Abstract
Streptozotocin is widely used to induce diabetes in laboratory animals through multiple low-dose or single high-dose intraperitoneal injections. HPLC analysis has shown that the composition of the solution may change considerably during the first 2 h after dissolution due to equilibration of the 2 anomers (α and β) of streptozotocin. Because of the drug's alleged instability in solution, the typical recommendation is to administer streptozotocin within 10 min after dissolution. We compared the induction of diabetes in NOD/SCID mice by injection of a single high dose of freshly made or anomer-equilibrated streptozotocin solution. Solutions were prepared from dry compound containing 85% of the α anomer, which is the more toxic of the 2. Body weight and nonfasting blood glucose levels were measured weekly for 8 wk. Both solutions induced long-term hyperglycemia, but blood glucose levels and mortality were higher and damage to pancreatic islands more pronounced in the mice receiving freshly prepared solution. A small proportion of mice did not respond in both treatment groups. If stored at 4 °C in the dark, the anomer-equilibrated solution retains its biologic activity for at least 40 d; under those conditions the streptozotocin content decreases by 0.1% daily, as determined by HPLC. Anomer-equilibrated streptozotocin solution has several practical advantages, and we recommend its use as standard for the induction of experimental diabetes because this practice may improve reproducibility and comparison of results between different laboratories.
Streptozotocin (2-deoxy-2-[3-methyl-3-nitrosourea] 1-D-glucopyranose) occurs in 2 anomeric forms, α and β, which can be separated by HPLC. This drug is used for treating islet cell tumors of the pancreas and some neuroendocrine tumors18 and has selective cytotoxicity for pancreatic β cells. It enters these cells by means of a glucose transporter (GLUT2)17 and causes alkylation of DNA, resulting in rapid and irreversible necrosis.
Streptozotocin is widely applied in medical research to generate diabetes mellitus in many experimental animal species.13 Most publications on diabetes induction by streptozotocin involve mice. A wide variety of dose schedules and routes of administration have been reported. The 2 most common protocols are intraperitoneal injection of a single high dose or multiple low doses.
For diabetes induction through the single-high–dose regimen, reported doses vary from 100 mg/kg5 to 220 mg/kg.2 The low-dose protocol typically involves intraperitoneal administration of 5 consecutive daily doses of 40 mg/kg streptozotocin,14 but the use of 4 (35 mg/kg)9 and 66 daily administrations as well as of 2 separate courses of 5 injections of 40 mg/kg3 have been reported also. An exceptionally aggressive regimen of 5 times 100 mg/kg induced diabetes in 90% of C3H mice,19 but in that case streptozotocin was dissolved in PBS instead of acidic citrate buffer, which allegedly rapidly inactivates the drug. Even though induction through multiple low doses of streptozotocin is more laborious, many investigators adhere to this regimen based on the belief that it is more consistent in its yield of hyperglycemic animals.
With both regimens, investigators seem to select the hyperglycemic responders, commonly defined as showing persistent blood glucose levels of at least 16.6 mmol/L (300 mg/dL), although only a few publications specifically address subject selection.6,8,19 Little information is provided on the percentage of nonresponders and reproducibility of the induction protocol in consecutive experiments. Mortality has been noted with both induction regimens, and all mortality reported to date appears to have been due to hyperglycemia. In addition, strain-, sex-, and age-associated differences in sensitivity to the streptozotocin induction regimens have been observed.4,5,20
Most investigators specify2,4,5,6,8,14,20 that streptozotocin solution (in citrate or acetate buffer, pH 4.5) was administered “immediately” but no later than 15 to 20 min after dissolving, as recommended by the National Institute of Diabetes and Digestive and Kidney Diseases Animal Models of Diabetic Complications Consortium.1 The reason underlying this timing is an alleged instability of streptozotocin in these buffers, an assumption that has not been supported by solid data. On the contrary, as first reported in 1978,11 the total amount of streptozotocin (as determined by HPLC) remains constant for several days at room temperature in acidic buffer solution. Due to mutarotation of the glucopyranose ring, approximate equimolar equilibrium between the 2 anomers is achieved 60 to 90 min after the powder goes into solution.11,12,14 However, the α anomer is more toxic than is the β anomer, as manifested by a difference of approximately 5.5 mmol/L (100 mg/dL) in nonfasting plasma glucose levels at 48 h after administration.14
This difference in blood glucose concentration was apparent in male Sprague–Dawley rats given 30 to 50 mg/kg intravenously after a 24-h fast.15 The authors also noted more severe β-cell necrosis in the pancreas of animals that received the α anomer,15 but no quantification of the damage was provided. The potential ramifications of anomer composition in the experimental induction of diabetes was not addressed again in the literature for 16 y, until a study involving Syrian hamsters.12 The authors of that study found no difference in glucose levels between Syrian hamsters treated with solutions stored for 2 h at room temperature or at 6 °C for 5 to 7 d.12 Those investigators went on to use HPLC to confirm equilibration of streptozotocin anomers in acid buffer solution within 2 h and recovered 100% and 94% of the initial streptozotocin concentration after storage of solutions at 6 °C for 88 and 177 d, respectively.12 The ratio of α to β anomer varies considerably between lots of streptozotocin,16 and similar variation likely persists soon after dissolving the powdered drug into its solvent.
In addition to facilitating induction, the use of stored equilibrated solutions would abolish variations in anomer composition due to differences between lots of streptozotocin and to time lapse when freshly prepared solutions are injected. If these factors contribute to the variation in diabetes induction, the use of anomer-equilibrated solutions might yield more uniform and reproducible results. Here we used a single high dose of streptozotocin to induce diabetes in NOD/SCID mice; we opted to use immunodeficient mice to exclude any influence of autoimmune reactions. By determining the concentrations of anomers and total streptozotocin in the solutions, we confirmed the equilibration and relative stability of streptozotocin after storage.
Materials and Methods
Animals.
Immunodeficient NOD/SCID mice (male, 59; female, 10; age, 5 to 20 wk) were obtained from the animal breeding facilities of Leiden University Medical Center (Leiden, The Netherlands). The mice were kept under specific pathogen-free conditions. The animal room was on a 12:12-h h light:dark cycle and kept at 22 °C. Mice were housed in a pressurized, individually ventilated caging system. Standard laboratory chow and sterile water were provided ad libitum. The drinking water contained 100 mg/L ciprofloxacin (Bayer, Leverkusen, Germany), 100 mg/L polymyxin B (Bufa, Uitgeest, The Netherlands), 100 mg/L amphotericin B (Bristol Myers Squibb, Princeton, NY), and 40 g/L sucrose. All experimental procedures were approved by the Experimental Animal Ethics Committee of Leiden University Medical Center.
Induction of diabetes.
Three different lots of streptozotocin (Sigma–Aldrich, St Louis, MO) were used and contained 88%, 84%, and 87% of the α anomer, respectively, as specified by the manufacturer. The dry powder was dissolved in 0.1 M citrate buffer, pH 4.5, and filtered. Control and experimental groups were injected intraperitoneally once with 300 µL citrate buffer or streptozotocin solution, respectively. All mice receiving the fresh solution of streptozotocin were injected within 15 min after dissolving the streptozotocin. The anomer-equilibrated solution was injected 2 to 3 h after dissolution. Stored streptozotocin solutions for animal experiments were kept in the dark at 4 °C.
Body weight and nonfasting blood glucose levels were monitored weekly. Blood samples were obtained from tail cuts under general anesthesia by inhalation of isoflurane (Piramal Healthcare, London, England). Glucose was determined by using a glucometer (Accu-Check, Roche, Germany); the upper limit of this assay is 33.3 mmol/L (600 mg/dL). Blood samples with this limit were recorded as such for the calculations. Mice with glucose levels greater than 16.6 mmol/L (300 mg/dL) for 2 consecutive weeks or longer and before day 30 after injection of streptozotocin were classified as diabetic responders.
Histology and immunohistochemistry.
Mice were euthanized by cervical dislocation after induction of isoflurane anesthesia at 25 and 70 d after diabetes induction. Pancreases were fixed in 4% paraformaldehyde overnight and embedded in paraffin. Tissue sections (thickness, 6 μm) were mounted on SuperFrost Plus slides (Menzel–Gläser, Germany), deparaffinized, rehydrated, and stained with hematoxylin–phloxin–saffron.
Immunohistochemistry was performed as described7 by using a polyclonal insulin-specific antiserum from guinea pig (Abcam, Cambridge, UK) and horseradish peroxidase-conjugated polyclonal rabbit serum directed against antiguinea pig IgG. The immunoreactivity of the antibodies was visualized with 3, 3′-diaminobenzidine (Sigma–Aldrich). The sections were counterstained with hematoxylin and saffron and mounted by using Pertex mounting medium (Histolab, Gothenburg, Sweden). Images (ColorView IIIu camera mounted BH2 microscope, Olympus, Hamburg, Germany) were processed by using imaging software (Cell F, Olympus).
HPLC analysis.
The chromatographic system consisted of a solvent delivery system (P680, Dionex, Sunnyvale, CA), an automatic sample injection device (ASI100, Dionex), and a diode array detector (UVD340U, Dionex) operated at 250 nm. The entire system was controlled by using Chromeleon software (Dionex). Streptozotocin solutions were kept and chromatographed at room temperature on a Microspher C18 column (length, 100 mm; inner diameter, 4.6 mm; mean particle size, 3 µm; Chrompack, Bergen op Zoom, The Netherlands). The mobile phase comprised 3% methanol–97% acetate buffer (pH 4.4). The injection volume was 20 µL, and a flow rate of 1.0 mL/min was used throughout the study.
Statistics.
Data were analyzed by t test and χ2 distribution; results are expressed as mean ± 1 SD. A P value less than 0.05 was considered significant.
Results
We performed 8 separate experiments with a total of 69 mice. The dose of streptozotocin was 150 or 160 mg/kg. Because of the small number of mice in each experiment and because the age and sex of the mice varied markedly between experiments, the results of all experiments were pooled (Table 1); combining the results of the 2 treatment doses seems justified because the difference in diabetes induction was only 7%. The freshly prepared streptozotocin solution led to a mortality of 36% compared with 7% for the anomer-equilibrated solution (P = 0.0021). We could not determine blood glucose levels in all mice before they were found dead, but most of these animals had at least 1 glucose value that exceeded 33.3 mmol/L (600 mg/dL) before death.
Table 1.
Effect of fresh and anomer-equilibrated streptozotocin solutions on induction of diabetes and related deaths
| No. of mice |
||||
| Streptozotocin | Total | Diabetic | Non-responders | Dieda |
| Fresh | 25 | 14 | 2 | 9 |
| Anomer-equilibrated | 44 | 36 | 7 | 3 |
Significant difference (χ2 = 9.449; P = 0.0021) between values for fresh and anomer-equilibrated solutions.
When we compared the glucose levels of the responders that received fresh solutions with those of diabetic mice that received anomer-equilibrated solutions (Table 2), the glycemia in mice treated with freshly dissolved streptozotocin was about 20% higher (P = 0.0026) than that in mice given anomer-equilibrated solutions. In addition, the frequency of maximal glucose values (as limited by the range of detection of glucometer) was significantly higher among the mice treated with the fresh solution (42%) than the anomer-equilibrated solution (11%; P = 0.0009).
Table 2.
Blood glucose levels (mean ± 1 SD; mmol/L) in responder mice
| Streptozotocin | No. of mice | Blood glucosea |
| Fresh | 12 | 28.9 ± 5.6 |
| Anomer-equilibrated | 36 | 23.4 ± 5.1 |
Glucose values reflect the average of 2 consecutive determinations in each mouse between days 17 and 31 after administration of streptozotocin.
Significant difference (t = 3.19; P = 0.0026) between values for fresh and anomer-equilibrated solutions.
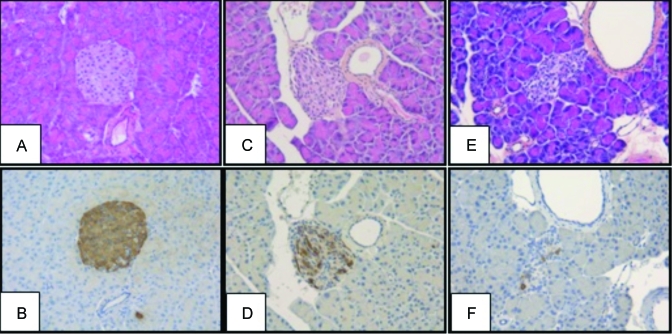
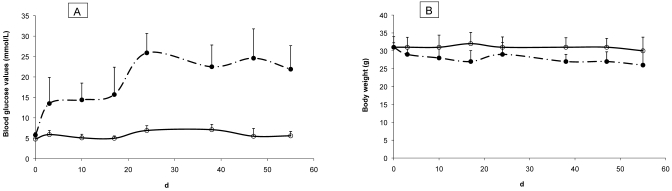
At 25 d after injection, pancreatic tissue from diabetic mice that received fresh streptozotocin solution revealed a decreased number of islets, disruption of islet cytoarchitecture, and weaker insulin staining of granulated β cells (Figure 1 E, F) compared with that from diabetic mice induced with the anomer-equilibrated streptozotocin solution. Compared with those of mice given freshly prepared solution, islet morphology and the insulin staining of the few residual islands (Figure 1 C, D) were better preserved, although insulin staining was weaker than in the nondiabetic control animals (Figure 1 A, B). These histologic differences between mice treated with fresh compared with anomer-equilibrated streptozotocin solution were similarly evident in the pancreas at 70 d after induction (not shown). Furthermore, the anomer-equilibrated streptozotocin solution induced persistent hyperglycemia (Figure 2 A) with only minimal loss of body weight (Figure 2 B).
Figure 1.
Histology and immunostaining of pancreases of diabetic mice after treatment with fresh or anomer-equilibrated streptozotocin solution. Microphotographs of sections of pancreas 25 d after injection of (A, B) citrate buffer, (C, D) anomer-equilibrated solution of streptozotocin, and (E, F) fresh streptozotocin solution. Sections in the upper row are stained with hematoxylin–phloxin–saffron; those in the lower row with an antibody specific for insulin. Note the irregular morphology of residual Langerhans islets of mice treated with streptozotocin solutions (C, E) as compared with the normal islets of untreated control mice (A). Fewer cells staining positive for insulin were seen in residual islands from mice treated with fresh streptozotocin (F) than in those from mice treated with anomer-equilibrated streptozotocin (D). Magnification, ×200.
Figure 2.
Mean (A) blood glucose levels and (B) body weight of mice treated with anomer-equilibrated streptozotocin solution. Diabetes was induced with anomer-equilibrated streptozotocin solution. Body weight of diabetic mice (n = 10) did not differ significantly from that of control mice injected with citrate buffer (n = 6). Open circle solid line (○): control group; closed circle broken line (•): experimental group. Error bars, 1 SD.
Both treatment groups contained a small proportion of nonresponders (Table 1). These mice had normal numbers of pancreatic islets that stained strongly for insulin at 25 d after treatment (not shown).
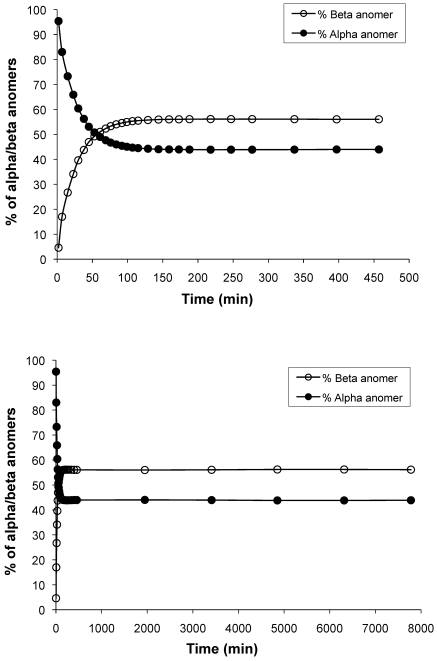
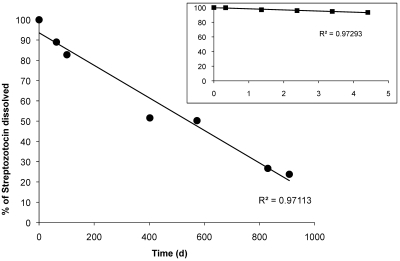
We then evaluated the relative anomer composition of freshly prepared and anomer-equilibrated streptozotocin solutions by HPLC. This assay revealed a 3- to 20-fold preponderance of the more toxic anomer (α) in the solutions (Figure 3) during the first 15 min after preparation. In separate experiments, we used our standard dose of 160 mg/kg to test equilibrated solutions that had been kept at 4 °C in the dark. All 8 mice treated with a solution that had been stored for 40 d developed diabetes as defined in Materials and Methods. However, a solution that had been stored for 300 d, leading to 30% degradation of streptozotocin, was inactive: none of the 8 mice injected became hyperglycemic (data not shown). HPLC measurements of 9 different streptozotocin solutions, derived from the same lot of streptozotocin and stored at 4 °C, revealed an average decrease in streptozotocin content of 0.1% daily (Figure 4). Over time, the heights of the 2 streptozotocin anomer peaks decreased as the number and area of smaller peaks (presumably degradation products) increased (not shown). In comparison, a decay of 1% daily occurred over 5 d of storage at room temperature (Figure 4 inset).
Figure 3.
Time-dependent changes in anomer composition of streptozotocin solutions. Streptozotocin was dissolved in citrate buffer and kept at room temperature in the dark. Samples were injected at the indicated intervals for measuring α and β anomers by HPLC. Equilibrium between anomers (50:50) was achieved 1 h after dissolution (upper graph). Equilibration of the solution at a ratio of 44% α: 56% β was reached after approximately 2 h (upper and lower graphs).
Figure 4.
Time-related degradation of anomer-equilibrated streptozotocin solution. Streptozotocin was dissolved in citrate buffer, and solutions were stored at 4 °C in the dark for as long as 27 mo. Total streptozotocin concentration was determined by HPLC. The calculated rate of degradation was 0.1% daily. Insert, Streptozotocin solutions were kept at room temperature and sampled over 5 d. The calculated rate of degradation was 1% daily.
Discussion
Freshly prepared solutions of streptozotocin explicitly are recommended for the induction of diabetes in laboratory animals.1 This protocol states that “the streptozotocin–sodium citrate buffer solution should only be prepared immediately before injection, as the drug degrades after 15 to 20 min in sodium citrate buffer.”1 In view of the data we present here, this recommendation seems inappropriate. In fact, our findings suggest that, depending on the composition of the dry powder lot, the anomer composition is most subject to change during the first 30 min after dissolution. This variation in composition may lead to considerable interexperimental variation, much of which might be avoided by letting the anomer distribution of the streptozotocin solution to stabilize.
One such variation is animal mortality: we found greater mortality associated with freshly prepared streptozotocin than with the anomer-equilibrated solution. In light of the rate of equilibration of streptozotocin anomers in acidic buffer,11,12 the mice treated with fresh solutions were exposed to a larger amount of the more toxic α anomer than were those treated with the anomer-equilibrated solution, given that the proportion of the α anomer in our streptozotocin lots was 84% or more. The only information available regarding the relative biologic effects of the α and β anomers is based on glucose levels in rats given much lower doses than those we used in our mice and which presumably did not cause any mortality.14 Our understanding of the factors that influence diabetes induction by streptozotocin might be advanced if authors routinely reported the incidence of mortality as well as of nonresponsiveness.
The use of anomer-equilibrated streptozotocin solution also saves time and labor, particularly when the multiple-injection induction regimen is followed. One possible reason for investigators preferring multiple low-dose injections of freshly prepared streptozotocin solution is the belief that this regimen lacks clinically significant podocyte, glomerular, or tubular toxicity; kidney damage is considered to be more probable with the SHD protocol.3 However, glomerular damage has been documented after the multiple–low-dose streptozotocin regimen in mice.9 Furthermore, we speculate that the occurrence of a small number of nonresponders, regardless of streptozotocin preparation or injection regimen, is a common phenomenon that has been ignored in the literature.
The rate of degradation at 4 °C that we measured (0. 1%) is 3 times that (0.03%) derived from a single HPLC measurement of a solution that had been stored for 177 d.12 Similarly we measured 1% degradation daily at room temperature as compared with 0.4% daily for 177 d of storage reported elsewhere.12 This difference may be due to the use of different buffers as the solvent—0.1 M sodium acetate buffer (pH 4.4) in the previous study10 compared with 0.1 M sodium citrate buffer (pH 4.5) in the current study. Only 1 published study12 addressed the usefulness of streptozotocin solutions stored beyond 25 d; the data indicated no change in the hyperglycemic response of Syrian hamsters after storage of streptozotocin solutions for 7 d at 6 °C in the dark.
One additional experimental detail that merits mention is that we induced diabetes in mice fed ad libitum. Many investigators fast their animals for 4 to 6 h prior to streptozotocin injection,3 a practice that is recommended by the Animal Models of Diabetic Complications Consortium.1 However such a brief fast is unlikely to influence blood glucose levels markedly.10 Therefore, investigators can consider eliminating fasting from the diabetes-induction protocol.
We recommend that diabetes induction with streptozotocin should be accomplished only by using anomer-equilibrated solutions. This practice would allow results from different laboratories to be compared more reliably, given that at least the time-dependent variable of relative anomer composition is eliminated.
Acknowledgments
We are grateful for the histology assistance of Hester Boersma (Virus and Stem Cell Biology Laboratory, Department of Molecular Cell Biology, Leiden University Medical Center, The Netherlands). This study was supported by a Scholarship to AS de la Garza-Rodea from the Universidad Autónoma de Nuevo León (Monterrey, México).
References
- 1.Animal Models of Diabetic Complications Consortium [Internet] 2003. Low-dose streptozotocin induction protocol (mouse). [Cited 15 Sep 2006]. Available at http://www.amdcc.org/shared/showFile.aspx?doctypeid=3&docid=19
- 2.Ceylan-Isik AF, LaCour KH, Ren J. 2006. Sex difference in cardiomyocyte function in normal and metallothionein transgenic mice: the effect of diabetes mellitus. J Appl Physiol 100:1638–1646 [DOI] [PubMed] [Google Scholar]
- 3.Brosius FC.2008. Personal communication.
- 4.Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM. 2006. Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol 290:F214–F222 [DOI] [PubMed] [Google Scholar]
- 5.Hayashi K, Kojima R, Ito M. 2006. Strain differences in the diabetogenic activity of streptozotocin in mice. Biol Pharm Bull 29:1110–1119 [DOI] [PubMed] [Google Scholar]
- 6.Hegde KR, Henein MG, Varma SD. 2003. Establishment of mouse as an animal model for study of diabetic cataracts: biochemical studies. Diabetes Obes Metab 5:113–119 [DOI] [PubMed] [Google Scholar]
- 7.Hermann M, Pirkebner D, Draxl A, Berger P, Untergasser G, Margreiter R, Hengster P. 2007. Dickkopf 3 is expressed in a subset of adult human pancreatic β cells. Histochem Cell Biol 127:513–521 [DOI] [PubMed] [Google Scholar]
- 8.Keswani SG, Katz AB, Lim FY, Zoltick P, Radu A, Alaee D, Herlyn M, Crombleholme TM. 2004. Adenoviral mediated gene transfer of PDGFB enhances wound healing in type I and type II diabetic wounds. Wound Repair Regen 12:497–504 [DOI] [PubMed] [Google Scholar]
- 9.Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, Prockop DJ. 2006. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/Scid mice. Proc Natl Acad Sci USA 103:17438–17443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leiter E.2008. Personal communication.
- 11.Oles PJ. 1978. High-pressure liquid chromatographic separation and determination of anomeric forms of streptozocin in a powder formulation. J Pharm Sci 67:1300–1302 [DOI] [PubMed] [Google Scholar]
- 12.Povoski SP, McCullough PJ, Zhou W, Bell RH., Jr 1993. Induction of diabetes mellitus in Syrian golden hamsters using stored equilibrium solutions of streptozotocin. Lab Anim Sci 43:310–314 [PubMed] [Google Scholar]
- 13.Rees DA, Alcolado JC. 2005. Animal models of diabetes mellitus. Diabet Med 22:359–370 [DOI] [PubMed] [Google Scholar]
- 14.Rossini AA, Like AA, Chick WL, Appel MC, Cahill GF., Jr 1977. Studies of streptozotocin-induced insulitis and diabetes. Proc Natl Acad Sci USA 74:2485–2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossini AA, Like AA, Dulin WE, Cahill GF., Jr 1977. Pancreatic beta cell toxicity by streptozotocin anomers. Diabetes 26:1120–1124 [DOI] [PubMed] [Google Scholar]
- 16.Sigma–Aldrich Chemical Company. 2008. Personal communication.
- 17.Schnedl WJ, Ferber S, Johnson JH, Newgard CB. 1994. STZ transport and cytotoxicity. Specific enhancement in GLUT2-expressing cells. Diabetes 43:1326–1333 [DOI] [PubMed] [Google Scholar]
- 18.Strosberg J, Hoffe S, Gardner N, Choi J, Kvols L. 2007. Effective treatment of locally advanced endocrine tumors of the pancreas with chemoradiotherapy. Neuroendocrinology 85:216–220 [DOI] [PubMed] [Google Scholar]
- 19.Vijayan S, Zhou P, Rajapaksha TW, Alegre ML, Peter ME. 2005. Transplanted islets from lpr mice are resistant to autoimmune destruction in a model of streptozotocin-induced type I diabetes. Apoptosis 10:725–730 [DOI] [PubMed] [Google Scholar]
- 20.Weber C, Pernis B, Ting W, Rosenkrantz K, Reemtsma K. 1984. Murine streptozotocin diabetes: influences of the major histocompatibility complex, genetic background, and blood transfusion. Diabetologia 27Suppl:160–162 [DOI] [PubMed] [Google Scholar]