Abstract
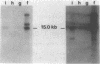
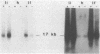
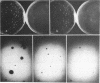
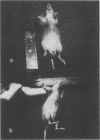
Many human tumors, particularly those of epithelial origin, appear to express greatly reduced levels of major histocompatibility complex class I antigens on their surface. It has been previously reported that the class I gene H-2Ld, introduced into adenovirus type 12-transformed mouse cells, induces reversal of oncogenesis in immunocompetent BALB/c mice. We have tested the hypothesis that the H-2Ld gene, when transfected into HCT colon cancer cells, may alter their transformed phenotype. Two H-2Ld transfectants, HCT-Ii and HCT-If, were found to exhibit a markedly reduced-to-virtually suppressed ability to form colonies in soft agar in comparison to a transfectant (HCTh) carrying only the neomycin-resistance gene. We also compared the tumorigenicity of HCTh vs. HCT-If cells in two different strains of immunodeficient mice: nude (T-) and triple-deficient mutants (T-, NK-, B-). At 28 days postinjection of 10(7) and 10(6) cells, the size and growth rate of HCT-If tumors were greatly reduced compared to HCTh cells. Therefore, as assayed in immunodeficient animals, expression of the class I H-2Ld gene in HCT cells appears to correlate with partial suppression of the tumorigenic phenotype, suggesting that the expression of a transfected class I gene may by itself alter the phenotype of the recipient cell and that such phenotypic changes may be independent of the immune system.
Full text
PDF
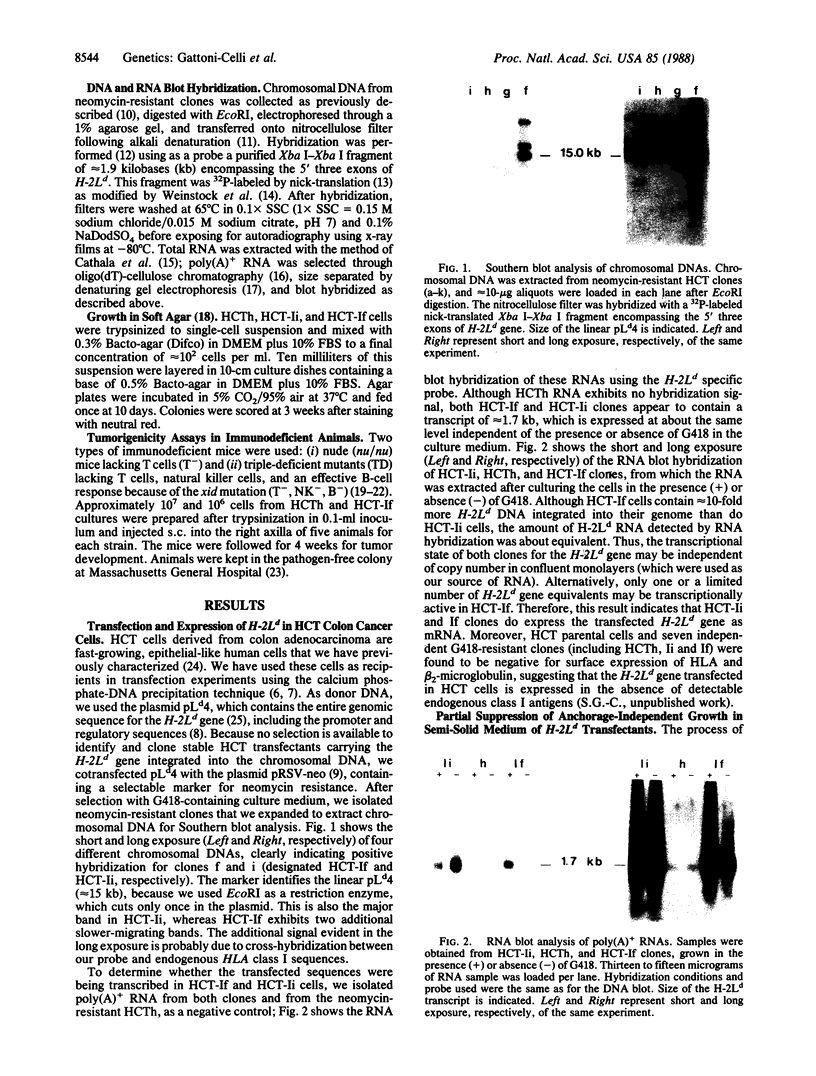
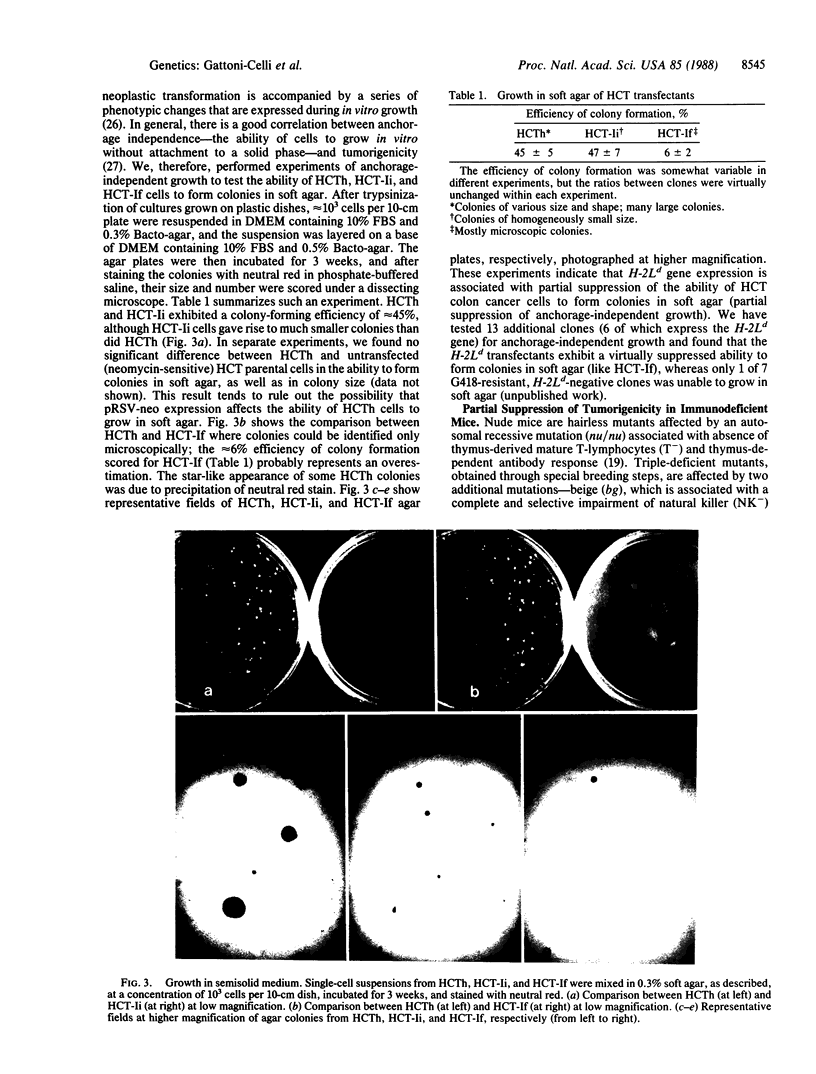
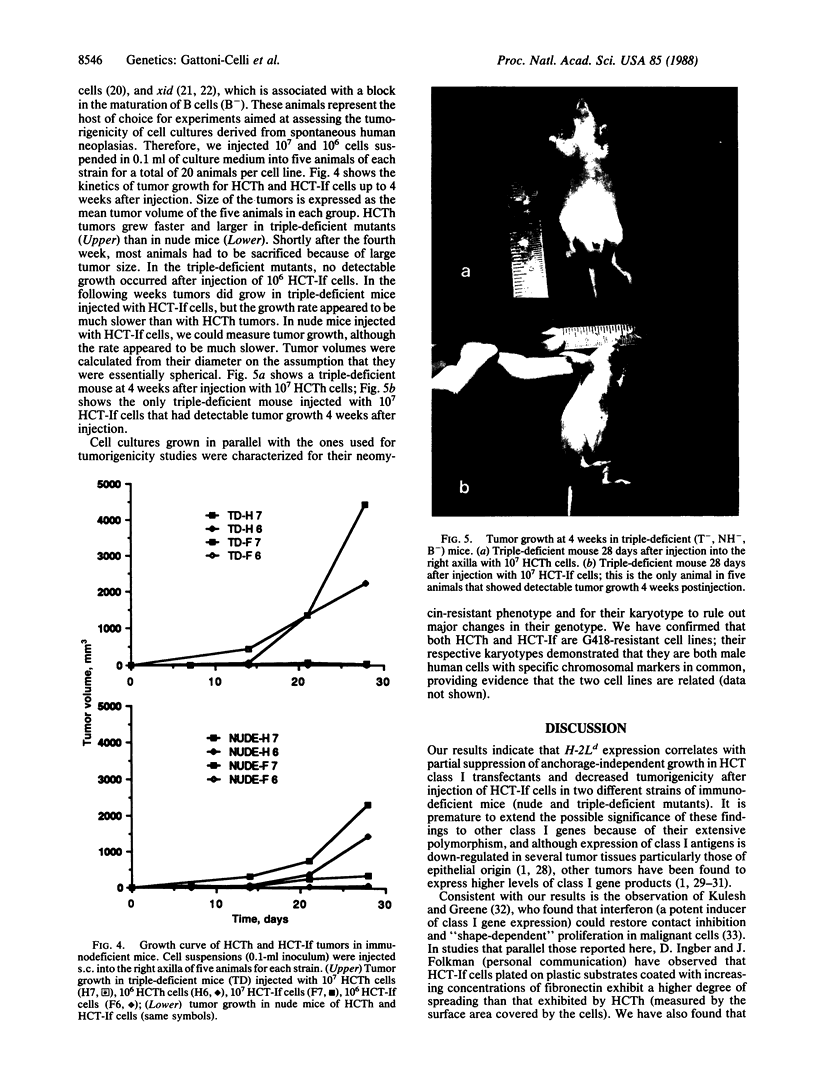

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards R., Schrier P. I., Houweling A., Bos J. L., van der Eb A. J., Zijlstra M., Melief C. J. Tumorigenicity of cells transformed by adenovirus type 12 by evasion of T-cell immunity. 1983 Oct 27-Nov 2Nature. 305(5937):776–779. doi: 10.1038/305776a0. [DOI] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Transformation of rat fibroblasts by FSV rapidly increases glucose transporter gene transcription. Science. 1987 Mar 20;235(4795):1495–1498. doi: 10.1126/science.3029870. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Dausset J. The major histocompatibility complex in man. Science. 1981 Sep 25;213(4515):1469–1474. doi: 10.1126/science.6792704. [DOI] [PubMed] [Google Scholar]
- Dexter D. L., Barbosa J. A., Calabresi P. N,N-dimethylformamide-induced alteration of cell culture characteristics and loss of tumorigenicity in cultured human colon carcinoma cells. Cancer Res. 1979 Mar;39(3):1020–1025. [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Camerini-Otero R. D., Ozato K., Seidman J. G. Structure and expression of a mouse major histocompatibility antigen gene, H-2Ld. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1994–1998. doi: 10.1073/pnas.79.6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Shykind B., Seidman J. G., Ozato K. Exon shuffling: mapping polymorphic determinants on hybrid mouse transplantation antigens. Nature. 1982 Dec 23;300(5894):755–757. doi: 10.1038/300755a0. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Mueckler M. M., Usher P., Lodish H. F. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science. 1987 Mar 20;235(4795):1492–1495. doi: 10.1126/science.3103217. [DOI] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Gattoni-Celli S., Kirsch K., Kalled S., Isselbacher K. J. Expression of type C-related endogenous retroviral sequences in human colon tumors and colon cancer cell lines. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6127–6131. doi: 10.1073/pnas.83.16.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattoni S., Kirschmeier P., Weinstein I. B., Escobedo J., Dina D. Cellular Moloney murine sarcoma (c-mos) sequences are hypermethylated and transcriptionally silent in normal and transformed rodent cells. Mol Cell Biol. 1982 Jan;2(1):42–51. doi: 10.1128/mcb.2.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Haddada H., Sogn J. A., Coligan J. E., Carbone M., Dixon K., Levine A. S., Lewis A. M., Jr Viral gene inhibition of class I major histocompatibility antigen expression: not a general mechanism governing the tumorigenicity of adenovirus type 2-, adenovirus type 12-, and simian virus 40-transformed Syrian hamster cells. J Virol. 1988 Aug;62(8):2755–2761. doi: 10.1128/jvi.62.8.2755-2761.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao W. L., Gattoni-Celli S., Weinstein I. B. Effects of 5-azacytidine on the progressive nature of cell transformation. Mol Cell Biol. 1985 Jul;5(7):1800–1803. doi: 10.1128/mcb.5.7.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagogeos D., Rosenberg N., Wortis H. H. Early arrest of B cell development in nude, X-linked immune-deficient mice. Eur J Immunol. 1986 Sep;16(9):1125–1130. doi: 10.1002/eji.1830160916. [DOI] [PubMed] [Google Scholar]
- Katzav S., Segal S., Feldman M. Immuno-selection in vivo of H-2D phenotypic variants from a metastatic clone of sarcoma cells results in cell lines of altered metastatic competence. Int J Cancer. 1984 Mar 15;33(3):407–415. doi: 10.1002/ijc.2910330320. [DOI] [PubMed] [Google Scholar]
- Kerbel R. S., Waghorne C., Man M. S., Elliott B., Breitman M. L. Alteration of the tumorigenic and metastatic properties of neoplastic cells is associated with the process of calcium phosphate-mediated DNA transfection. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1263–1267. doi: 10.1073/pnas.84.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesh D. A., Greene J. J. Shape-dependent regulation of proliferation in normal and malignant human cells and its alteration by interferon. Cancer Res. 1986 Jun;46(6):2793–2797. [PubMed] [Google Scholar]
- Ljunggren H. G., Kärre K. Experimental strategies and interpretations in the analysis of changes in MHC gene expression during tumour progression. Opposing influences of T cell and natural killer mediated resistance? J Immunogenet. 1986 Apr-Jun;13(2-3):141–151. doi: 10.1111/j.1744-313x.1986.tb01095.x. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Momburg F., Möller P., Moldenhauer G., Hämmerling G. J. Loss of HLA-A,B,C in colorectal carcinoma is related to the degree of de-differentiation. J Immunogenet. 1986 Apr-Jun;13(2-3):195–199. doi: 10.1111/j.1744-313x.1986.tb01101.x. [DOI] [PubMed] [Google Scholar]
- Ohno S. The original function of MHC antigens as the general plasma membrane anchorage site of organogenesis-directing proteins. Immunol Rev. 1977 Jan;33:59–69. [PubMed] [Google Scholar]
- Pantelouris E. M. Absence of thymus in a mouse mutant. Nature. 1968 Jan 27;217(5126):370–371. doi: 10.1038/217370a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roder J., Duwe A. The beige mutation in the mouse selectively impairs natural killer cell function. Nature. 1979 Mar 29;278(5703):451–453. doi: 10.1038/278451a0. [DOI] [PubMed] [Google Scholar]
- Scher I., Sharrow S. O., Paul W. E. X-linked B-lymphocyte defect in CBA/N mice. III. Abnormal development of B-lymphocyte populations defined by their density of surface immunoglobulin. J Exp Med. 1976 Aug 1;144(2):507–518. doi: 10.1084/jem.144.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier P. I., Bernards R., Vaessen R. T., Houweling A., van der Eb A. J. Expression of class I major histocompatibility antigens switched off by highly oncogenic adenovirus 12 in transformed rat cells. 1983 Oct 27-Nov 2Nature. 305(5937):771–775. doi: 10.1038/305771a0. [DOI] [PubMed] [Google Scholar]
- Sedlacek R. S., Mason K. A. A simple and inexpensive method for maintaining a defined flora mouse colony. Lab Anim Sci. 1977 Oct;27(5 Pt 1):667–670. [PubMed] [Google Scholar]
- Shin S. I., Freedman V. H., Risser R., Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano A. R., Cremaschi G., Sánchez M. L., Borda E., Sterin-Borda L., Podestá E. J. Molecular and biological interaction between major histocompatibility complex class I antigens and luteinizing hormone receptors or beta-adrenergic receptors triggers cellular response in mice. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5087–5091. doi: 10.1073/pnas.85.14.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Isselbacher K. J., Khoury G., Jay G. Reversal of oncogenesis by the expression of a major histocompatibility complex class I gene. Science. 1985 Apr 5;228(4695):26–30. doi: 10.1126/science.3975631. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Yoshioka T., Bieberich C., Jay G. Role of the major histocompatibility complex class I antigens in tumor growth and metastasis. Annu Rev Immunol. 1988;6:359–380. doi: 10.1146/annurev.iy.06.040188.002043. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock R., Sweet R., Weiss M., Cedar H., Axel R. Intragenic DNA spacers interrupt the ovalbumin gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1299–1303. doi: 10.1073/pnas.75.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]