SUMMARY
Activation of AMP-activated protein kinase (AMPK) is thought to convey many of the beneficial effects of exercise via its inhibitory effect on acetyl-CoA carboxylase 2 (ACC2) and promotion of fatty acid oxidation. Hence, AMPK and ACC have become major drug targets for weight loss and improved insulin action. However, it remains unclear if or how activation of the fatty acid oxidation pathway without a concomitant increase in energy expenditure could be beneficial. In this study we have used either pharmacological (administration of the AMPK agonist 5′ aminoimidazole-4-carboxamide-riboside (AICAR)) or genetic means (mutation of the ACC2 gene in mice) to manipulate fatty acid oxidation to determine if this is sufficient to promote leanness. Both of these strategies increased whole body fatty acid oxidation without altering energy expenditure or adiposity. We conclude that negative energy balance is a pre-requisite for weight reduction and increased fatty acid oxidation per se has little, if any, effect to reduce adiposity.
Keywords: Adiposity, Energy Expenditure, Acetyl-CoA Carboxylase, ACC2, Malonyl-CoA, DNP, AICAR
INTRODUCTION
Obesity is linked to a number of metabolic diseases such as type 2 diabetes (T2D) and cardiovascular disease. Energy imbalance, due to either reduced energy expenditure or increased energy consumption, is the ultimate cause of obesity with excess nutrients being channeled into lipid depots. While adipose tissue is an ideal energy store over a certain ideal body weight range, excess lipid is thought to contribute to disease either via modulating the circulating adipokine profile or by directly inducing damage in non adipose tissues (lipotoxicity). In view of the poor success rate of lifestyle change in managing human obesity alternate solutions have been sought.
Impaired mitochondrial lipid metabolism has been linked to metabolic disease (Savage et al., 2007). Mitochondria are the major site for fatty acid oxidation (FAO) and defects in this process may contribute to non-oxidative utilization of fatty acids such as triglyceride storage or conversion to lipotoxic intermediates, both of which may increase adiposity and metabolic disease. A decline in mitochondrial function has been observed both during ageing (Petersen et al., 2003), and in individuals with T2D (Kelley et al., 2002) suggesting that increasing FAO may prevent lipid storage and improve metabolism. A major caveat is that thermodynamically the only way to achieve net weight loss, assuming energy intake remains constant, is to increase energy usage or decrease energy efficiency by diverting metabolic energy to heat. Normally electron transport via the mitochondrial electron transport chain (ETC) is tightly coupled to ATP synthesis and so increased oxidation of nutrients would require increased ATP turnover. In the absence of exercise or other forms of ATP hydrolysis, one way to circumvent an oversupply of fuel substrates is to uncouple the mitochondrial ETC from ATP production. Despite an obvious thermodynamic requirement for increased energy expenditure to promote leanness, it is still widely held that increased muscle mitochondrial FAO per se is sufficient to reduce whole body adiposity.
AMPK regulates mitochondrial FAO by phosphorylating and inhibiting the activity of the mitochondrial enzyme ACC2 leading to reduced malonyl-CoA production. This leads to de-repression of CPT1 activity and increased long-chain FA entry into mitochondria. Treatment of rodents with AMPK agonists is associated with leanness (Narkar et al., 2008) and deletion of the ACC2 gene in mice has been reported to result in increased FAO coupled with elevated whole-body energy expenditure (Choi et al., 2007) and a marked reduction in whole body adiposity (Abu-Elheiga et al., 2001). These studies support the notion that increased flux of FA into mitochondria is sufficient to trigger increased whole body energy expenditure and leanness. However, how such manipulations trigger increased energy expenditure remains uncertain.
In the current report we have re-examined the relationship between increased fatty acid oxidation and reduced adiposity using both pharmacologic and genetic approaches. Our results do not support the hypothesis that increased fatty acid oxidation causes leanness. Rather, as predicted (Randle, 1963), increasing the flux of FA into mitochondria without a concomitant increase in energy demand resulted in a shift in substrate oxidation from glucose and other fuels to FA, with no net change in energy balance.
RESULTS
FAO is Independent of Energy Expenditure
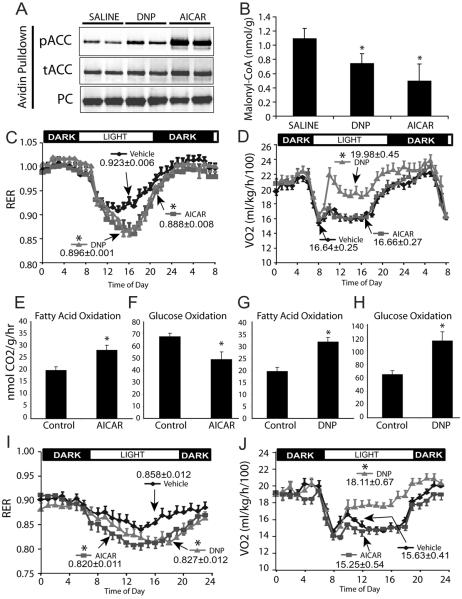
To assess whether increased FAO is associated with increased energy expenditure we treated rats with a single dose of the AMPK activator AICAR (250 mg/kg sc). AICAR increased ACC2 phosphorylation, decreased malonyl-CoA levels by 55% within 60 min of injection (Figs 1A and 1B) and increased whole body FAO as shown by a drop in the respiratory exchange ratio (RER) (Fig 1C), without any change in energy expenditure (VO2) in the 10 h following dosing (Fig 1D). This suggests that there was a reciprocal decrease in the oxidation of other substrates. To test this, isolated EDL muscles were treated with AICAR (2 mM) ex vivo in the presence of either 14C-palmitate or 14C-glucose tracers. Consistent with the in vivo data, AICAR increased palmitate oxidation (+31%, p < 0.001, Fig 1E) at the expense of glucose oxidation (−30%, p < 0.01, Fig 1F). This substrate switching effect of AICAR was due to increased fat oxidation rather than impaired glucose availability or glycolysis since lactate levels in were elevated 30 minutes post AICAR treatment in vivo (2.4 ± 0.3 vs 1.5 ± 0.3 mM, AICAR vs control, p < 0.05) and in the media after palmitate oxidation (27.5 ± 1.3 vs 23.2 ± 1.6 μmol lactate released/g EDL, AICAR vs control, p < 0.05).
Figure 1. AMPK activation drives FAO but not energy expenditure.
Rats were acclimatized overnight and at 10 am dosed with either vehicle (saline), AICAR (250mg/kg), or DNP (30mg/kg).
(A) Avidin pulldown from skeletal muscle. ACC phosphorylation and expression 60 min after treatment. Pyruvate carboxylase (PC) is shown as a loading control for the pulldown.
B) Malonyl-CoA levels in skeletal muscle described in (A) above.
C–D) Indirect calorimetry measurement of RER and VO2 before and after treatment with saline, DNP, or AICAR. Values indicated in the figure represent the average of the 10 h following dosing.
E–H) EDL muscle strips were isolated and incubated ex vivo in KHB media with 5mM glucose and 0.5mM palmitate and either AICAR (2mM), or DNP (0.5mM). Rates of FA and glucose oxidation were measured as described in experimental procedures.
I–J) Indirect calorimetry measurement of RER and VO2 before and after treatment with saline, DNP, or AICAR in rats fed a high fat diet for 21 days. Values indicated in the figure represent the average of the 10 h following dosing.
All results are displayed as means −/+ s.e.m. n>5, *P<0.05 for the 10 hr period following dosing.
As a control, rats were treated with the mitochondrial uncoupler dinitrophenol (DNP; 30mg/kg po). DNP increased ACC2 phosphorylation, reduced cellular malonyl-CoA levels by 32% (Figs 1A and 1B), increased FAO to a similar extent as AICAR, and increased VO2 (Figs 1C and 1D). As predicted, in ex vivo experiments DNP (0.5 mM) caused a significant increase in energy demand and increased the oxidation of both palmitate (+62%, p < 0.01, Fig 1G) and glucose (+77%, p < 0.01, Fig 1H). We observed similar results in rats fed a HFD for 21 days; AICAR and DNP increased FAO, while only DNP increased VO2 (Figs 1I–J).
Since activation of AMPK with AICAR did not increase energy expenditure in either chow or HFD rats, it is unlikely that this treatment would result in reduced body weight and/or adiposity. Consistent with this, chronic treatment with AICAR (250 mg/kg sc for 10 days) did not result in any difference in body weight gain, food intake, fat mass or whole-body VO2 and RER (Supplementary Fig S1). As predicted (Winder et al., 2000), chronic AICAR increased the activity of several oxidative enzymes and lipid metabolic enzymes in muscle consistent with increased muscle mitochondrial biogenesis (Supplementary Fig S2). While we cannot exclude that longer term activation of AMPK may drive changes in body composition and energy metabolism via a separate AMPK target, such as SIRT1 and/or PGC-1α (Canto et al., 2009; Jager et al., 2007) our studies do not support a major role for AMPK mediated changes in FAO via ACC inhibition in reduced adiposity. To determine the effect of prolonged FAO on leanness and energy expenditure independently of changes in mitochondrial capacity we created a mouse model of chronic FAO by deleting the ACC2 gene.
Preservation of Energy Neutrality despite Chronic FAO
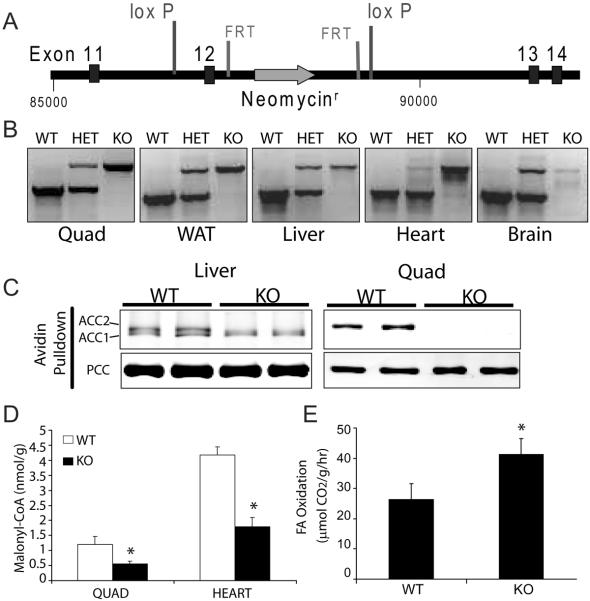
We created ACC2 deficient mice on a pure C57BL/6 background. We flanked exon 12 of the ACC2 gene, encoding a critical region of the biotin carboxylase motif, with loxP sites and crossed these mice onto a cre-deleter strain to remove exon 12 and introduce an early stop codon rendering the ACC2 protein inactive (Fig 2A). Cre was bred out and the resulting mice were confirmed to be deficient in ACC2 by Southern blot (not shown), PCR (Fig 2B), and Western blotting (Fig 2C). Loss of ACC2 resulted in decreased malonyl-CoA levels in skeletal and cardiac muscle, where ACC2 is the predominant isoform (Fig 2D), and a 57% increase in FAO in isolated soleus muscle (Fig 2E).
Figure 2. Confirmation of ACC2 deletion and phenotype.
A) Exon 12 encodes an essential region of the biotin carboxylase motif and was targeted for deletion. Exon 12 was flanked with loxP sites and subsequently bred with mice expressing Cre recombinase driven by the early acting PGK promoter. Cre-mediated recombination removes exon 12, the Neomycin selection cassette, and causes a frameshift mutation and an early stop codon. The PGK-Cre allele was subsequently bred out. The embryonic stem cells and all crosses were performed with pure C57BL/6 lineage.
B) PCR confirmation of exon 12 deletion as described in experimental procedures.
C) Protein confirmation of ACC2 deletion by monomeric avidin pulldown (see experimental procedures). Membranes were probed with streptavidin-800 and ACC2, ACC1, and propionyl-CoA Carboxylase (PCC) enzymes were identified based on molecular weight.
D) Malonyl-CoA levels in skeletal and cardiac muscle of WT and ACC2−/− mice.
E) FAO rates in isolated soleus muscle from WT and ACC2−/− mice. *p<0.05.
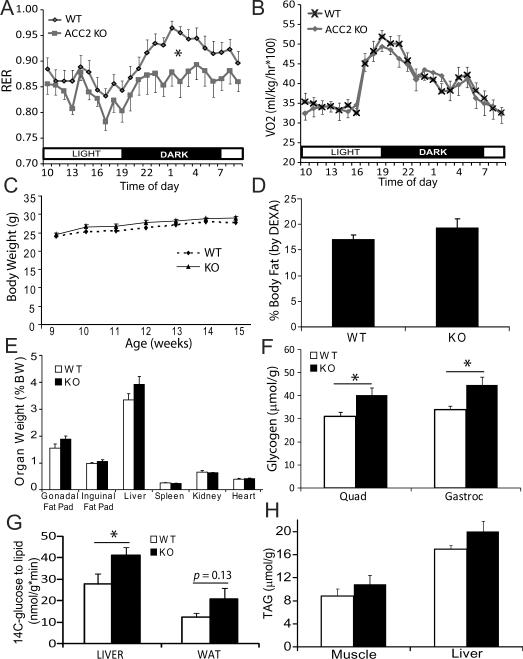
The functional consequences of ACC2 deletion on whole-body metabolic parameters were then studied in WT and ACC2−/− littermates. ACC2−/− mice had increased (p<0.05) whole body FAO in the dark (0.87 ± 0.02 vs. 0.92 ± 0.01, ACC2−/− vs. WT) cycle and a trend for increased FAO during the light cycle (0.85 ± 0.01 vs. 0.88 ± 0.02, ACC2−/− vs. WT) (Fig 3A), however this was not accompanied by a change in energy expenditure (WT light 34.2±0.8 and dark 41.9±1.6; KO light 33.6±0.7 and dark 42.2±0.8 ml O2/kg/hr/100) (Fig 3B). Despite preferential oxidation of FAs in ACC2−/− mice compared to WT controls, we observed no difference in body weight (Fig 3C) or adiposity between the two groups (Figs 3D–E). Although there was no change in serum lactate between genotypes (data not shown), muscle glycogen stores were elevated and the utilization of glucose for lipid synthesis was increased in the ACC2−/− mice, providing evidence that the excess available glucose was being used non-oxidatively (Figs 3F–G). Since there was no increase in total TAG accumulation in liver or muscle (Fig 3H), the increased amount of lipid being synthesized was likely being used to support FAO. These data confirm our acute experiments with AICAR in Fig 1 and show that long-term changes in FAO are not linked to significant alterations in energy expenditure and adiposity.
Figure 3. Chronic FAO increases glycogen storage but not energy expenditure.
A–B) Indirect calorimetry measurements were used to determine RER and VO2 in male WT and ACC2−/− mice. *p<0.05 for ACC2−/− vs. WT in the dark cycle.
C–E) Body weight, body fat (by DEXA), and organ size (by dissection) were measured in male littermate mice 18–22 weeks of age, n>5.
F) Glycogen content in hind limb skeletal muscle from male WT and ACC2−/− mice, n=6. *p<0.05.
G) 14C-glucose incorporation into lipid in liver and white adipose tissue in WT and ACC2−/− mice following a glucose load (1.5 g/kg), n=4–7. *p<0.05.
H) Triacylglycerol content per g of liver tissue in WT and ACC2−/− mice, n=4.
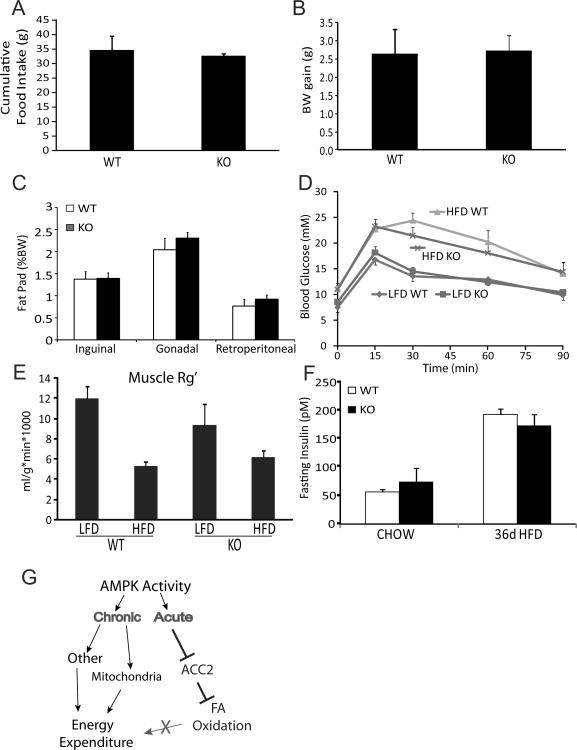
To explore whether the chronic elevation in FAO observed with ACC2 deletion could prevent the metabolic defects induced by a high-fat diet (HFD), we challenged WT and ACC2−/− mice with 36 d of high-fat feeding. During this feeding regime, both genotypes consumed similar quantities of food (Fig 4A), gained a similar amount of body weight (Fig 4B), and exhibited similar fat deposition in 3 separate adipose depots (Fig 4C). There was also no indication that ACC2−/− mice were protected from HFD-induced insulin resistance as they displayed similar glucose and insulin tolerance as WT mice (Fig 4D and Supplementary Fig S3) and they had similar rates of glucose disposal into skeletal muscle during the glucose tolerance test (Fig 4E) with similar insulin levels to control mice (Fig 4F). Collectively, these findings suggest that despite the fact that ACC2−/− mice display increased lipid oxidation they are not resistant to weight gain or insulin resistance caused by 36 d of a HFD.
Figure 4. Effects of chronic FAO on body weight gain and insulin sensitivity with 36 days of high fat feeding.
A–C) Food intake, body weight gain, and fat pad weights were measured over 36 days of high fat feeding.
D) Glucose tolerance test of 16 week old male WT and ACC2−/− mice fed a chow diet or high fat diet, n=5–7 per group.
E) Skeletal muscle insulin resistance was determined by measuring glucose disposal into skeletal muscle during the glucose tolerance test as described in experimental procedures.
F) Insulin levels were not altered between WT and ACC2−/− mice fed either a chow or high fat diet.
G) Inhibition of ACC2 increases FAO without altering energy expenditure. These data suggest that other effects of AMPK agonists, possibly involving their effects on mitochondrial metabolism (Supplementary Fig S3) drive an increase in energy expenditure.
DISCUSSION
Body weight and fat mass in mammals are highly robust parameters. Fat mass is very well defended rendering many lifestyle interventions ineffective and so there is a growing need for new therapies. The hypothesis that manipulations which enhance fat burning will reduce adiposity has provided novel avenues and new hope for the treatment of obesity. Indeed there is a link between AMPK activation and leanness (Lage et al., 2008; Narkar et al., 2008). Currently the precise mechanism linking AMPK to leanness is unknown as AMPK activity has both short and long-term effects. On one hand AMPK rapidly increases FAO, primarily via its inhibition of ACC. On the other its prolonged activity regulates many other pathways such as mitochondrial biogenesis (Bergeron et al., 2001; Winder et al., 2000), uncoupling protein expression (Narkar et al., 2008; Suwa et al., 2003), GLUT4 expression (Winder et al., 2000), and the SIRT1 pathway (Canto et al., 2009). Hence, it is unclear if AMPK agonists promote leanness via the FAO pathway or an alternate route (Fig 4G).
To address this question we used pharmacological and genetic manipulations in rodents to increase FAO upstream or downstream of AMPK. We used AICAR to activate AMPK acutely and chronically for 10 days. There was no change in body composition, substrate utilization, or energy expenditure following AICAR treatment at either time point, but there was a clear stimulation of mitochondrial biogenesis in skeletal muscle after 10 days. To test the effects of long-term changes in FAO independently of AMPK we directly targeted ACC2 in mice. ACC2 inhibition led to reduced intracellular malonyl-CoA levels and a concomitant increase in FAO in skeletal muscle without any change in energy expenditure or adiposity. Rather, in both acute and chronic settings, our data show that increased FAO is offset by an alteration in the handling of other macronutrients. Our findings are consistent with previous studies using a non-selective ACC1/2 inhibitor in rats. This inhibitor decreased RER over a 3 h period without altering energy expenditure (Harwood et al., 2003). Secondly, reduced ACC1/2 expression in rat liver and fat using antisense oligonucleotides was without effect on body weight (Savage et al., 2006). This illustrates that increased FAO is insufficient to promote energy expenditure or weight loss. Rather, the inherent flexibility in the metabolic system compensates for enforced FAO by altering carbohydrate metabolism. Such an effect is consistent with the glucose-FA cycle first proposed by Randle (Randle, 1963).
Our data are in contrast to a previous mouse model of ACC2 deficiency that displayed increased energy expenditure (Choi et al., 2007) and a lean phenotype despite hyperphagia (Abu-Elheiga et al., 2001). Although no mechanism for this effect has been described this mouse model has provided a major incentive for targeting FAO as a treatment for human obesity. Why have we been unable to observe changes in energy expenditure or a lean phenotype in our ACC2−/− mice despite the fact that they are metabolically similar in almost every other respect to those reported previously (Abu-Elheiga et al., 2001; Choi et al., 2007)? This is unlikely due to a more modest phenotype in our animals since the increase in muscle and whole body FAO in the ACC2−/− mice described here was more robust than that reported previously (Abu-Elheiga et al., 2001; Choi et al., 2007). There are a number of technical differences between the two studies that may account for this difference. In our studies we constructed the ACC2 deletion on a genetically pure C57BL/6 mouse background and performed our studies using littermates to avoid founder effects. We also removed the stem cell selection marker from the genome prior to study to avoid potential artifacts whereas this was not reported in the case of the previous studies (Abu-Elheiga et al., 2001) raising the possibility that all tissues in that animal overexpressed an enzyme involved in purine biosynthesis (HPRT). In addition, we targeted an exon closer to the 5' end of the gene at an upstream biotin carboxylase motif and we could find no evidence for any ACC2 polypeptide expressed in tissues from our animals. Finally, one cannot ignore the fact that of ~2,000 knock out mouse models examined, 30% exhibit a lean phenotype raising the possibility that either 30% of all genes independently regulate leanness or other processes involved in genetic engineering and selection somehow contribute to leanness (Reed et al., 2008). This, combined with the fact that our ACC2−/− mice phenocopy long term administration of AICAR in rats, provides strong support for the theory that was advanced by Randle nearly 50 years ago – that energy demand drives energy expenditure and that different substrates compete for entry into the energy utilization pathway in a mutually exclusive manner. Our results provide new evidence to indicate that FAO and energy expenditure are not coupled and thus the belief that FAO drives leanness is likely incorrect or at least an oversimplification. The implications of these findings are considerable particularly for those investing effort to develop ACC2 inhibitors for the treatment of obesity and type 2 diabetes (Corbett, 2009).
Excess adiposity and lipid accumulation in non-adipose tissues are linked to insulin resistance (Savage et al., 2007). However the effect of increasing FAO on insulin sensitivity is controversial in that some studies indicate that enhancing FAO protects against HFD-induced insulin resistance (Bruce et al., 2009), while other studies show that excessive FAO in fact promotes insulin resistance (Koves et al., 2008). In our study we were unable to observe any protective effect of increased FAO against HFD-induced insulin resistance.
Based on the present study it is unlikely that increasing lipid oxidation alone is sufficient to cause leanness. In view of the fact that increased FAO was considered one of the major mechanisms of AMPK in fat reduction and leanness, this leaves open the possibility that the adipose lowering effects of chronic AMPK activation are mediated via an alternate pathway such as increased mitochondrial biogenesis or increased expression of uncoupling proteins. Hence, this study supports the traditional view of energy homeostasis which is that under normal conditions of thermoneutrality, body mass is principally controlled by the balance between food consumption and ATP hydrolysis and that coercing the animal to metabolize one nutrient in favor of another, as in the case of the ACC2−/− mouse, is compensated for simply by downregulating the use of alternate fuels, with the end result being no change in net energy balance.
EXPERIMENTAL PROCEDURES
Materials
Antibodies against ACC and phospho-S79 ACC were from Cell Signaling (Beverly, MA). DNP was from Sigma and AICAR was from Toronto Research Chemicals. Monomeric avidin beads were from Pierce (Rockford, IL). Streptavidin conjugated IRDye-800 was from Rockland Chemicals (Gilbertsville, PA).
Animals
Food and water were provided ad libitum until the date of study and all animal care was in compliance with the Australian National Health and Medical Research Council guidelines, as well as institutional guidelines at Garvan. The high- and low-fat diets (45% and 8% kcal as fat respectively) used are described previously (Bruce et al., 2009). Animals were maintained on a 12/12 light/dark schedule such that lights go out at 7pm and on a 7am.
Generation of ACC2 floxed mice
The ACC2 gene has 52 exons. We targeted exon 12 because its deletion will destroy the biotin carboxylase motif and will result in a frame shift mutation and an early stop codon. Ozgene Pty Ltd. (Murdoch, Australia) was employed to develop these mice. They designed the ACC2 targeting vector FLSniper in 3 fragments; the 3' arm, the 5' arm, and the loxP arm by PCR using PGK-Neo-pA-SD-IS as the selection cassette. A BglII site was introduced in the 5' loxP site and was used for targeting confirmation by Southern blotting. The targeting vector was used for homologous recombination by electroporation in Bruce4 embryonic stem (ES) cells. ACC2-targeted ES cell clones were identified by PCR and Southern blot hybridization using 5'- and 3'-flanking probes. Screening with the endogenous probe revealed a 13.8-kb wild type band, a 7.1-kb targeted band, and a 2.9-kb fragment when the floxed region was deleted by Crerecombinase (data not shown). The ES cells carrying the correct mutation were injected into BALB/c blastocysts and implanted into foster mothers. Chimeric progeny were bred with C57/BL6 mice to obtain germ-line founders. These were then bred with heterozygous Cre-deleter mice on a BL/6 background (ROSA locus knockin driven by a PGK promoter) and genotyping of progeny was performed by Southern blot or PCR (Fig 2B). The PCR primers for Cre were CCGGTCGATGCAACGAGTGAT and ACCAGAGTCATCCTTAGCGCC. Primer sets to differentiate between ACC2 wild type, heterozygous, and homozygous knockout were ACC2 Common forward primer AGGATTTGAACTCAGGTCATCAGGCTTGGC, wild type reverse primer GTACAGAAGCCGTATGTCCTTCAGTCGGTG, and knockout reverse primer CCTGAGCCGAGTGCTGGGCACCGTTTAGAC. These 3 primers were all used in the same PCR reaction and generated products of 750bp for WT, 1091bp for KO, and both 750bp and 1091bp for HET (Fig 2B). Mice were maintained on a C57BL/6 background and bred with our in-house C57BL/6 line. The Cre allele was bred out for all mice in this study.
Glucose and Insulin Tolerance Testing
Mice were fasted for 6 h and were then injected ip with glucose (1.5g/kg) or insulin (0.5U/kg) and blood glucose levels were monitored over time using an Accu-check II glucometer (Roche Diagnostics, Castle Hill, Australia). Clearance of 14C-glucose (10 μCi/animal) into lipid and [3H]-2-DOG (10 μCi/animal) into glucose-6-phospate during the glucose tolerance test were determined using methods described previously (Bruce et al., 2009; Cooney et al., 2004).
Avidin Pulldown
Protein lysates were prepared by homogenizing tissues in NP-40 lysis buffer. 300μg protein was incubated with monomeric avidin beads overnight at 4°C. The beads were then washed and the biotin containing proteins were eluted with 1× Laemmli buffer at 65°C for 5 minutes. Eluate was run on a 6% SDS-PAGE gel, transferred to PVDF, probed with streptavidin conjugated IRDye 800 and biotinylated proteins were detected on a LiCOR Odyssey Infrared Scanner.
Enzyme activity measurements and immunoblotting in muscle
Measurement of the activity of oxidative enzymes and immunoblotting of proteins involved in mitochondrial function and lipid metabolism was conducted as described previously (Bruce et al., 2009; MacArthur et al., 2008).
Respirometry
Oxygen consumption rate (VO2) and respiratory exchange ratio (RER) were measured under a consistent environmental temperature (22°C) using an indirect calorimetry system (Oxymax series, Columbus Instruments, Columbus, OH). For mice, studies were commenced after 2 h of acclimation to the metabolic chamber using an air flow of 0.6 l/min. VO2 was measured in individual mice at 27-min intervals over a 24 h period. For the rat studies, the air flow was 1.5 l/min and measurements were made at 15-min intervals. Animals were placed in the chamber in the late afternoon and the following morning were dosed with vehicle, AICAR or DNP at the doses detailed in the figure legends. During the studies both mice and rats had ad libitum access to food and water.
Ex vivo muscle substrate oxidation
Palmitate and glucose oxidation were determined in muscle strips as described (Bruce et al., 2009; Hoy et al., 2007). Briefly whole soleus from mice or EDL strips from rats were dissected tendon-to-tendon and placed in a vial containing warmed (30–34°C), pregassed (95% O2-5% CO2, pH 7.4), modified Krebs-Henseleit buffer containing 4% FA-free BSA, 5 mM glucose, and 0.5 mM palmitate giving a palmitate:BSA molar ratio of 1:1. Following a 30 min pre-incubation period, muscle strips were transferred to vials containing 0.5 μCi/mL of [1-14C] palmitate or 2 μCi/mL of [U-14C] glucose (GE Healthcare Life Sciences, Buckinghamshire, UK) for 60 min. At the completion of this phase, the medium was acidified with 1M HClO4 and evolved 14CO2 was captured in 1M NaOH.
Blood and Tissue Metabolites
Insulin was measured from whole blood by ELISA (Crystal Chem, Downers Grove, IL). NEFAs were measured colorimetrically (WAKO diagnostics, Osaka Japan). TAG and glycogen contents were measured in tissues as described previously (Bruce et al., 2009; Hoy et al., 2007). Malonyl-CoA was measured from rat skeletal muscle 60 min after saline, DNP, or AICAR injection, and from wild type or ACC2KO mice fasted for 12h. Malonyl-CoA was measured by LC-MS/MS using a modification of the method described (Minkler et al., 2006). Specific details of the malonyl-CoA assays can be found in the Supplementary file.
Statistical Analyses
Data are expressed as means ± standard error. p-values were calculated by two-tailed Student's t-test or one-way ANOVA with Fisher's PLSD post-hoc test. Statistical significance was set at P<0.05. All results are displayed as means −/+ s.e.m.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the US National Institute of Health (DK067509 to DEJ, F32 DK075249 to KLH), National Health and Medical Research Council of Australia (NHMRC) (DEJ, GJC, NT, EWK), Diabetes Australia Research Trust and Viertel Trust (KLH). AJH is supported by a University of New South Wales Australian Postgraduate Award. NT is supported by a Career Development Award and EWK, GJC, and DEJ by Research Fellowships from the NHMRC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M, Young LH, Semenkovich CF, Shulman GI. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2001;281:E1340–1346. doi: 10.1152/ajpendo.2001.281.6.E1340. [DOI] [PubMed] [Google Scholar]
- Bruce CR, Hoy AJ, Turner N, Watt MJ, Allen TL, Carpenter K, Cooney GJ, Febbraio MA, Kraegen EW. Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance. Diabetes. 2009;58:550–558. doi: 10.2337/db08-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD(+) metabolism and SIRT1 activity. Nature. 2009 doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CS, Savage DB, Abu-Elheiga L, Liu ZX, Kim S, Kulkarni A, Distefano A, Hwang YJ, Reznick RM, Codella R, et al. Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc Natl Acad Sci U S A. 2007;104:16480–16485. doi: 10.1073/pnas.0706794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney GJ, Lyons RJ, Crew AJ, Jensen TE, Molero JC, Mitchell CJ, Biden TJ, Ormandy CJ, James DE, Daly RJ. Improved glucose homeostasis and enhanced insulin signalling in Grb14-deficient mice. EMBO J. 2004;23:582–593. doi: 10.1038/sj.emboj.7600082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett JW. Review of recent acetyl-CoA carboxylase inhibitor patents: mid-2007 – 2008. Expert Opin Ther Pat. 2009;19:943–956. doi: 10.1517/13543770902862180. [DOI] [PubMed] [Google Scholar]
- Harwood HJ, Jr., Petras SF, Shelly LD, Zaccaro LM, Perry DA, Makowski MR, Hargrove DM, Martin KA, Tracey WR, Chapman JG, et al. Isozyme-nonselective N-substituted bipiperidylcarboxamide acetyl-CoA carboxylase inhibitors reduce tissue malonyl-CoA concentrations, inhibit fatty acid synthesis, and increase fatty acid oxidation in cultured cells and in experimental animals. J Biol Chem. 2003;278:37099–37111. doi: 10.1074/jbc.M304481200. [DOI] [PubMed] [Google Scholar]
- Hoy AJ, Bruce CR, Cederberg A, Turner N, James DE, Cooney GJ, Kraegen EW. Glucose infusion causes insulin resistance in skeletal muscle of rats without changes in Akt and AS160 phosphorylation. Am J Physiol Endocrinol Metab. 2007;293:E1358–1364. doi: 10.1152/ajpendo.00133.2007. [DOI] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Lage R, Dieguez C, Vidal-Puig A, Lopez M. AMPK: a metabolic gauge regulating whole-body energy homeostasis. Trends Mol Med. 2008;14:539–549. doi: 10.1016/j.molmed.2008.09.007. [DOI] [PubMed] [Google Scholar]
- MacArthur DG, Seto JT, Chan S, Quinlan KG, Raftery JM, Turner N, Nicholson MD, Kee AJ, Hardeman EC, Gunning PW, et al. An Actn3 knockout mouse provides mechanistic insights into the association between alpha-actinin-3 deficiency and human athletic performance. Hum Mol Genet. 2008;17:1076–1086. doi: 10.1093/hmg/ddm380. [DOI] [PubMed] [Google Scholar]
- Minkler PE, Kerner J, Kasumov T, Parland W, Hoppel CL. Quantification of malonyl-coenzyme A in tissue specimens by high-performance liquid chromatography/mass spectrometry. Anal Biochem. 2006;352:24–32. doi: 10.1016/j.ab.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle PJ, Garland P, Hales LN, Newsholme EA. The glucose fatty acid cycle, its role in insulin sensitivity and the metabolic distrubances of diabetes mellitus. Lancet. 1963;i:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Reed DR, Lawler MP, Tordoff MG. Reduced body weight is a common effect of gene knockout in mice. BMC Genet. 2008;9:4. doi: 10.1186/1471-2156-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage DB, Choi CS, Samuel VT, Liu ZX, Zhang D, Wang A, Zhang XM, Cline GW, Yu XX, Geisler JG, et al. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest. 2006;116:817–824. doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–520. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwa M, Nakano H, Kumagai S. Effects of chronic AICAR treatment on fiber composition, enzyme activity, UCP3, and PGC-1 in rat muscles. J Appl Physiol. 2003;95:960–968. doi: 10.1152/japplphysiol.00349.2003. [DOI] [PubMed] [Google Scholar]
- Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol. 2000;88:2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.