Abstract
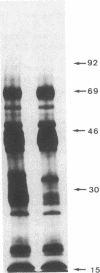
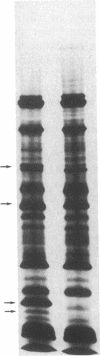
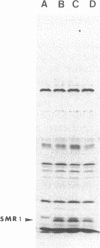
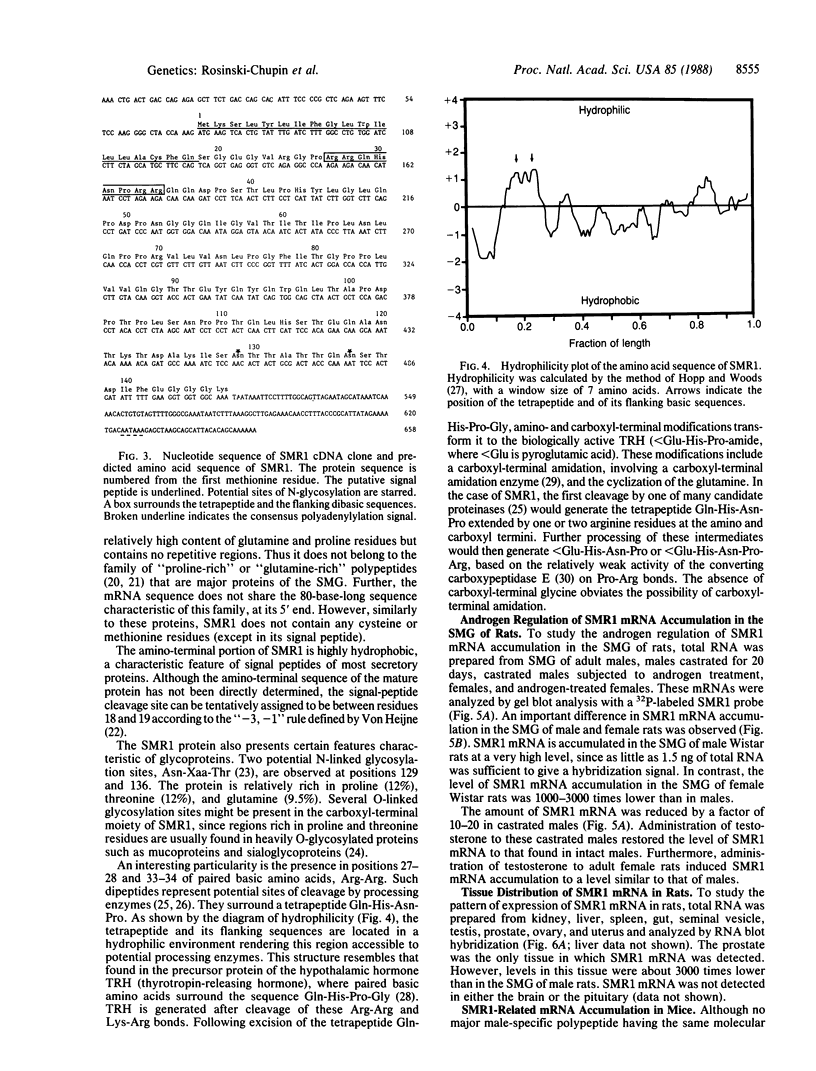
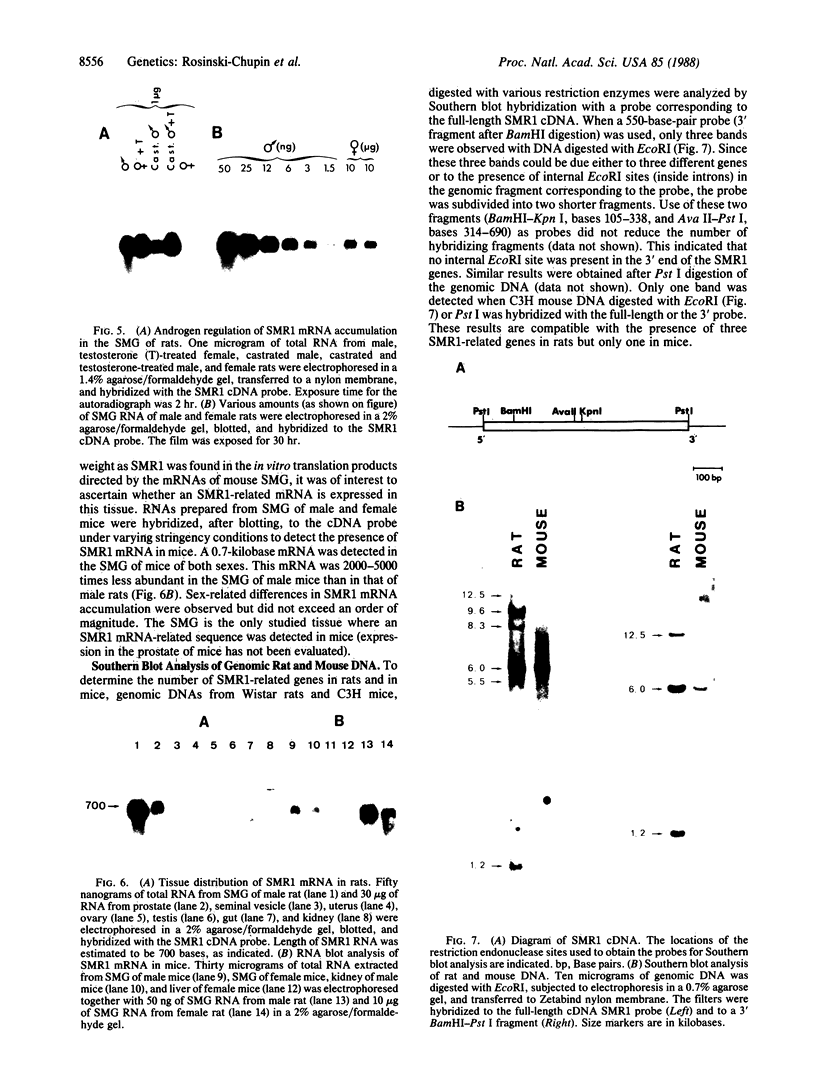
NaDodSO4/PAGE analysis of in vitro translation products of rat submaxillary gland (SMG) mRNAs has revealed an important sexual dimorphism. Moreover, most of the rat male-specific major translation products differ in size from those translated from male mouse SMG mRNAs. To characterize proteins accumulated in the rat SMG under androgen control, a cDNA library was constructed. Here we report the nucleotide sequence of a 0.7-kilobase mRNA that is 1000-3000 times more abundant in male rats than in female rats. The predicted corresponding protein, SMR1, has a molecular weight of 16,000 and contains a signal peptide for secretion and potential signals for glycosylation. An interesting feature of SMR1 is the presence, in a hydrophilic region, of the tetrapeptide Gln-His-Asn-Pro surrounded by two pairs of basic residues that represent potential cleavage sites for maturation enzymes. In rats, the tissue distribution of the SMR1 mRNA is restricted to the SMG and the prostate. Only very low amounts of SMR1 mRNA can be detected in the SMG of male or female mice. Southern blot analysis indicates the presence of three genes in rats but only one in mice. Hypotheses on the physiological role of SMR1-derived peptides in male rats are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloe L., Alleva E., Böhm A., Levi-Montalcini R. Aggressive behavior induces release of nerve growth factor from mouse salivary gland into the bloodstream. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6184–6187. doi: 10.1073/pnas.83.16.6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley P. L., MacDonald R. J. Tissue-specific expression of kallikrein-related genes in the rat. Biochemistry. 1985 Aug 13;24(17):4520–4527. doi: 10.1021/bi00338a006. [DOI] [PubMed] [Google Scholar]
- Barka T. Biologically active polypeptides in submandibular glands. J Histochem Cytochem. 1980 Aug;28(8):836–859. doi: 10.1177/28.8.7003006. [DOI] [PubMed] [Google Scholar]
- Bing J., Poulsen K., Hackenthal E., Rix E., Taugner R. Renin in the submaxillary gland: a review. J Histochem Cytochem. 1980 Aug;28(8):874–880. doi: 10.1177/28.8.7003007. [DOI] [PubMed] [Google Scholar]
- Bradbury A. F., Finnie M. D., Smyth D. G. Mechanism of C-terminal amide formation by pituitary enzymes. Nature. 1982 Aug 12;298(5875):686–688. doi: 10.1038/298686a0. [DOI] [PubMed] [Google Scholar]
- Catterall J. F., Kontula K. K., Watson C. S., Seppänen P. J., Funkenstein B., Melanitou E., Hickok N. J., Bardin C. W., Jänne O. A. Regulation of gene expression by androgens in murine kidney. Recent Prog Horm Res. 1986;42:71–109. doi: 10.1016/b978-0-12-571142-5.50006-9. [DOI] [PubMed] [Google Scholar]
- Claverie J. M., Bricault L. PseqIP: a nonredundant and exhaustive protein sequence data bank generated from 4 major existing collections. Proteins. 1986 Sep;1(1):60–65. doi: 10.1002/prot.340010110. [DOI] [PubMed] [Google Scholar]
- Clements S., Mehansho H., Carlson D. M. Novel multigene families encoding highly repetitive peptide sequences. Sequence analyses of rat and mouse proline-rich protein cDNAs. J Biol Chem. 1985 Nov 5;260(25):13471–13477. [PubMed] [Google Scholar]
- Docherty K., Steiner D. F. Post-translational proteolysis in polypeptide hormone biosynthesis. Annu Rev Physiol. 1982;44:625–638. doi: 10.1146/annurev.ph.44.030182.003205. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Field L. J., Philbrick W. M., Howles P. N., Dickinson D. P., McGowan R. A., Gross K. W. Expression of tissue-specific Ren-1 and Ren-2 genes of mice: comparative analysis of 5'-proximal flanking regions. Mol Cell Biol. 1984 Nov;4(11):2321–2331. doi: 10.1128/mcb.4.11.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker L. D., Snyder S. H. Purification and characterization of enkephalin convertase, an enkephalin-synthesizing carboxypeptidase. J Biol Chem. 1983 Sep 25;258(18):10950–10955. [PubMed] [Google Scholar]
- Gresik E. W. Postnatal developmental changes in submandibular glands of rats and mice. J Histochem Cytochem. 1980 Aug;28(8):860–870. doi: 10.1177/28.8.6160181. [DOI] [PubMed] [Google Scholar]
- Heinrich G., Habener J. F. Genes encoding proteins with homologous contiguous repeat sequences are highly expressed in the serous cells of the rat submandibular gland. J Biol Chem. 1987 Apr 15;262(11):5262–5270. [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Killeen N., Barclay A. N., Willis A. C., Williams A. F. The sequence of rat leukosialin (W3/13 antigen) reveals a molecule with O-linked glycosylation of one third of its extracellular amino acids. EMBO J. 1987 Dec 20;6(13):4029–4034. doi: 10.1002/j.1460-2075.1987.tb02747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazure C., Seidah N. G., Pélaprat D., Chrétien M. Proteases and posttranslational processing of prohormones: a review. Can J Biochem Cell Biol. 1983 Jul;61(7):501–515. doi: 10.1139/o83-066. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987 Sep 4;237(4819):1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Mirels L., Bedi G. S., Dickinson D. P., Gross K. W., Tabak L. A. Molecular characterization of glutamic acid/glutamine-rich secretory proteins from rat submandibular glands. J Biol Chem. 1987 May 25;262(15):7289–7297. [PubMed] [Google Scholar]
- Mullins J. J., Burt D. W., Windass J. D., McTurk P., George H., Brammar W. J. Molecular cloning of two distinct renin genes from the DBA/2 mouse. EMBO J. 1982;1(11):1461–1466. doi: 10.1002/j.1460-2075.1982.tb01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panthier J. J., Holm I., Rougeon F. The mouse Rn locus: S allele of the renin regulator gene results from a single structural gene duplication. EMBO J. 1982;1(11):1417–1421. doi: 10.1002/j.1460-2075.1982.tb01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Kuff E. L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Piccini N., Knopf J. L., Gross K. W. A DNA polymorphism, consistent with gene duplication, correlates with high renin levels in the mouse submaxillary gland. Cell. 1982 Aug;30(1):205–213. doi: 10.1016/0092-8674(82)90026-5. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Richter K., Kawashima E., Egger R., Kreil G. Biosynthesis of thyrotropin releasing hormone in the skin of Xenopus laevis: partial sequence of the precursor deduced from cloned cDNA. EMBO J. 1984 Mar;3(3):617–621. doi: 10.1002/j.1460-2075.1984.tb01857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougeon F., Chambraud B., Foote S., Panthier J. J., Nageotte R., Corvol P. Molecular cloning of a mouse submaxillary gland renin cDNA fragment. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6367–6371. doi: 10.1073/pnas.78.10.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronik D., Dreyfus M., Babinet C., Rougeon F. Regulated expression of the Ren-2 gene in transgenic mice derived from parental strains carrying only the Ren-1 gene. EMBO J. 1987 Apr;6(4):983–987. doi: 10.1002/j.1460-2075.1987.tb04848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronik D., Ekker M., Rougeon F. Structural analysis of 5'-flanking regions of rat, mouse and human renin genes reveals the presence of a transposable-like element in the two mouse genes. Gene. 1988 Sep 15;69(1):71–80. doi: 10.1016/0378-1119(88)90379-4. [DOI] [PubMed] [Google Scholar]
- Windass J. D., Mullins J. J., Beecroft L. J., George H., Meacock P. A., Williams B. R., Brammar W. J. Molecular cloning of cDNAs from androgen-independent mRNA species of DBA/2 mouse sub-maxillary glands. Nucleic Acids Res. 1984 Feb 10;12(3):1361–1376. doi: 10.1093/nar/12.3.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen B. H., Penschow J. D., Coghlan J. P., Richards R. I. Cellular basis for the differential response of mouse kallikrein genes to hormonal induction. EMBO J. 1987 Jun;6(6):1705–1713. doi: 10.1002/j.1460-2075.1987.tb02421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]