Abstract
Suppuration of the preputial gland in mice occurs as a septic complication of fight wounds around the external genitalia. Currently reported bacterial isolates from these lesions are limited to Staphylococcus aureus, Pasteurella pneumotropica, and Klebsiella oxytoca. In the context of a pilot experiment aimed at defining the aging phenotype of estrogen receptor β knockout (BERKO) mice, 2 male mice (1 of the BERKO line and the other from the age- and sex-matched wild-type control group) were discovered at necropsy to have preputial gland lesions. In both cases, histopathologic examination confirmed severe suppuration and abscesses of the preputial glands associated with systemic reactive (secondary) amyloidosis. Both Gram staining and Bacillus Calmette-Guérin immunohistochemistry highlighted the presence of numerous bacillary to rod-shaped bacteria within the preputial lesions. Subsequent PCR analysis coupled with denaturing gradient gel electrophoresis identified Corynebacterium mastitidis in the preputial gland abscesses. This organism is isolated infrequently from the milk of sheep with subclinical mastitis and was identified as part of the normal microflora of the human ocular surface. No information regarding the epidemiology and pathogenesis of C. mastitidis infection in laboratory animals is currently available, and to our knowledge this report is the first description of C. mastitidis infection in mice.
Abbreviation: BCG, Bacillus Calmette-Guérin; BERKO, estrogen receptor β knockout mouse; DGGE, denaturing gradient gel electrophoresis
Corynebacteria are small, pleomorphic gram-positive bacteria with irregular (coryneform) morphology, including coccoid, club, and rod forms.8 Many members of the genus are commensal and colonize the skin and mucous membranes of humans and animals. Corynebacteria are well known to cause opportunistic infections in humans and animals, particularly immunocompromised subjects.1
Only a few Corynebacteria currently are recognized as pathogenic in laboratory mice. Corynebacterium bovis has been identified as the cause of ‘scaly skin disease,’ a diffuse hyperplastic and hyperkeratotic dermatitis that typically affects athymic nude mice.19,20 Corynebacterium kutscheri is the agent of pseudotuberculosis, 1 of the first multisystemic septic syndrome to be recognized in laboratory mice and rats.9,17 Corynebacterium hoffmani is suspected to play a role in conjunctivitis in BALB/c mice,17 and uncharacterized Corynebacteria represent the most common isolates in aged C57BL/6J mice with ulcerative keratoconjunctivitis.15
Suppurative inflammation and abscesses of the preputial gland, although infrequently investigated in mice, often result as a long-term septic complication of fight wounds around the external genitalia.17 Little information exists on the bacteria involved in this kind of lesion.10,17
Standard diagnostic tests, generally based on cultivation, are often suboptimal in the detection of bacteria in suppurative inflammation. The difficulty in culturing some types (particularly fastidious) of organisms means that some strains of bacteria might be overlooked and that the more easily grown species in a mixed microbial community are likely to be overrepresented. Cultivation-based limitations can be overcome by analyzing microbial DNA directly extracted from tissue. Several molecular techniques involve the use of rRNA gene sequences as tools for species identification by means of phylogenetic sequence analysis. Coupling PCR amplification of the microbial 16S rRNA gene with denaturing gradient gel electrophoresis (DGGE) generates ‘fingerprints’ of bacterial communities. In the current study, we applied the DGGE technique to investigate the microflora associated with suppurative inflammation by comparing the microbial communities present in the preputial gland tissue of affected and nonaffected mice.
Materials and Methods
Animals and husbandry.
In the context of a pilot experiment aimed at defining the aging phenotype of estrogen receptor β knockout [BERKO (129P2/OlaHsd*C57BL/6 Esr2tm1Unc/Esr2tm1Unc)] mice,13 a total of 8 male mice (BERKO, n = 4; age, 15 to 20 mo; sex- and age-matched C57BL/6 wild-type (WT), n = 4) were examined. Two mice (BERKO1 and WT1) were discovered at necropsy to have preputial gland lesions. Mice originated from the same facility where they were maintained under standard conditions (22 ± 5 °C; relative humidity, 30% to 70%; 12:12-h light:dark cycle) in 2 separate cohorts based on their BERKO or wild-type status. In both cohorts, male mice were cohoused in groups of 5 in plastic cages on nonautoclaved sawdust bedding (Lignocel 3–4; Rettenmaier and Sohne, Ellwangen-Holzmühle, Germany) and were provided standard chow (Teklad Global Rodent Diet 2018, Harlan Laboratories, Milan, Italy) and tap water ad libitum. In both groups, manifestations of aggressive behavior with occasional evidence of fight wounds on the back, tail, and external genitalia were reported sporadically.
The facility of origin provided a health monitoring system (Harlan UK Technical Services, Loughborough, UK) that complied with guidelines from the Federation of European Laboratory Animal Science Associations. The colony tested negative for ectoparasites and for the following viruses and bacteria: ectromelia virus, lymphocytic choriomeningitis virus, minute virus of mice, mouse adenovirus type 1 (MAd FL), mouse adenovirus type 2 (MAd K87), mouse cytomegalovirus, mouse hepatitis virus, mouse norovirus, mouse parvovirus, mouse rotavirus, pneumonia virus of mice, reovirus type 3, Sendai virus, Theiler murine encephalomyelitis virus, Clostridium piliforme, Bordetella bronchiseptica, Citrobacter rodentium, Corynebacterium kurcheri, Mycoplasma spp., Pasteurella spp., Salmonella sp., Streptobacillus moniliformis, β-hemolytic streptococci, and Streptococcus pneumoniae. The colony tested positive for Helicobacter rodentium, Syphacia obvelata, Spironucleus spp., and Tritrichomonas spp. On entering the animal rooms, personnel donned disposable gowns, shoe covers, latex gloves, face masks, and hair covers. The cages were changed twice each week without using a changing station; caretakers disinfected their latex gloves with Tego Spray (Johnson Diversey, Sturtevant, WI) during cage changing. Procedures involving animals and their care conformed to institutional guidelines in compliance with national and international laws andregulations.4,11,18
Necropsy, histopathology, and immunohistochemistry.
Mice were euthanized by CO2 asphyxiation followed by cervical dislocation, and a complete necropsy was performed. Salivary glands, thyroids, lungs, heart, liver, spleen, kidneys, pancreas, stomach, small intestine, large intestine, seminal vesicles, prostate, urinary bladder, preputial glands, bulbourethral glands, cervical and mesenteric lymph nodes, brain, and sternum and vertebrae with bone marrow were collected in 10% neutral buffered formalin and routinely processed with hematoxylin and eosin for histologic evaluation. In BERKO1 and WT1, additional histologic sections from spleen, liver, and kidneys were stained with Congo Red for amyloid detection. Serial sections from preputial gland lesions were Gram stained and immunostained by using a rabbit polyclonal primary antibody raised against Mycobacterium bovis(Bacillus Calmette–Guérin, BCG; Dako, Glostrup, Denmark) by using a standard avidin–biotin complex immunoperoxidase technique (ABC Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA). Serial sections of formalin-fixed, paraffin-embedded, unaffected preputial glands from 2 BERKO mice (BERKO2 and BERKO3) and 1 wild-type mouse (WT2) of the same pilot study were used as controls.
Cytology.
Air-dried smears of bone marrow from the femurs of the mice were stained with May–Grunwald–Giemsa for cytologic examination.
PCR and sequencing.
DNA was extracted from formalin-fixed, paraffin-embedded tissues. Sterile razor blades were used to harvest 30-μg samples of tissue from the centers of the preputial gland abscesses of the 2 affected mice (BERKO1 and WT1). Similar samples of formalin-fixed, paraffin-embedded, unaffected preputial glands from 2 BERKO mice (BERKO2 and BERKO3) and 1 wild-type mouse (WT2) of the same pilot study were used as controls. Samples were collected in sterile 1.5-mL tubes and dewaxed in 1 mL xylene (VWR BDH Prolabo, Arlington Heights, IL) for 20 min, centrifuged at 1000 × g for 3 min, and washed twice with 100% ethanol (Merck KGaA, Darmstadt, Germany). DNA extracted from the pellet by using a DNA minikit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. 16S rRNA fragments were amplified with primers EUBf933 (5′ CAC AAG CGG TGG AGC ATG TGG 3′) and EUBr1387 (5′ GCC CGG GAA CGT ATT CAC CG 3′), which are specific for universally conserved bacterial 16S rRNA sequences.12 Amplifications were performed in 50 μL PCR buffer (Eppendorf, Hamburg, Germany) containing 1.5 mM MgCl2, 20 pmol each primer, 200 μM each dNTP and 1.25 U Taq polymerase (Eppendorf, Hamburg, Germany). Incubation conditions consisted of an initial incubation at 95 °C for 3 min, followed by 35 cycles of 95 °C for 35 s, 64 °C for 30 s and 72 °C for 40 s, and then a final incubation at 72 °C for 8 min.
The amplicons were separated on the basis of their melting behavior by DGGE. Polyacrylamide gels (7% of a 37:1 acrylamide–bisacrylamide mixture in 1× Tris–acetate–EDTA buffer) were prepared by using a gradient maker (BioRad, Milan, Italy) according to the manufacturer's guidelines to achieve a gradient of 40% (top) to 60% (bottom) denaturant, where 100% denaturant is 7 M urea in 40% formamide.16 Gels were run for 15 h at 90 V in 1× TAE buffer at a constant temperature of 60 °C in (D-Code Electrophoresis System, BioRad). The gels were stained for 30 min in 1× TAE buffer containing SYBR Green (Molecular Probes, Leiden, Netherlands). Gels were visualized and digitally imaged (GelDoc 2000, BioRad) by using Diversity Database software (Bio-Rad). Major bands were excised from the gel by using a sterile blade, transferred to 50 µL deionized double-distilled water, incubated at 4 °C overnight, and frozen at –20 °C prior to reamplification by using the same primers and protocol as previously. A 10-μL sample of the PCR products was electrophoresed in 2% agarose (Promega, Milan, Italy) and stained with ethidium bromide (Euroclone, Milan, Italy). Negative controls consisting of the PCR master mixture without genomic DNA were included in all amplifications. Amplification products were purified by using QIAquick PCR Purification Kit (Qiagen) and sequenced by BMR Genomics (Padova, Italy) on an automated sequencer (ABI377, Applied Biosystems, Foster City, CA) by using a dye-terminator cycle sequencing kit (ABI Prism, Applied Biosystems) with Amplitaq DNA polymerase (Applied Biosystems). After manual editing and exclusion of the primer regions, the percentage of similarity with reference sequences was evaluated through BLAST homology analysis (www.ncbi.nlm.nih.gov/BLAST). The sequences were submitted to the GenBank database, with accession numbers FJ970963 for BERKO mouse A1 and FJ970964 for WT mouse A2
Results
For both affected mice (BERKO1 and WT1), gross findings at necropsy included mild dehydration, patchy alopecic areas with skin scaling and crusting along the tail base or perianal region, diffuse moderate visceral serous fat atrophy, mild diffuse splenomegaly, and pitted renal capsular surface. Both lobes of the preputial gland had multiple fibrous adhesions with surrounding panniculus and appeared replaced and expanded (approximately 3 times normal) by multinodular capsulated cavitations containing suppurative exudate (abscesses).
Histopathologic examination confirmed that both the mice with preputial gland abscesses (BERKO1 and WT1) had concurrent systemic amyloidosis with prominent deposits in the renal glomeruli (Figure 1), splenic marginal zone, hepatic vessel adventitia, and intestinal lamina propria. The deposits stained light pink to orange with Congo red and demonstrated green birefringence under polarized light. Most of the tissue samples examined (particularly lungs, kidneys, and mesenteric ligaments) had multifocal perivascular reactive infiltrates of lymphocytes and plasma cells with scattered Mott cells and histiocytes. Examined nodes displayed variable degree of sinus histiocytosis and plasmocytosis of medullary cords. Bone marrow cytology revealed an increased number of plasma cells with numerous Mott cells and reactive myeloid hyperplasia. None of the BERKO and WT mice without preputial gland abscessation displayed systemic amyloidosis, plasmocytosis of lymphoid organs, or infiltration of reactive mononuclear cells into multiple tissues. In the affected preputial glands, lobules were almost completely effaced by a prominent necrosuppurative infiltrate. Ducts were segmentally dilated with marked irregular hyperplasia of the squamous epithelium, hyperkeratosis, and luminal accumulation of keratosebaceous and suppurative material. Surrounding interstitium was diffusely expanded by granulation tissue proliferation, lymphohistiocytic and plasmacytic infiltrates, and fibrosis extending into the adjacent subcutis (Figure 2). Both Gram staining and BCG immunohistochemistry highlighted the presence numerous irregular 0.5 × 1 μm bacillary to rod-shaped bacteria within the keratosebaceous and suppurative infiltrates (Figures 3 and 4). No gram-positive or BCG-immunoreactive microorganisms were present in the normal preputial glands from control animals (BERKO2, BERKO3, and WT2). Additional histologic findings common to all the examined BERKO and WT mice included massive mucosal colonization of the small and large intestine by flagellate protozoan parasites (Spironucleus spp. and Tritrichomonas spp., respectively), massive colonization of crypts of large intestine by spiral shaped bacteria (Helicobacter spp.), and occasional cross- and tangential sections of pinworms (Syphacia obvelata) in the colon.
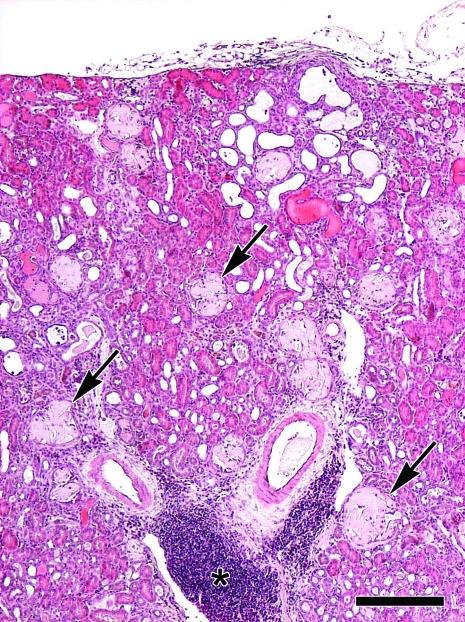
Figure 1.
Male 15-mo-old BERKO mouse with suppuration of the preputial gland. Histopathologic examination of the kidney shows severe multifocal global expansion of renal glomeruli (arrows) and, to a lesser extent, perivascular interstitium by dense hypocellular pale eosinophilic amorphous material consistent with amyloid. Note the prominent perivascular pseudofollicular infiltrates of reactive lymphocytes and plasma cells (asterisk). Hematoxylin and eosin stain; bar, 250 μm.
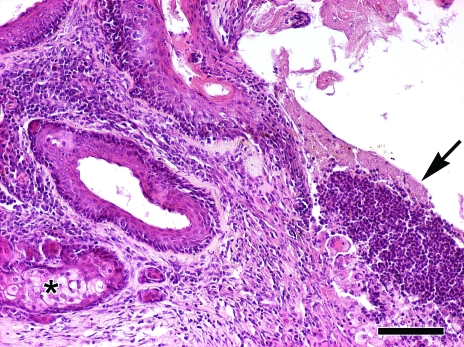
Figure 2.
Male 15-mo-old BERKO mouse with suppuration of the preputial gland. Histopathologic examination of the affected preputial glands shows severely distended ducts with hyperplasia and hyperkeratosis of the lining squamous epithelium, and luminal accumulation of keratosebaceous and suppurative material (arrow). Residual holocrine lobules (asterisk) are surrounded by fibrosis and sparse infiltration of lymphocytes and plasma cells. Hematoxylin and eosin stain; bar, 100 μm.
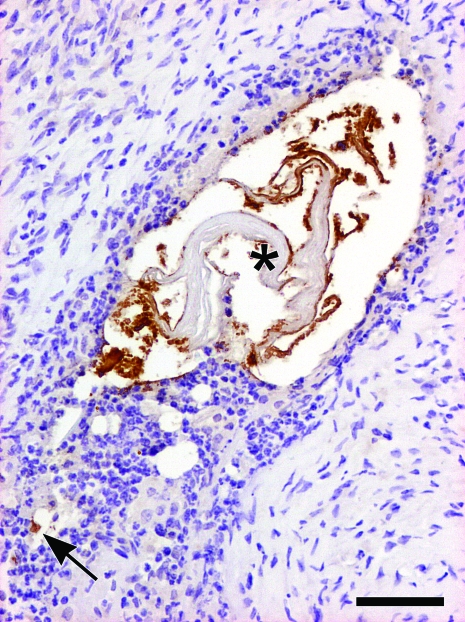
Figure 3.
Male 17-mo-old C57BL/6 WT control mouse with suppuration of the preputial gland. The duct of the preputial gland is disrupted by suppuration with numerous BCG-positive bacteria within the karyolytic neutrophils (arrow) and along the keratin lamellae in the lumen (asterisk). Immunohistochemical stain for BCG; Mayer hematoxylin counterstain; bar, 50 μm.
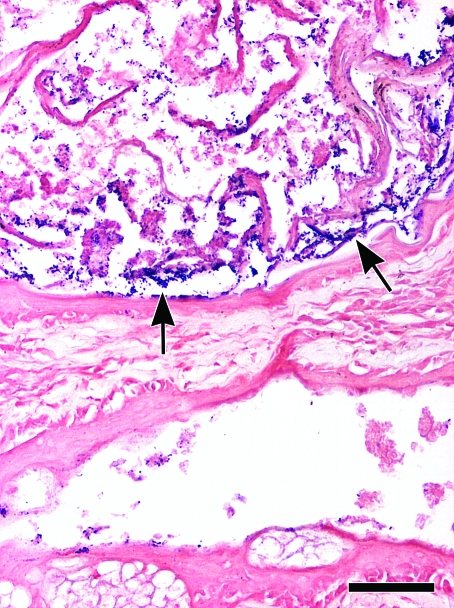
Figure 4.
17-mo-old C57BL/6 WT control mouse with suppuration of the preputial gland. Numerous gram-positive bacillary to rod-shaped bacteria within the keratosebaceous and suppurative collections of the affected preputial glands. As seen with BCG immuhistochemistry (Figure 3), note the peculiar pattern of distribution of the bacteria along the keratin lamellae (arrows). Gram stain; bar, 50 μm.
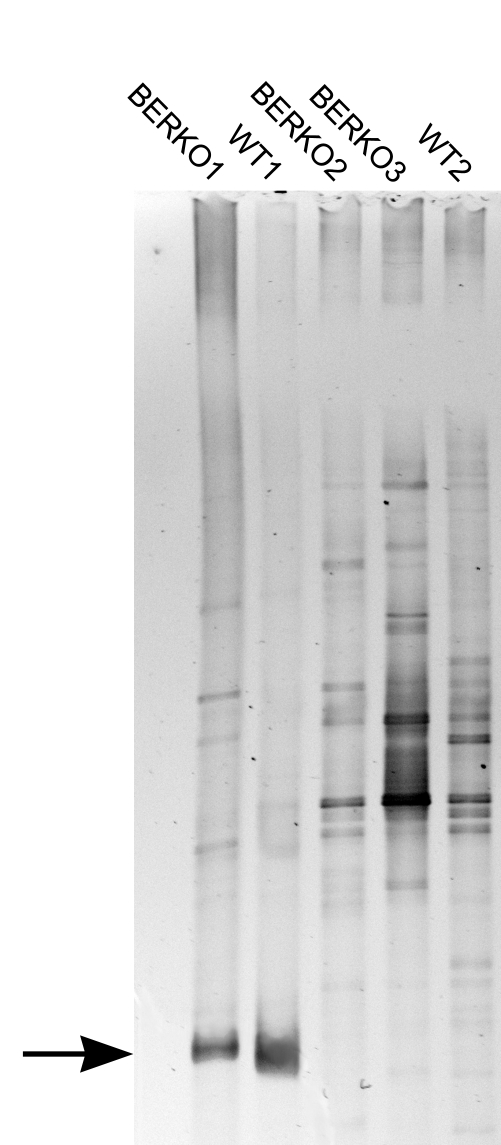
PCR–DGGE analysis targeting the microbial 16S rRNA gene showed numerous bands with similar migration patterns in both affected (Figure 5; BERKO1 and WT1) and nonaffected (Figure 5; BERKO2, BERKO3 and WT2) mice. In addition, 2 major bands with identical migration patterns appeared exclusively in the samples from the mice affected by suppurative adenitis of preputial glands (Figure 5; BERKO1 and WT1). Reamplification and sequencing of these 2 dominant bands excised from PCR–DGGE gels exhibited 99.5% similarity at the nucleic acid level with Corynebacterium mastitidis.
Figure 5.
PCR–DGGE gel showing the bacterial communities in the preputial glands of affected (BERKO1 and WT1) and nonaffected (BERKO2, BERKO3, and WT2) mice. Two dominant bands (arrow) are unique to samples from the preputial glands abscesses.
Discussion
In mice, preputial glands are modified sebaceous glands that produce various pheromones that influence important social features including sexual attractiveness, promotion of the estrous cycle in grouped females, dominance, and aggression.22 Aggressive behavior (‘pugilistic’ activity) is common among cohoused adult laboratory mice, and suppurative inflammation and abscesses of the preputial gland, although infrequently reported in the current literature, often results from long-term septic complications of fight wounds oriented around the external genitalia.17 Reported bacterial isolates from preputial gland abscesses currently are limited to Staphylococcus aureus, Pasteurella pneumotropica and Klebsiella oxytoca.10,17 To our knowledge, this report is the first description of C. mastitidis infection in mice.
The etiologic diagnosis for the mice we present was achieved by combining morphologic (Gram stain and BCG immunohistochemistry) identification of the bacterium within the exudate of the affected glands and PCR–DGGE analysis. BCG immunohistochemistry is a valuable diagnostic tool because, for unknown reasons, it specifically recognizes a broad spectrum of pathogenic microorganisms including bacteria, fungi, and protozoa in tissue sections.2,14,21 We combined PCR–DGGE analysis targeting the microbial 16S rRNA gene by using universal eubacterial primers with reamplification and sequencing of the dominant bands to identify C. mastitidis in the lesions of the preputial glands. Numerous bands with comparable migration features were apparent among all the samples. This result was expected given the heterogeneous microbial communities of the normal prepuce and associated glands. Interestingly, the bands corresponding to C. mastitidis were present only in the 2 samples from the preputial abscesses, suggesting that this bacterium had a key role in the development of the lesions.
The origin of the C. mastitidis organism in our mice is uncertain. Many corynebacteria are well known to be commensal in humans and animals.8 C. mastitidis was first isolated from the milk of sheep with subclinical mastitis, and the organism subsequently was identified in as part of the normal microflora of the ocular surface in humans, but no information regarding C. mastitidis in either healthy or sick mice is available.5-7 We cannot exclude the possibility that C. mastitidis is present in healthy murine preputial glands, where it sporadically can cause abscesses in cases of fight lesions. Similarly, C. mastitidis may belong to the commensal flora of the murine oral cavity and may reach the preputial glands through bites during the so-called pugilistic activity. In fact, organisms similar to C. mastitidis, such as C. kutscheri, are commonly isolated from the oral cavity of healthy rodents, and bite wounds by animals harboring oral C. kutscherihave been reported to develop cutaneous infections.9 Because the normal microflora of the ocular surface of humans includes C. mastitidis,5 oculonasal secretions and skin from personnel should also be considered potential sources of infection. However, this route of transmission seems unlikely in our cases, given that personnel were equipped with disposable gown, disinfected gloves, face masks, and hair covers during routine husbandry procedures. In the current study, only formalin-fixed paraffin embedded material was available, which prevented us from better detailing the origin and role of C. mastitidis in mice; specific investigations aimed at defining these aspects seem necessary. Nevertheless, parallels between C. bovis and C. mastitidis exist: both can infect mice and cause mastitis in ruminants.3,6,19,20
In laboratory mice, chronic suppurative lesions of the preputial glands represent a leading cause of reactive systemic (secondary) amyloidosis.17 Systemic amyloidosis was confirmed in our cases as well. Although we did not definitively characterize the type of amyloid deposits in our mice, the concurrent preputial gland abscesses and disseminated deposits of amyloid favored a diagnosis of reactive systemic (secondary) amyloidosis in the context of a chronic septic inflammatory condition.
Acknowledgments
We wish to express our gratitude to Dr Robert D Cardiff (Center for Comparative Medicine, University of California, Davis, CA), Dr Alberto Gobbi (Department of Experimental Oncology, European Institute of Oncology, Milan, Italy), and Professor Daniele Giuseppe Daffonchio (Dipartimento di Scienze e Tecnologie alimentari e Microbiologiche, Università degli Studi di Milano, Milan, Italy) for their critical review of the manuscript and insightful comments. We also gratefully acknowledge Drs Barbara Banco, Camilla Recordati, and Vittoria Castiglioni and Mr Marco Brevi (Department of Veterinary Pathology, Hygiene, and Public Health, Università degli Studi di Milano) for assistance with necropsy sessions and immunohistochemical procedures. We declare no competing interests that might be perceived to influence the results and discussion reported in this manuscript.
References
- 1.Adderson EE, Boudreaux JW, Hayden RT. 2008. Infections caused by coryneform bacteria in pediatric oncology patients. Pediatr Infect Dis J 27:136–141 [DOI] [PubMed] [Google Scholar]
- 2.Bonenberger TE, Ihrke PJ, Naydan DK, Affolter VK. 2001. Rapid identification of tissue microorganisms in skin biopsy specimens from domestic animals using polyclonal BCG antibody. Vet Dermatol 12:41–47 [DOI] [PubMed] [Google Scholar]
- 3.Boyer P. 1998. Mastitis in dairy herds associated with Corynebacterium bovis. Vet Rec 143:175–176 [PubMed] [Google Scholar]
- 4.Council of the European Communities Council Directive 86/609/EEC of 24 November 1986 on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes. Off J Eur Communities L358: 1–28 [Google Scholar]
- 5.Eguchi H, Kuwahara T, Miyamoto T, Nakayama-Imaohji H, Ichimura M, Hayashi T, Shiota H. 2008. High-level fluoroquinolone resistance in ophthalmic clinical isolates belonging to the species Corynebacterium macginleyi. J Clin Microbiol 46:527–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez-Garayzabal JF, Collins MD, Hutson RA, Fernandez E, Monasterio R, Marco J, Dominguez L. 1997. Corynebacterium mastitidis sp. nov., isolated from milk of sheep with subclinical mastitis. Int J Syst Bacteriol 47:1082–1085 [DOI] [PubMed] [Google Scholar]
- 7.Fernández-Garayzábal JF, Vela AI, Egido R, Hutson RA, Lanzarot MP, Fernández-García M, Collins MD. 2004. Corynebacterium ciconiae sp. nov. isolated from the trachea of black storks (Ciconia nigra). Int J Syst Evol Microbiol 54:2191–2195 [DOI] [PubMed] [Google Scholar]
- 8.Funke G, von Graevenitz A, Clarridge JE, 3rd, Bernard KA. 1997. Clinical microbiology of coryneform bacteria. Clin Microbiol Rev 10:125–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmes NE, Korman TM. 2007. Corynebacterium kutscheriinfection of skin and soft tissue following rat bite. J Clin Microbiol 45:3468–3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong CC, Ediger RD. 1978. Perputial gland abscess in mice. Lab Anim Sci 28:153–156 [PubMed] [Google Scholar]
- 11.Institute of Laboratory Animal Resources 1996. Guide for the care and use of laboratory animals Washington (DC): National Academies Press; [DOI] [PubMed] [Google Scholar]
- 12.Iwamoto T, Tani K, Nakamura K, Suzuki Y, Kitagawa M, Eguchi M, Nasu M. 2000. Monitoring impact of in situ biostimulation treatment on groundwater bacterial community by DGGE. FEMS Microbiol Ecol 32:129–141 [DOI] [PubMed] [Google Scholar]
- 13.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. 1998. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA 95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kutzner H, Argenyi ZB, Requena L, Rütten A, Hügel H. 1998. A new application of BCG antibody for rapid screening of various tissue microorganisms. J Am Acad Dermatol 38:56–60 [DOI] [PubMed] [Google Scholar]
- 15.McWilliams TS, Waggie KS, Luzarraga MB, French AW, Adams RJ. 1993. Corynebacterium species-associated keratoconjunctivitis in aged male C57BL/6J mice. Lab Anim Sci 43:509–512 [PubMed] [Google Scholar]
- 16.Muyzer G, de Waal EC, Uitterlinden AG. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of PCR-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Percy DH, Barthold SW. 2007. Mouse, p 3–124 : Percy DH, Barthold SW. Pathology of laboratory rodents and rabbits, 3rd ed Ames (IA): Wiley–Blackwell [Google Scholar]
- 18.Presidenza della Repubblica Italiana Gazzetta Ufficiale. 1992. ;(supplement 40) D.L. N.116, 18-2-1992 [Google Scholar]
- 19.Scanziani E, Gobbi A, Crippa L, Giusti AM, Giavazzi R, Cavalletti E, Luini M. 1997. Outbreaks of hyperkeratotic dermatitis of athymic nude mice in northern Italy. Lab Anim 31:206–211 [DOI] [PubMed] [Google Scholar]
- 20.Scanziani E, Gobbi A, Crippa L, Giusti AM, Pesenti E, Cavalletti E, Luini M. 1998. Hyperkeratosis-associated coryneform infection in severe combined immunodeficient mice. Lab Anim 32:330–336 [DOI] [PubMed] [Google Scholar]
- 21.Szeredi L, Glávits R, Tenk M, Jánosi S. 2008. Application of antiBCG antibody for rapid immunohistochemical detection of bacteria, fungi, and protozoa in formalin-fixed paraffin-embedded tissue samples. Acta Vet Hung 56:89–99 [DOI] [PubMed] [Google Scholar]
- 22.Zhang JX, Rao XP, Sun L, Zhao CH, Qin XW. 2007. Putative chemical signals about sex, individuality, and genetic background in the preputial gland and urine of the house mouse (Mus musculus). Chem Senses 32:293–303 [DOI] [PubMed] [Google Scholar]