Abstract
Insufficient wear of rodent incisors often results in malocclusion and rapid tooth elongation. This condition may go undetected for a prolonged time and have a negative effect on affected animals’ welfare. Dental overgrowth can lead to undernutrition due to chronic difficulty in feeding and may cause trauma to the surrounding tissues, potentially ultimately resulting in death. Here we describe the increased incidence of malocclusion observed during a longitudinal study of the normal growth and aging of Wistar rats. Histologic examination of the temporomandibular joint of affected animals did not reveal any inflammatory or degenerative changes. Because no environmental factor could be implicated in the condition, we considered that aging or genetic factors were responsible for its appearance. We conclude that special attention should be given to the potential appearance of malocclusion during long-term rodent studies, because its incidence may adversely affect the animals’ health and general wellbeing.
The incisor teeth of rodents grow continuously throughout their lives. Optimal occlusion of the incisors is necessary for a balance between their rapid extrusive growth and normal attrition. To maintain normal occlusion of their incisors, rodents require material to gnaw on. Incisor malocclusion and overgrowth are common among rodents,1,3 reaching an incidence of approximately 1% in 2-y-old Wistar rats.21 Malocclusion is considered to result from a variety of hereditary and environmental causes.6,7,18 This condition can lead rapidly to incisor overgrowth, which can cause trauma to the surrounding tissues and lead to undernutrition due to chronic difficulty in feeding. If undetected and left untreated, rodents with overgrown incisors may be unable to eat; death may follow as a potential consequence.6,7
In rats, class II malocclusion (also called overbite or mandibular brachygnathism) is present when the lower (mandibular) incisors retrude in relation to the upper (maxillary) and the maxillary molars are rostrad to the mandibular molars; in class III malocclusion (also called underbite or mandibular prognathism), the lower incisors protrude in relation to the upper, and the mandibular molars lie rostrad to the maxillary molars.17,22 In rats, class I malocclusion is normal, whereas in companion animals, the class I change is a subtle shift in the relationship of the maxillary and mandibular premolar interdigitation.17
The present report describes the incidence of malocclusion in a longitudinal study of the normal growth and aging of Wistar rats carried out in our laboratory. Although the rats received a pelleted balanced diet and did not undergo any invasive experimental procedure during their entire life span, malocclusion occurred in these animals more frequently than reported in the literature.
Materials and Methods
Laboratory animals.
Wistar rats (HsdHan:WIST; 20 male, 20 female, from 4 litters; age, 3 wk) were obtained from the specific-pathogen-free breeding facilities of the Hellenic Pasteur Institute (Athens, Greece). The animals were housed 4 to a cage (transparent polycarbonate, 45 × 30 × 20 cm; Iffa Credo, Arbresle, France), under standard laboratory conditions with room temperature ranging between 19 and 22 °C, relative humidity between 50% and 65%, 15 air changes hourly, 12:12-h photoperiod, and daily visual monitoring. These animals were used in a study addressing skeletal development and aging; the study protocol was approved by the Athens Directorate of Veterinary Services (permit no. K/954/1999) according to Greek legislation.15 The experimental facilities are accredited by the Athens Veterinary Directorate.
The rats were fed ad libitum a pelleted commercial balanced rat diet (Elviz, Imathia, Greece) procured from the same supplier for all studies over several years at the laboratory and consisting of 21% protein, 6.2% fat, 4.5% fiber, 7.5% ash, 1.1% calcium, 0.9% phosphorus, 0.35% sodium, and 1.1% methionine. This diet is similar to other commercial rat pelleted diets for growing rats. The rats were weighed weekly during the first 3 mo and then monthly throughout their lifespan. Body and tail length measurements were obtained monthly during the first 3 mo and every 6 mo thereafter from rats lightly anesthetized with isoflurane. These were the only procedures the rats underwent during the study. The rats were left to age as long as their health condition permitted. The animals were euthanized by isoflurane inhalation when terminal end points were noted by the authorized veterinarian, such as decreased food intake or rapid weight loss, hunched position, and lethargy. For euthanasia, the rat was placed in a special acrylic euthanasia chamber where isoflurane (2% to 3%) was administered by a vaporizer and oxygen at a flow rate of 2 L/min. The rat was observed for 5 to 10 min, after which its death was confirmed.
When malocclusion was observed (usually first noticed by the animal caretaker during the animals’ handling for cage cleaning, which was conducted twice a week), affected rats were treated by trimming their teeth regularly throughout their aging. Incisors were trimmed approximately every 2 to 4 wk, depending on the rate of growth of the incisors of each animal. The decision to trim was made when the incisors either came into contact with the buccal soft tissues before causing trauma or when their length was such that they protruded outwardly, placing the rat at risk for accidents. Affected rats were anesthetized briefly with isoflurane, and a low-speed dental bur was used for trimming. Care was taken to hold the base of the incisor steadily during trimming, to avoid luxation of the tooth. When malocclusion was present, powdered rat diet was added to the pelleted diet, in case the rats could not use their incisors adequately to remove and gnaw on the pellets; the rats could use their molars to chew the powdered diet.
Specimen collection.
For the skeletal aging study, the femurs and tibias were removed and processed for histology and histomorphometry. The soft tissues of the heads were removed carefully, leaving the mandibular condyles intact. The skulls were fixed in 10% buffered formalin for 36 h, decalcified in 10% nitric acid buffer for 48 h, dehydrated in ethanol and xylene, and embedded in paraffin. Sagittal sections (thickness, 4 to 5 μm) were stained with hematoxylin and eosin and evaluated under light microscopy.
Statistical analysis.
The statistical software package SSPS (version 13.0 for Windows, SSPS, Chicago, IL) was used to analyze the data. A significance level of 5% was set.
Results
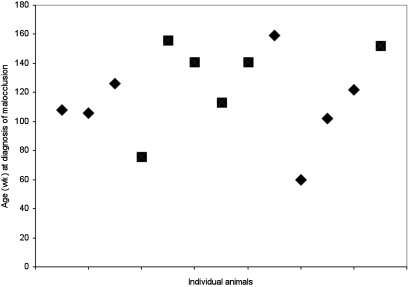
During the first year, all rats showed normal overall growth. Malocclusion of the incisors was first diagnosed during the 60th week of age in a male rat and the 76th week of age in a female rat. Thereafter, the cases of malocclusion increased (Table 1). By the age of 3 y, 13 of the 40 rats (32.5%) in the study had incisor malocclusion. All 4 litters were represented: 4 rats presented with malocclusion from each of litters A, C, and D, but only 1 rat belonged to litter B. However, the incidence of malocclusion did not differ significantly with the affected animal's litter of origin. Nor did the sex of the rat influence malocclusion: 46% (6 of13) affected rats were female and 54% (7 of 13) were male. Most of the cases of incisor malocclusion were diagnosed in rats 100 to 160 wk old (Figure 1).
Table 1.
Demographics of rats with malocclusion of the incisors
| Rat no. | Age (wk) at diagnosis of malocclusion | Age (wk) at euthanasia | Sex | Siblings |
| 1 | 108 | 136 | Male | Litter A |
| 4 | 106 | 165 | Male | Litter A |
| 7 | 126 | 133 | Male | Litter A |
| 8 | 76 | 145 | Female | Litter A |
| 16 | 156 | 160 | Female | Litter B |
| 21 | 141 | 145 | Female | Litter C |
| 22 | 113 | 117 | Female | Litter C |
| 23 | 141 | 145 | Female | Litter C |
| 28 | 159 | 163 | Male | Litter C |
| 31 | 60 | 79 | Male | Litter D |
| 33 | 102 | 106 | Male | Litter D |
| 34 | 122 | 126 | Male | Litter D |
| 40 | 152 | 156 | Female | Litter D |
Figure 1.
Graph of the 13 cases of malocclusion in the present rat study indicating the animal's age (in weeks) when the condition was diagnosed. Squares, female rats; diamonds, male rats.
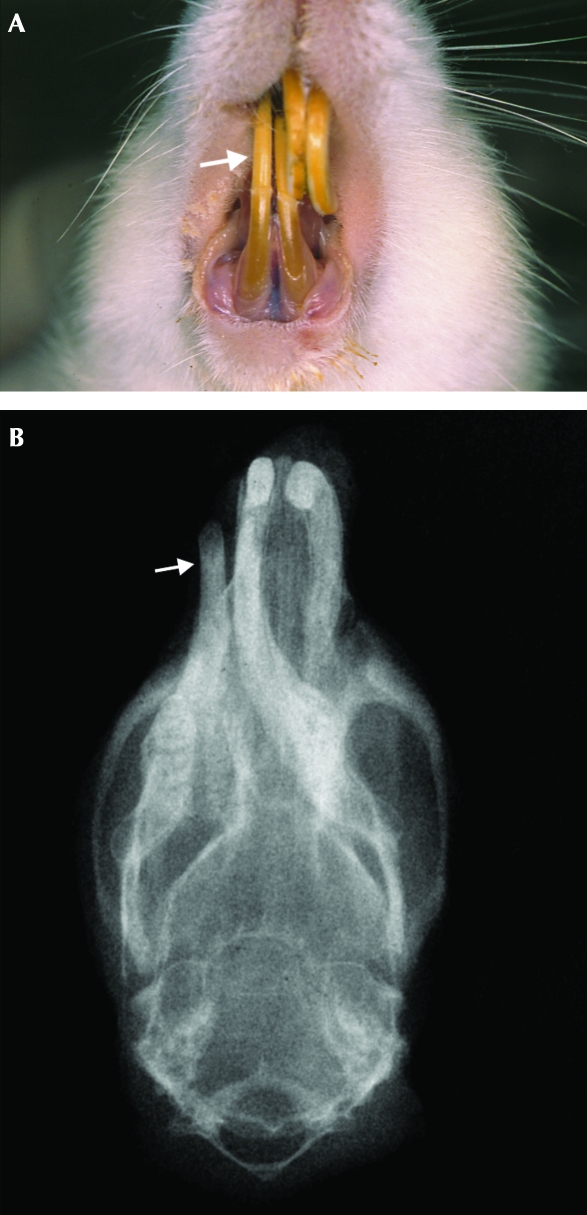
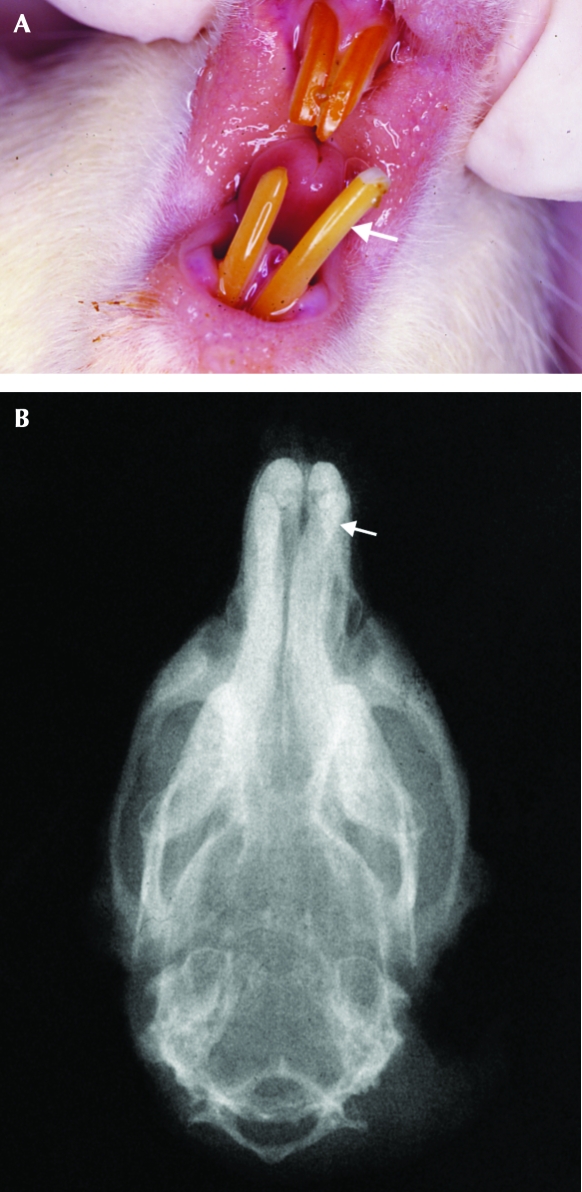
All of the affected rats presented class II malocclusion, distinguished into 2 subcategories. Whereas 80% of these rats presented an incisor overbite deviating toward 1 side, the remaining 20% presented with widening between the lower incisors. Three of the affected rats presented mild (less than 5%) weight loss just before malocclusion was diagnosed. Images of representative female rats with class II malocclusion (nos. 16 and 21; 156 and 141 wk of age, respectively) before trimming of their incisors (Figures 2A and 3A) and radiographs at euthanasia (Figures 2B and 3B) are shown.
Figure 2.
(A) Representative rat (no. 16) with class II malocclusion (incisor overbite). The lower incisors deviate to the right side of the animal (subcategory 1). The white arrow indicates the lower right incisor. (B) Corresponding frontal radiograph of the head of rat 16, showing the deviation of the mandibular incisors to the right side of the animal. The white arrow indicates the lower right incisor. Note the misalignment of the mandible.
Figure 3.
(A) Representative rat (no. 21) with class II malocclusion (incisor overbite). The lower left incisor deviates to the left side of the animal, resulting in widening between the mandibular incisors (subcategory 2). The white arrow indicates the lower left incisor. (B) Corresponding radiograph of rat 21. The white arrow indicates the lower left incisor. Note that the jaws are well aligned.
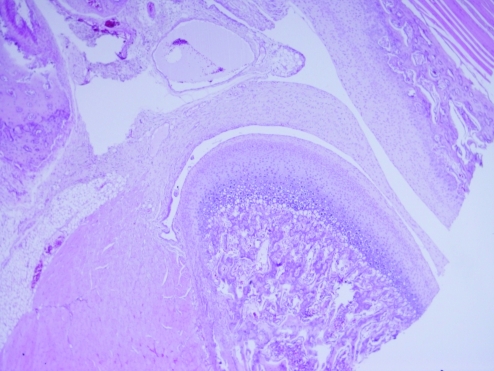
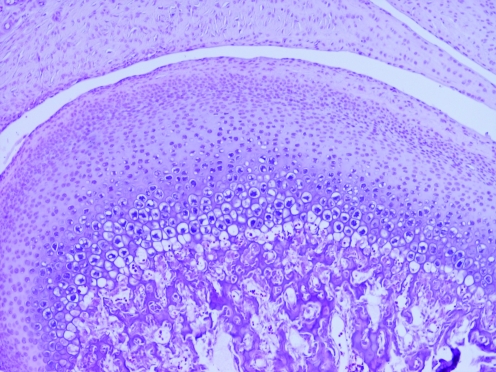
The mandibular condyles of all affected animals were examined histologically. All rats yielded similar findings of integral cartilage, which was composed of fibrocartilage, prechondroblastic and cartilaginous layers, as well as an endochondral ossification zone and subchondral bone (Figures 4 and 5). No osteoarthritic changes were observed.
Figure 4.
This mandibular condyle of an affected animal (rat 21) is normal in appearance. Hematoxylin and eosin stain; magnification, ×4.
Figure 5.
Higher magnification (×10) of the mandibular condyle of rat 21, showing normal component cartilage layers. Hematoxylin and eosin stain.
Discussion
The incisors of rodents are continuously growing and wearing structures that are renewed every 40 to 50 d.7 Insufficient wear of the rodent incisors often results in an abnormal occlusal wear pattern and rapid tooth elongation. Unopposed, continuously growing incisors can erupt as much as 1 mm daily.1 These overgrown teeth can cause cheek, tongue, or palatal ulcers and prevent rats from feeding.
Malocclusion may result from a variety of hereditary and environmental causes. Malocclusion can be due to congenital or functional misalignment of the jaw.7,18,19 Environmental causes of malocclusion include mechanical trauma, soft diet, and insufficient dietary intake of minerals and vitamins. When mechanical tooth or jaw trauma occurs, the incidence of malocclusion reportedly increases.7 The fracture or loss of a tooth results in the accelerated growth of the opposing tooth, due to loss of contact and wear. In addition, a soft diet has been shown to cause abnormal incisor wear and affect mandibular condylar growth.4,5 Firm pelleted diet has been considered to reduce dental problems in Wistar rats by providing appropriate wearing of the occlusal surfaces and preventing overgrowth.21 Even though the rats in the present study received standard pelleted diet and were allowed to follow normal dental attrition, malocclusion occurred in 13 of the 40 animals. Dietary problems, such as calcium deficiency leading to alveolar bone resorption and tooth loosening, also can result in lack of wear and tooth overgrowth. Finally, the nutritional status of the animal can be reflected in incisor morphology. Because the dentin of rat incisors is continuously being formed, changes in calcium homeostasis will be apparent; for example, hypocalcemia will result in undercalcified newly formed dentin. Hypovitaminosis A, D, or E or Mg deficiency will produce incisor lesions such as changes in dentin thickness or stratification, enamel atrophy, and hypoplasia.7 Other causes of incisor malocclusion include tooth root infection, pulp infection secondary to tooth fracture, and neoplasia of the jaws.3,6
Experimentally induced incisor disocclusion in rats can affect the mandibular condylar cartilage at the cellular level, by increasing mitotic activity and accelerating chondrocyte maturation.16 Normally, the condylar cartilage is composed of 5 layers; changes in thickness of the condylar cartilage overall can be the result of low masticatory function.5 Another cause of malocclusion is osteoarthritis of the temporomandibular joint, which is apparent radiographically as flattening or irregularities of the mandibular condyle, reduction of the joint space, the appearance of osteophytes, and subchondral bone sclerosis.9 Implicating factors include gender, age, dietary preferences, and psychologic stress expressed against a genetic background, demonstrated in human and rodent studies.9 In all our animals that presented class II malocclusion, the histologic picture of the condyle was normal, showing no abnormal morphologic changes. The absence of condylar changes may be due to the fact that we treated malocclusion by regularly trimming the incisors, thus allowing the rats to eat normally. Therefore, given that no osteoarthritic changes or inflammatory infiltration were apparent radiographically or histologically, we hypothesized that aging or genetic factors were responsible for the incidence of malocclusion in this group of rats.
Malocclusion has been reported to occur in approximately 1% of rats of Wistar origin (1% in male rats, 0.3% in female rats) during the first 2 y of age.19 In contrast, by the end of our aging study and especially during their third year of age, a large percentage of our Wistar rats—32.5% (13 of the 40 animals)—developed malocclusion of their incisors; the incidence of incisor malocclusion was equivalent between male (7 of 40, 17.5%) and female (6 of 40, 15%) rats.
Treatment of incisor malocclusion in rats consists of periodic tooth trimming or permanent removal of the incisors. If tooth trimming is selected, rats must be monitored frequently to determine when the next session should be. Because incisor growth rates can differ between animals, defining a universally applicable trimming schedule is impossible. Depending on the rate of growth, trimming every 2 to 4 wk (in the present study) or 4 to 6 wk6 may be necessary. Ideally, a low-speed or water-cooled high-speed dental burr should be used on an anesthetized rat. Care must be taken so that the overgrown tooth is trimmed to an appropriate length to bring the teeth into occlusion without overtrimming, which may expose the pulp cavity and potentially lead to infection. In addition, the surrounding soft tissues must be protected during the procedure. The use of clippers or rongeurs has been described6,22 but should be avoided because they may produce jagged edges, thereby fracturing the teeth longitudinally and predisposing animals to apical abscess formation.6,13 Rasping of the teeth similarly should also be avoided because luxation and the energy transmitted into the teeth can damage the periodontal and periapical tissues; this injury can result in deformity of the newly formed dental tissue.1,7 Treatment of incisor malocclusion through removal of the overgrown tooth is an option, but one that involves an aseptic surgical procedure. A harm–benefit analysis comparing the risks of the repeated handling involved with trimming versus those of surgery will reveal the best route for maximizing the animal's welfare. Extraction of a single mandibular incisor is possible, because most rats will use both maxillary incisors with the remaining mandibular incisor; the maxillary incisors may elongate or develop oblique wear patterns and need monitoring. Extracting all the incisors may be considered best, to avoid secondary problems with those remaining.1
Malocclusion of the incisors in mice has a similar etiology as in rats. In mice, incisor malocclusion occasionally is a feature of a mutant phenotype and may become evident around the time of weaning because of the mouse's difficulty in eating a normal pelleted diet.14,22 Class III malocclusion (underbite) occurs more often in mice than in rats; as regards aging, we were unable to identify a reference to the appearance of malocclusion in aged mice. Similarly to rats, mice require regular tooth-trimming. The use of a dental burr necessitates anesthesia; trimming with rongeurs, nail clippers, or scissors can be done without anesthesia, but these instruments carry a risk of tooth fracture. In mice, either the overgrown or all incisors can be removed surgically; providing softened or mashed pellets is advisable when all 4 incisors are removed.14,22
Dental malocclusion in guinea pigs is attributed to genetic causes, diet, trauma, and infection.12,20 Because the incisors and cheek teeth (premolars and molars) of guinea pigs grow continuously, overgrowth of any of these teeth is possible, with the cheek teeth involved most frequently.20 No references to malocclusion specifically in aging guinea pigs were located in the available literature. The first clinical signs may be reduced food intake, difficulty in chewing, anorexia, excess salivation, wetting of the chin and forepaws, and secondary moist dermatitis, followed by penetration of soft tissues by the overgrown teeth.12 Malocclusion of the incisors in guinea pigs can traumatize the lips and palate; the maxillary cheek teeth overgrow laterally traumatizing the cheeks, whereas the mandibular cheek teeth overgrow medially traumatizing the tongue. Treatment consists of regular tooth-trimming (every 4 to 6 wk) with a dental burr. In addition, vitamin C can be added to the diet to prevent decreased collagen formation leading to tooth movement.12
Malocclusion of the incisors also occurs in rabbits, which also have continuously growing incisors, premolars, and molars. As in rodents, incisor malocclusion in rabbits is attributed to hereditary and environmental causes.1,2,8,11 In this animal species, this condition can occur as early as the second month of age, particularly when the etiology is congenital, as in mandibular prognathism.2,8 In addition to congenital reasons, incisor malocclusion in adult rabbits can be due to dietary factors (such as high-energy diets that can lead to insufficient wear and incisor tooth overgrowth), coronal elongation of the cheek teeth leading to secondary incisor malocclusion, and calcium deficiency which can lead to alveolar bone resorption and tooth loosening.1,2,13 In addition, trauma of the teeth or (more rarely) the jaw or skull, infection of the tooth root or pulp, and tumors in the jaws all can lead to malocclusion in rabbits.1,2,11 Aging (not associated with metabolic diseases, such as calcium deficiency, or neoplasia) has not been suggested as a possible factor for malocclusion in rabbits in the available literature. As in rodents, treatment of malocclusion in rabbits consists of periodic incisor trimming with a dental burr or permanent removal of the incisors.2
In the present study, the first case of malocclusion appeared at the age of 60 wk, while the rats were on a pelleted diet that had been used in our laboratory for many years without problem. The number of cases of malocclusion increased during the second and third years of the animals’ life, reaching an incidence of 32.5%. The small number of animals used in the study (40) limits the conclusions we can draw from our findings. However, this increased incidence of malocclusion relative to that in the literature18 may indicate a previously unappreciated welfare issue in aging rats that needs to be considered in depth.
Aging is often accompanied by dysfunction or disease of bodily systems, manifesting as cardiovascular system disorders, renal and respiratory function decline, osteoarthritis, osteoporosis, cataracts, disease-promoting mutations, oxidative damage, and others.10 Because we could identify no other environmental factor that led to the condition and because the diagnosed cases of malocclusion belonged mainly to 3 of the 4 litters in the study, we conclude that aging or some genetic factor is responsible for the increased incidence of incisor malocclusion in our rats. We further conclude that special attention should be given to the potential for malocclusion during long-term rodent studies, because its increased incidence may constitute a threat to the animals’ health and general wellbeing.
References
- 1.Crossley DA, Aiken S. 2004. Small mammal dentistry, p370–382 : Quesenberry KE, Carpenter JW, Ferrets, rabbits and rodents. Clinical medicine and surgery, 2nd ed St Louis (MO): Saunders [Google Scholar]
- 2.Flecknell PA. 2000. BSAVA manual of rabbit medicine and surgery. Gloucester (UK): British Small Animal Veterinary Association [Google Scholar]
- 3.Harkness JE. 1994. Small rodents. Vet Clin North Am Small Anim Pract 24:89–102 [DOI] [PubMed] [Google Scholar]
- 4.Kiliaridis S. 1986. The relationship between masticatory function and craniofacial morphology. III. The eruption pattern of the incisors in the growing rat fed a soft diet. The relationship between masticatory function and craniofacial morphology. J Orthod 8:71–79 [DOI] [PubMed] [Google Scholar]
- 5.Kiliaridis S, Thilander B, Kjellberg H, Topouzelis N, Zafiriadis A. 1999. Effect of low masticatory function on condylar growth: a morphometric study in the rat. Am J Orthod Dentofacial Orthop 116:121–125 [DOI] [PubMed] [Google Scholar]
- 6.King WW, Russell SP. 2006. Metabolic, traumatic, and miscellaneous diseases, p513–546 : Suckow MA, Weisbroth SH, Franklin CL, The laboratory rat Burlington (MA): Elsevier Academic Press [Google Scholar]
- 7.Kuijpers MH, van de Kooij AJ, Slootweg PJ. 1996. Review article. The rat incisor in toxicologic pathology. Toxicol Pathol 24:346–360 [DOI] [PubMed] [Google Scholar]
- 8.Lindsey JR, Fox RR. 1994. Inherited diseases and variations, p293–320 : Manning PJ, Ringler DH, Newcomer CE, The biology of the laboratory rabbit, 2nd ed London (UK): Academic Press [Google Scholar]
- 9.Milam SB. 2006. TMJ osteoarthritis, p105–123 : Laskin DM, Greene CS, Hylander WL, Temporomandibular disorders Hanover Park (IL): Quintessence Publishing [Google Scholar]
- 10.Miller RA. 2009. ‘Dividends’ from research on aging: can biogerontologists, at long last, find something useful to do? J Gerontol A Biol Sci Med Sci 64:157–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okuda A, Hori Y, Ichihara N, Asari M, Wiggs RB. 2007. Comparative observation of skeletal–dental abnormalities in wild, domestic, and laboratory rabbits. J Vet Dent 24:224–229 [DOI] [PubMed] [Google Scholar]
- 12.O'Rourke DP. 2004. Disease problems of guinea pigs, p245–254 : Quesenberry KE, Carpenter JW, Ferrets, rabbits and rodents. Clinical medicine and surgery, 2nd ed St Louis (MO): Saunders [Google Scholar]
- 13.Papadimitriou S, Thomas S, Kouki M. 2008. Dental problems in rabbits and rodents. J Hellenic Vet Med Soc 59:225–238 [Google Scholar]
- 14.Papaioannou VE, Behringer RR. 2005. Mouse phenotypes—a handbook of mutation analysis. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press [Google Scholar]
- 15.Presidential Decree 160/91 1991. Protection of vertebrate animals used for experimental or other scientific purposes, in compliance with European Union Directive 86/609/EEC. Official Journal 64A [Google Scholar]
- 16.Ramirez-Yanez GO, Daley TJ, Symon L, Young WG. 2004. Incisor disocclusion in rats affects mandibular condylar cartilage at the cellular level. Arch Oral Biol 49:393–400 [DOI] [PubMed] [Google Scholar]
- 17.Saidla JE. 2001. Dentistry: genetic, environmental, and other considerations, p430–432 : Ettinger SJ, Pocket companion to textbook of veterinary internal medicine, 5th ed Philadelphia (PA): WB Saunders [Google Scholar]
- 18.Sharp PE, LaRegina MC. 1998. The laboratory rat. : Suckow MA, Boca Raton (FL): CRC Press [Google Scholar]
- 19.Spyropoulos MN, Tsolakis AI. 1997. Altered mandibular function and prevention of skeletal assymetries after unilateral condylectomy in rats. Eur J Orthod 19:211–218 [DOI] [PubMed] [Google Scholar]
- 20.Terril LA, Clemons DJ. 1997. The laboratory guinea pig. Boca Raton (FL): CRC Press [Google Scholar]
- 21.Tucker MJ. 1997. Diseases of the Wistar rat. London (UK): Taylor and Francis [Google Scholar]
- 22.Vasquez SX, Bahadur AN, Johnson JT, Rodriguez DR, Keller C. 2007. Severe runting in a laboratory mouse (Mus musculus). Lab Animal (NY) 36:19, 22–23 [DOI] [PubMed] [Google Scholar]