Candida spp.: microdilution, time-kill, and postantifungal-effect studies. Antimicrob Agents Chemother. 2002;46:3846–53. doi: 10.1128/AAC.46.12.3846-3853.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messer SA, Diekema DJ, Boyken L, Tendolkar S, Hollis RJ, Pfaller MA. Activities of micafungin against 315 invasive clinical isolates of fluconazole-resistant Candida spp. J Clin Microbiol. 2006;44:324–6. doi: 10.1128/JCM.44.2.324-326.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petraitis V, et al. Comparative antifungal activities and plasma pharmacokinetics of micafungin (FK463) against disseminated candidiasis and invasive pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob Agents Chemother. 2002;46:1857–69. doi: 10.1128/AAC.46.6.1857-1869.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfaller MA, Boyken L, Hollis RJ, Messer SA, Tendolkar S, Diekema DJ. Global surveillance of in vitro activity of micafungin against Candida: a comparison with caspofungin by CLSI-recommended methods. J Clin Microbiol. 2006;44:3533–8. doi: 10.1128/JCM.00872-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tawara S, et al. In vitro activities of a new lipopeptide antifungal agent, FK463, against a variety of clinically important fungi. Antimicrob Agents Chemother. 2000;44:57–62. doi: 10.1128/aac.44.1.57-62.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dannaoui E, et al. Comparative in vitro activities of caspofungin and micafungin, determined using the method of the European Committee on Antimicrobial Susceptibility Testing, against yeast isolates obtained in France in 2005–2006. Antimicrob Agents Chemother. 2008;52:778–81. doi: 10.1128/AAC.01140-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfaller MA, et al. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J Clin Microbiol. 2008;46:150–6. doi: 10.1128/JCM.01901-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller MA, et al. Candida krusei, a multidrug-resistant opportunistic fungal pathogen: geographic and temporal trends from the ARTEMIS DISK Antifungal Surveillance Program, 2001 to 2005. J Clin Microbiol. 2008;46:515–21. doi: 10.1128/JCM.01915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfaller MA, et al. Geographic and temporal trends in isolation and antifungal susceptibility of Candida parapsilosis: a global assessment from the ARTEMIS DISK Antifungal Surveillance Program, 2001 to 2005. J Clin Microbiol. 2008;46:842–9. doi: 10.1128/JCM.02122-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takakura S, Fujihara N, Saito T, Kudo T, Iinuma Y, Ichiyama S. National surveillance of species distribution in blood isolates of Candida species in Japan and their susceptibility to six antifungal agents including voriconazole and micafungin. J Antimicrob Chemother. 2004;53:283–9. doi: 10.1093/jac/dkh053. [DOI] [PubMed] [Google Scholar]
- 16.Hatano K, Morishita Y, Nakai T, Ikeda F. Antifungal mechanism of FK463 against Candida albicans and Aspergillus fumigatus. J Antibiot (Tokyo) 2002;55:219–22. doi: 10.7164/antibiotics.55.219. [DOI] [PubMed] [Google Scholar]
- 17.Hiemenz J, et al. Pharmacokinetic and maximum tolerated dose study of micafungin in combination with fluconazole versus fluconazole alone for prophylaxis of fungal infections in adult patients undergoing a bone marrow or peripheral stem cell transplant. Antimicrob Agents Chemother. 2005;49:1331–6. doi: 10.1128/AAC.49.4.1331-1336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuse ER, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet. 2007;369:1519–27. doi: 10.1016/S0140-6736(07)60605-9. [DOI] [PubMed] [Google Scholar]
- 19.Pappas PG, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis. 2007;45:883–93. doi: 10.1086/520980. [DOI] [PubMed] [Google Scholar]
- 20.Seibel NL, et al. Safety, tolerability, and pharmacokinetics of micafungin (FK463) in febrile neutropenic pediatric patients. Antimicrob Agents Chemother. 2005;49:3317–24. doi: 10.1128/AAC.49.8.3317-3324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Queiroz-Telles F, et al. Micafungin versus liposomal amphotericin B for pediatric patients with invasive candidiasis: substudy of a randomized double-blind trial. The Pediatric infectious disease journal. 2008;27:820–6. doi: 10.1097/INF.0b013e31817275e6. [DOI] [PubMed] [Google Scholar]
- 22.Smith PB, et al. Pharmacokinetics of an elevated dosage of micafungin in premature neonates. The Pediatric infectious disease journal. 2009;28:412–5. doi: 10.1097/INF.0b013e3181910e2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deerfield I. Astellas Pharma Inc. USA. Mycamine (US Prescribing Information) 2008. [Google Scholar]
- 24.Munich G. Astellas Pharma GmbH. Mycamine (Summary of Product Characteristics) 2008. [Google Scholar]
- 25.Heresi GP, et al. The pharmacokinetics and safety of micafungin, a novel echinocandin, in premature infants. The Pediatric infectious disease journal. 2006;25:1110–5. doi: 10.1097/01.inf.0000245103.07614.e1. [DOI] [PubMed] [Google Scholar]
- 26.Hope WW, et al. The pharmacokinetics and pharmacodynamics of micafungin in experimental hematogenous Candida meningoencephalitis: implications for echinocandin therapy in neonates. J Infect Dis. 2008;197:163–71. doi: 10.1086/524063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odio CM, et al. Caspofungin therapy of neonates with invasive candidiasis. The Pediatric infectious disease journal. 2004;23:1093–7. [PubMed] [Google Scholar]
- 28.Groll AH, Stergiopoulou T, Roilides E, Walsh TJ. Micafungin: pharmacology, experimental therapeutics and clinical applications. Exp Opin Investig Drugs. 2005;14:489–509. doi: 10.1517/13543784.14.4.489. [DOI] [PubMed] [Google Scholar]
- 29.Sirohi B, et al. A study to determine the safety profile and maximum tolerated dose of micafungin (FK463) in patients undergoing haematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;38:47–51. doi: 10.1038/sj.bmt.1705398. [DOI] [PubMed] [Google Scholar]
- 30.Tabata K, Katashima M, Kawamura A, Tanigawara Y, Sunagawa K. Linear pharmacokinetics of micafungin and its active metabolites in Japanese pediatric patients with fungal infections. Biol Pharm Bull. 2006;29:1706–11. doi: 10.1248/bpb.29.1706. [DOI] [PubMed] [Google Scholar]
- 31.Food and Drug Administration. [Accessed 9th August 2008];MedDRA: medical dictionary for regulatory activities. 2004 Available online from URL: http://www.fda.gov/medwatch/report/meddra.htm.