Abstract
The chemistry and hemostatic parameters of class B vendor cats (Felis catus) can show wide levels of variation, possibly because of initial health status. We compared prothrombin time, partial thromboplastin time, common pathway assay and thrombin time between Class B vendor cats (n = 30) and a control group of healthy cats (n = 16). The antiprotease activities of antiXa, antiIIa, heparin cofactor II, and antithrombin were measured also. Plasma samples from citrated blood were analyzed by using standard clotting assays and commercially available chromogenic substrate assays. Tests for homogeneity of variances and 1-way ANOVA were used to test for significant differences between groups. Results of ANOVA were highly significant between groups for heparin cofactor II and Heptest activity levels. Variances were significantly different between groups for prothrombin time; therefore, an ANOVA was not done. These studies suggest that the class B cats exhibited sufficiently wide variations in their coagulation parameters that they may not be optimal subjects for vascular or cardiovascular research.
Abbreviations: aPTT, activated partial thromboplastin time; USDA, United States Department of Agriculture
For many years the domestic cat (Felis catus) has been used by research institutions for a variety of studies,12 including cardiovascular research,5 leading to advancements in both human and feline healthcare.17 Cats that are available to research institutes are acquired through 1 of 2 groups approved by the US Department of Agriculture (USDA) and protected by a division of the USDA, the Animal and Plant Health Inspection Service.19 Class A dealers supply purpose-bred animals and provide information that describes the genetic and pathogen status of these animals and assures the investigator that any data obtained by using these animals will be similar in results.10 Alternatively, class B dealers supply random-source animals and are required to supply information regarding the name and address of the seller or donor, USDA license number of the dealer, vehicle license plate number, the animal's birth date, and either the purchase price of the animal or whether the animal was donated. However, class B vendors are not required to provide a long-term history of an animal's health.19 Of the 34 research universities that responded to a survey question in 2006 regarding the purchase of random-source animals, 10% indicated that they in fact did purchase animals from licensed class B vendors.9
When cats are used for cardiovascular or hematologic studies, the investigator should know the health status of the animal, because various illnesses can alter the coagulation cascade and affect the final data. The coagulation system typically is evaluated by the use of clotting tests,4 which screen the different pathways of the coagulation cascade. In particular, prothrombin time is used to screen the extrinsic pathway which includes factors X, VII, V, prothrombin, and fibrinogen;15 activated partial thromboplastin time (aPTT) screens the intrinsic pathway comprising of factors XII, XI, X, IX, VIII, V, prothrombin, and fibrinogen;14 a proprietary commercial assay (Heptest, Hemochem, St Louis, MO) which measures clotting time relative to components of the common coagulation pathway, including factors X and V, prothrombin, and fibrinogen;21 and thrombin time measures the conversion of fibrinogen into fibrin.3
Serine protease inhibitors target chymotrypsin-like serine proteases including thrombin1 and play an important role in the regulation of coagulation.7 These inhibitors are evaluated by the use of chromogenic substrate assays and include antiXa,20 antiIIa,8 antithrombin (which targets factors X, IX, XI, XII, and II of the intrinsic clotting pathway as well as factor VII of the extrinsic pathway),13 and heparin cofactor II which specifically inhibits thrombin18.
Results from earlier clinical studies in cats demonstrated that 1 or more abnormalities in the coagulation profiles, including prolonged prothrombin time and prolonged aPTT, can be associated with hypertrophic cardiomyopathy, but the cats were asymptomatic.2 Other diseases that can be associated with hemostatic abnormalities include neoplasia and diabetes mellitus.17 An individual cat's health status can affect each step of the hemostatic process,12 and underlying conditions may exist that could compromise the use of coagulation markers, even though the cat itself is asymptomatic.17 The health status of every cat should be known before data collection can begin. However, depending on the source of the cat, that information may or may not be readily available. We hypothesized that because underlying disease can affect various laboratory parameters, including hematologic and clinical chemistry values, and because random-source cats, depending on the quality of care prior to purchase, have unknown initial health status upon arrival at research facilities, the hemostatic system of random-source cats could be compromised unknowingly. The purpose of this study was to investigate whether the health of class B cats affects the range of variation in coagulation parameters and serine protease inhibitors compared with those of cats with a known health history. Our findings will help researchers determine what source of cat best suits their study requirements.
Materials and Methods
Source of study animals and sample collection.
Two groups of cats were profiled. One group comprised of random-source cats (2 male, 28 female) was purchased from R and R Research (Howard City, MI). Each cat in this group had documentation stating from whom the cat had been purchased, how long it had been at the class B facility (mean, 65 d), what vaccines and parasitic control had been administered at the vendor's facility, and the results of a physical exam given by the company's attending veterinarian before the animal left the facility. The only information provided regarding the actual age of the cats was that they were all adults, and all were intact. The cats arrived at Loyola University Medical Center (Maywood, IL) in 3 groups of 10 each for use in a terminal study and received a complete physical exam in accordance with the Comparative Medicine Department's standard operating procedure for quarantine, conditioning, and preventive health program for laboratory cats. Specifically, cats were anesthetized by using isoflurane, and blood was drawn for routine screening, including CBC; complete chemistry panel; and feline leukemia virus, feline infectious peritonitis, and FIV assays (IDEXX, Elmhurst, IL). Parasite screening was performed inhouse.
The cats (8 male, 8 female; age, 1 to 6 y) used as a control group were housed at the Joliet Junior College Department of Veterinary Technology (Joliet, IL). The control cats had stayed at the facility for approximately 120 d and were used as training models for future certified veterinary technicians. All cats were intact. As part of the program at Joliet, each cat had weekly physical exams, individually prescribed diets, regular baths, regular flea control, and routine health screening including CBC; complete chemistry panel; and feline leukemia virus, feline infectious peritonitis, and FIV assays (IDEXX, Elmhurst, IL). Inhouse parasite screening was performed; in addition, as part of the certification program, students performed manual blood counts on all cats in the control group. All control cats were being prepared for adoption at the end of the semester. Blood was drawn from control cats under manual restraint, as part of the Veterinary Technology Department's courses in Small Animal Nursing and Surgical Technology.
Sample preparation.
The blood collection procedures were under each institution's IACUC approved protocols. Blood (total, 5 mL) was drawn from the femoral vein by using a 21-gauge 3/4-in. needle; the initial 0.5 mL blood was discarded and the remainder immediately transferred into tubes containing 3.2% sodium citrate in a ratio of 9 parts blood to 1 part anticoagulant. Tubes were centrifuged for a total of 15 min to obtain plasma samples. Plasma was collected and aliquoted; pools were made from each group at the same time, and samples were stored at –70 °C until tests were run.
Coagulation analyses.
For each group of cats, 2 sets of 4 tests each were run. The first set consisted of 4 standard clotting tests (prothrombin time, thrombin time, aPTT, and common pathway assay) and were run on manual fibrometers (BBL Fibro System, Becton Dickinson, Franklin Lakes, NJ). For each test, measurement of the clotting time was stopped if the time exceeded 300 s, because this time was outside the linear range of the instrument. All manual clotting tests were carried out by using procedures established for the use of human plasma. Study tests included pooled human plasma as a validation control. The prothrombin time test15 consisted of sample plasma and thromboplastin–calcium reagent (Thromboplastin C+, Dade, Miami, FL), which consisted of lyophilized acetone-dehydrated rabbit brain thromboplastin, 11.6 µM Ca2+, stabilizers, and preservative rehydrated in 10 mL distilled H2O. The test for thrombin time3 consisted of the sample plasma and human α-thrombin (50 U/mL, Enzyme Research, South Bend, IN); each vial was reconstituted with a 1:10 dilution of 0.9% NaCl to yield a final concentration of 5 U/mL. The aPTT test14 consisted of the sample plasma and 2 reagents: calcium chloride (0.025 M) and aPTT reagent (BioMeriuex, Durham, NC), which contained rabbit brain phospholipids plus micronized silica (surface contact) as an activator in N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid buffer. The common pathway assay (Heptest, Hemochem) consisted of sample plasma and 2 reagents: purified bovine factor Xa (lyophilized and stabilized in a buffer containing serum albumin, PEG, NaCl, and Tris maleate; pH 7.5) and Recalmix (bovine plasma fraction containing factor V, fibrinogen, rabbit brain cephalin, and calcium chloride; lyophilized and buffered; pH 7.5). Both reagents were reconstituted with 2 mL distilled H2O before the test was performed according to the manufacturer's instructions.
The second set of tests consisted of the chromogenic substrate assays for antiXa, antiIIa, antithrombin, and heparin cofactor II. For all 4 tests, the optical density at an endpoint setting of 405 nm was read (SpectraMax Plus, Molecular Devices, Sunnyvale, CA) by using SoftMax Pro (version 5, Molecular Devices) software, and the average of duplicate samples was calculated. All assays used commercially available kits that had been validated for human plasma; results from pooled human plasma included as a validation control were compared with those from the assay standard. Kits for the antiXa assay (Actichrome Heparin Assay, catalog no. 832, American Diagnostica, Greenwich, CT) and antiIIa test (Actichrome Heparin Assay, catalog no. 820, American Diagnostica) were used according to the manufacturer's instructions. The OD values were then converted to percentage inhibition of factor Xa or IIa relative to baseline values (pooled control cat plasma) by using the following formula:
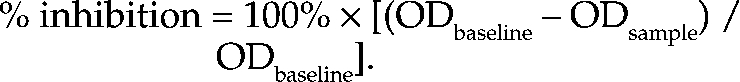 |
Kits for the antithrombin assay (Stachrom ATIII, catalog no. 0596, Diagnostica Stago, Asnieres-sur-Seine, France) and heparin cofactor II test (Stachrom Heparin Cofactor II, catalog no. 0851, Diagnostica Stago) were used according to the manufacturer's instructions. Pooled samples from each group of cats were serially diluted in 0.9% NaCl in a range consisting of 100%, 75%, 50%, 25%, and 0. For each sample, 250 µL was placed (in duplicate) into a well of a 98-well microtiter plate and the percentage of antithrombin or heparin cofactor II activity was read.
Statistical analyses.
Mean and standard deviation were calculated for each parameter by using Excel (Microsoft, Redmond, WA). Because both the antiXa and antiIIa assays showed no difference in percentage of inhibition between the 2 groups of cats, these parameters were not included in Table 1 or any further analysis. Percentage of inhibition for heparin cofactor II was plotted on a linear graph by using Excel (Microsoft); the standard line was generated from the serial dilutions of pooled control plasma. A graphic test of the cumulative frequency distribution was conducted by using rankits for small sample sizes to check for normality of the data, and a Scheffé–Box test was used to evaluate homogeneity of variances between the 2 groups of cats for each parameter.16 This method was chosen because it is less sensitive to slight deviations from normality that might occur with small sample sizes.16 One-way ANOVA was used to test for significant differences between study groups for those assays that had nonsignificant Scheffé–Box tests.16 A P value of 0.05 defined statistical significance.
Table 1.
Coagulation parameters (mean ± 1SD) in control (n = 16) and random-source (n = 30) cats.
| Control | Random-source | |
| Heparin cofactor II (μg/mL)a | 84.90 ± 0.10 | 72.20 ± 0.14 |
| Antithrombin (μg/mL) | 54.90 ± 0.28 | 53.60 ± 0.20 |
| Activated partial thromboplastin time (s) | 30.6 ± 19.9 | 26.7 ± 12.2 |
| Heptest (s)a | 29.9 ± 9.9 | 24.2 ± 2.7 |
| Prothrombin time (s)b | 10.1 ± 1.7 | 8.5 ± 0.6 |
| Thrombin time (5 U/mL; s) | 24.5 ± 10.2 | 22.6 ± 9.6 |
Significant (P < 0.05) difference between groups by ANOVA
Significant (P < 0.05) difference between groups by Scheffé–Box test
Results
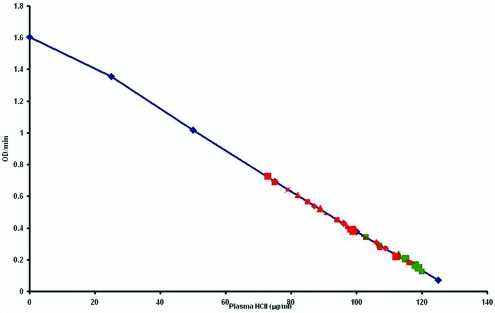
The mean values and standard deviations for the coagulation parameters varied between the 2 groups of cats (P < 0.05), but there was no consistent directional pattern of 1 group having a greater variance (Table 1). In a linear plot of heparin cofactor II mean percentage of inhibition (Figure 1), data from the control cats grouped at the higher end of the scale, indicating increased inhibition, whereas those from the random-source cats demonstrated lower inhibition. The graphic analysis showed the data to be normally distributed, with a slight deviation for heparin cofactor II possibly due to small sample size. Results from the Scheffé–Box test indicated that 5 of the 6 coagulation variables had homogeneous variances. Because the variances for prothrombin time differed significantly (P < 0.001) between the groups of cats, ANOVA of the prothrombin time data was not done. However ANOVA showed highly significant differences (P < 0.001) between groups for the heparin cofactor II levels and results of the common pathway assay (Table 2). Between-group differences for the remaining parameters were nonsignificant.
Figure 1.
Comparison of heparin cofactor II inhibition in random-source cats (n = 30) and control cats (n = 16). The blue calibration line represents the set of serially diluted control pooled plasma diluted in 0.9% NaCl. Red marks represent individual random source cats; green marks represent individual control cats.
Table 2.
Scheffé–Box test and 1-way ANOVA F values for the 2 test groups.
| Scheffé–Box test (df= 1,8) | ANOVA (df=1,44) | |
| Heparin cofactor II | F = 0.017; nonsignificant | F = 26.3; P < 0.001 |
| Antithrombin | F = 3.24; nonsignificant | F = 0.17; nonsignificant |
| Activated partial thromboplastin time | F = 2.67; nonsignificant | F = 0.39; nonsignificant |
| Heptest | F = 3.91; nonsignificant | F = 11.5; P < 0.001 |
| Prothrombin time | F = 21.4; P < 0.001 | not done |
| Thrombin time | F = 1.09; nonsignificant | F = 0.14; nonsignificant |
Discussion
Compared with healthy control cats, random-source cats had significantly lower coagulation and serine protease inhibitor activity. In particular, results of heparin cofactor II and common coagulation pathway assays were significantly different between groups. In addition, the variances for the prothrombin time results differed between the 2 groups, with the standard deviation for the random-source cats more than twice as large as that measured in the control cats. Although the reason for these results in the current study is unknown, previous studies6,11 reported that prolonged clotting times for prothrombin time and aPTT were part of a coagulation profile that met the criteria for disseminated intravascular coagulation in cats and that could also be associated with liver disease. In another study,2 despite marked overlap with values from clinically healthy cats, median antithrombin activity was lower in cats with cardiomyopathy. Our current study showed that antithrombin activity was not significantly different between control and random-source cats.
The results from this study indicate that cats of undetermined health history can have significant alterations or abnormalities of their hemostatic system. Other recent studies have shown a direct correlation between changes in coagulation factors and heart disease.2 Cardiomyopathy and hypercoagulability are 2 conditions that directly affect coagulation factors,17 but if the cat is asymptomatic, discovery of the disease and its potential effect on future data at the time of the animal's arrival at a facility is unlikely. The physical examination and parasitic screening that typically are performed likely would be insufficient to reveal underlying coagulation disorders prior to a cat's use in a study. Much more sophisticated methods of evaluation would be required to determine the cat's suitability, and the cost of these assessments would fall to the investigator.
In conclusion, the investigator has 2 options when considering the use of Felis catus for research studies: to acquire animals that have a predetermined and reliable health status and can be used immediately for studies or to use a random-source cat. If an investigator decides to use random-source cats, carefully determining the care the animal received prior to arrival for the study will help to ensure the quality of the cat being purchased. Even with the small sample we evaluated here, various coagulation markers differed sufficiently to distinguish random-source from healthy cats. Future research should address whether the differences noted are region-specific, perhaps expanding to include a wider geographic sampling area and test the null hypothesis for gender bias.
Acknowledgments
We acknowledge Scott Keller, DVM; Susan Ripper, CVT; Joy Barriball, CVT; and the Department of Veterinary Technology at Joliet Junior College (Joliet, IL) for their assistance in collecting the control plasma samples.
References
- 1.Barrett AJ, Rowlings ND. 1995. Families and clans of serine peptidases. Arch Biochem Biophys 318:247–250 [DOI] [PubMed] [Google Scholar]
- 2.Bedard C, Lanevschi-Pietersma A, Dunn M. 2007. Evaluation of coagulation markers in the plasma of healthy cats and cats with asymptomatic hypertrophic cardiomyopathy. Vet Clin Pathol 36:167–172 [DOI] [PubMed] [Google Scholar]
- 3.Bonsnes RW, Sweeney WJ., 3rd 1955. A rapid, simple semiquantitative test for fibrinogen employing thrombin. Amer J Obstet Gynecol 70:334–340 [DOI] [PubMed] [Google Scholar]
- 4.Brazzell JL, Borjesson DL. 2007. Evaluation of plasma antithrombin activity and D-dimer concentration in populations of healthy cats, clinically ill cats, and cats with cardiomyopathy. Vet Clin Pathol 36:79–84 [DOI] [PubMed] [Google Scholar]
- 5.Dedkova EN, Wang YG, Ji X, Blatter LA, Samarel AM, Lipsius SL. 2007. Signalling mechanisms in contraction-mediated stimulation of intracellular NO production in cat ventricular myocytes. J Physiol 580:327–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estrin MA, Wehausen CE, Jessen CR, Lee JA. 2006. Disseminated intravascular coagulation in cats. J Vet Intern Med 20:1334–1339 [DOI] [PubMed] [Google Scholar]
- 7.Griffith MJ, Noyes CM, Church FC. 1985. Reactive site peptide structural similarity between heparin cofactor II and antithrombin III. J Biol Chem 260:2218–2225 [PubMed] [Google Scholar]
- 8.Hoppensteadt DA, Walenga JM, Fareed J. 1985. Validity of serine protease inhibition test in the evaluation and monitoring of the effect of heparin and its fractions. Semin Thromb Hemost 11: 112–120 [DOI] [PubMed] [Google Scholar]
- 9.Humane Society of the United States. [Internet]. Does your university buy companion animals from Class B dealers? 2007. [Cited 27 Aug 2007]. Available at www.hsus.org.
- 10.Institute of Laboratory Animal Resources 1996. Guide for the care and use of laboratory animals, p57 Washington (DC): National Academies Press [Google Scholar]
- 11.Lisciandro SC, Hohenhaus A, Brooks M. 1998. Coagulation abnormalities in 22 cats with naturally occurring liver disease. J Vet Intern Med 12:71–75 [DOI] [PubMed] [Google Scholar]
- 12.O'Brien M, Murphy MG, Lowe JA. 1998. Hematology and clinical chemistry parameters in the cat (Felis domesticus). J Nutr 128(12 Suppl): 2678S–2679S [DOI] [PubMed] [Google Scholar]
- 13.Persson E, Bak H, Olsen OH. 2001. Substitution of valine for leucine 305 in factor VIIa increases the intrinsic enzymatic activity. J Biol Chem 276:29195–29199 [DOI] [PubMed] [Google Scholar]
- 14.Proctor RR, Rapaport SI. 1961. The partial thromboplastin time with kaolin–A simple screening test for first stage plasma clotting factor deficiencies. Am J Clin Pathol 36:212–219 [DOI] [PubMed] [Google Scholar]
- 15.Quick AJ. 1966. Hypoprothrombinemia states, p60 : Quick AJ. Hemorrhagic disease and thrombosis Philadelphia (PA): Lea and Febiger [Google Scholar]
- 16.Sokal RR, Rohlf HJ. 1995. Biometry, 3rd ed, p208–217 New York (NY): WH Freeman [Google Scholar]
- 17.Stokol T, Brooks M, Rush JE, Rishniw M, Erb H, Rozanski E, Kraus MS, Gelzer AL. 2008. Hypercoagulability in cats with cardiomyopathy. J Vet Intern Med 22:546–552 [DOI] [PubMed] [Google Scholar]
- 18.Tollefsen DM. 2007. Heparin cofactor II modulates the response to vascular injury. Arterioscler Thromb Vasc Biol 27:454–460 [DOI] [PubMed] [Google Scholar]
- 19.United States Department of Agriculture 2007. [Internet]. Random-source dog and cat dealer inspection. animal care resource guide 8.10.1–8.10.6 [Cited 21 Mar 2007]. Available at: www.aphis.usda.gov
- 20.Walenga JM, Bara L, Samama MM, Fareed J. 1985Amidolytic antifactor Xa assays in the laboratory evaluation of heparin and low-molecular–weight fractions. Semin Thromb Hemost 11: 100–107 [DOI] [PubMed] [Google Scholar]
- 21.Yin ET, Wessler S, Butler J. 1973. Plasma heparin: a unique, practical submicrogram-sensitive assay. J Lab Clin Med 81:298–310 [PubMed] [Google Scholar]