Abstract
Objective:
To evaluate the leishmanicidal activity of saponin, dasyscyphin C of Eclipta prostrata and sapogenin, gymnemagenol from Gymnema sylvestre leaves under in vitro conditions.
Materials and Methods:
Dasyscyphin C/Gymnemagenol were dissolved in phosphate buffered saline (PBS) and diluted with liquid medium to obtain concentrations ranging from 1000 to 15 μg /ml. The leishmanicidal activity against leishmanial parasites, Leishmania major, Leishmania aethiopica and Leishmania tropica promastigotes was studied by the MTS assay.
Result:
The Dasyscyphin C isolated from E. prostrata showed good leishmanicidal activity at 1000μg/ml concentration, with the IC50 value of 450μg/ml against L. major promastigote and the percentage of parasitic death was 73; whereas, gymnemagenol of G. sylvestre showed only 52% parasitic death at 1000 μg/ml concentration. The other Leishmania species, L. aethiopica and L. tropica promastigotes, were less sensitive to the saponins of E. prostrata and G. sylvestre.
Conclusion:
From this study, it can be concluded that the dasyscyphin C of E. prostrata has significant leishmanicidal activity against L. major promastigote.
Keywords: Eclipta prostrata, Gymnema sylvestre, leishmania promastigotes, Leishmanicidal activity, saponins
Introduction
Leishmaniasis is one of the leading causes of morbidity and mortality and it has been found to be a major global health problem, with 1.5 to 2 million humans affected by the disease annually. Nearly 350 million people in 88 countries are estimated to be threatened by the disease.[1] It is caused by Leishmania, a protozoa transmitted by Phlebotomus sp., commonly called sand flies. There are more than thirty species of sand flies responsible for the spread of Leishmaniasis. The available chemotherapy still relies on pentavalent antimonials, amphotericin B or pentamidine. Besides, they are reported to be associated with numerous toxic side effects, on prolonged use.[2,3] Hence, a search for a new active compound with potential leishmanicidal property remains essential for the development of a new antileishmanial therapy. Extracts from medicinal plants are being widely tested for leishmanicidal activity.[4] Studies on the antileishmanial activity of isolated active compounds from medicinal plants are scanty.
G. sylvestre leaves are traditionally used as medicine for the control of diabetes mellitus and stomach ache. They are also often used as a diuretic agent.[5] The decoction of E. prostrata leaves has long been used orally to control jaundice.[6] Earlier reports from our laboratory revealed the antimicrobial activity of gymnemagenol and dasyscyphin C.[7]
In the present study, the leishmanicidal activity of dasyscyphin C and gymnemagenol was evaluated against four Leishmania promastigotes under in vitro conditions.
Materials and Methods
Plant material
E. prostrata (Asteraceae) and G. sylvestre (Asclepiadaceae) leaves were collected in December 2007, from Jawadi Hills, Eastern Ghats, Tamil Nadu, India. The Voucher specimens were prepared and deposited at the Herbarium section of the VIT University, Tamil Nadu, India. The leaves of G. sylvestre and E. prostrata were washed with distilled water, shade dried, powdered and stored in an air- tight container until further use.
Isolation and Purification of saponin
The powdered sample was defatted by petroleum ether for 3 h at 40°C. After filtering the petroleum ether, the sample was extracted with methanol for 3 h, with mild heating. The methanol extract was concentrated and re-extracted with methanol and acetone (1: 5 v/v) as described by Yan et al.[8] The precipitate obtained was dried under vacuum, which turned to a whitish amorphous powder after complete drying. It was loaded on Merck silica gel-60 (230-400 mesh) column and eluted with chloroform-methanol-water (70: 30: 10), as described by Favel et al.[9] The first fraction collected was air dried at room temperature (28°C) and the residue obtained was treated as the pure saponin of G. sylvestre and E. prostrata. The purity of the saponin isolated was analyzed by thin layer chromatography, using chloroform and methanol (7 : 3) as solvent system.
Source of Leishmania species
All the cultures of the promastigote forms of Leishmania were obtained from the London School of Hygiene and Tropical Medicine and Armauer Hansen Research Institute, Addis Ababa, Ethiopia.
Assay of Leishmanicidal activity
Saponins were dissolved in phosphate buffered saline (PBS) and diluted with liquid medium, to obtain the concentrations ranging from 1000 to 15 μg/ml. The parasites were distributed in 96 well plates (Nunc, UK) (1×106 parasites/ml) in triplicate, and each experiment was repeated twice. The plates were incubated for 24 h, at 22°C or 26°C, depending on the species being tested. Inhibition of promastigote growth was determined by the MTS assay.[10] The percentage of growth inhibition was determined by comparing the treated groups with the untreated controls after 48 hours.
MTS assay
A solution of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl) -2- (4-sulfophenyl)-2H-tetrazolium, inert salt (MTS) was prepared (2mg/ml in PBS 0.02 M, pH 7.2) and stored at −20°C. Phenazine methosulfate (PMS, 0.92 mg/ml) was similarly prepared in PBS (0.02 M, pH 7.2) and stored at −20°C. For the evaluation, 20 μl of a MTS/PMS (5:1) mixture was added to each well containing 200 μl of treated and/or untreated Leishmania cultures, seeded at an initial concentration of 106 cells/ml. The entire plate was incubated at 37°C for 3 h. The absorbance was measured at 490 nm and the percentage of viability calculated as described by Ganguly et al.[10]
Structural Elucidation
The purified saponins were subjected to structural elucidation by Ultraviolet-visible spectroscopy (TECHCOMP), Fourier transform - Infrared spectroscopy (THERMO NICOLET -330), 1H-Nuclear Magnetic Resonance spectroscopy (JEOL GSX-500), 13C-Nuclear Magnetic Resonance spectroscopy (JEOL GSX-500) and Mass spectroscopy (FINNIGAN MAT-8230). All the chemicals used for extraction and purification were of analytical grade, obtained from SRL, Mumbai, India.
Results
The leishmanicidal activity of saponins dasyscyphin C and gymnemagenol isolated from E. prostrata and G. sylvestre was tested against three Leishmania species. The viable parasites were counted in the culture media, to calculate the percentage of parasitic death at different concentrations of dasyscyphin C and gymnemagenol used to test the leishmanicidal activity. The effect of the different concentrations (1000, 500, 250, 125, 62.5, 31.25 and 15.625 μg/ml) of dasyscyphin C of E. prostrata and gymnemagenol of G. sylvestre on the viability of L. major is shown in Table 1. The IC50 value for the inhibition of the growth of L. major promastigote was calculated to be 450 and 965 μg/ml respectively for dasyscyphin C and gymnemagenol.
Table 1.
Effect of dasyscyphin C and gymnemagenol isolated from the leaves of Gymnema sylvestre and Eclipta prostrata on Leishmania major promastigote
| Saponin/Sapogenin (μg/ml) | % Growth of Leishmania major | |
|---|---|---|
| Dasyscyphin C | Gymnemagenol | |
| 1000 | 27.01±3.04 | 48.39±8.53 |
| 500 | 51.33±13.70 | 76.77±6.71 |
| 250 | 62.41±10.06 | 81.10±2.38 |
| 125 | 69.96±4.29 | 90.26±4.00 |
| 62.5 | 69.50±13.30 | 98.76±8.24 |
| 31.25 | 76.06±7.10 | 96.84±2.00 |
| 15.625 | 88.82±3.30 | 110.73±12.66 |
| 0 (control) | 100 | 100 |
Values are mean ± S.D. of six experiments.
The dasyscyphin C and the gymnemagenol significantly inhibited (73 and 52% respectively) the growth of L. major promastigote at 1000 μg/ml concentration [Figure 1]. The reduction in the growth of L. aethiopica and L. tropica promastigote forms was minimal (24 and 22% respectively) at 1000 μg/ml concentration of dasyscyphin C. The gymnemagenol at 1000 μg/ml concentration inhibited (22 and 16% respectively) minimally the growth of L. aethiopica and L. tropica promastigotes. The reduction in parasitic growth was maximal, when the parasitic culture was exposed to dasyscyphin C. Further, both dasyscyphin C and the gymnemagenol exhibited concentration dependent inhibition on the growth of L. major.
Figure 1.
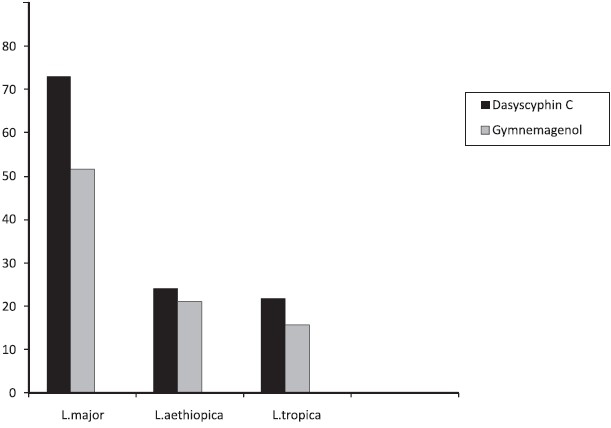
Effect of Dasyscyphin C and Gymnemagenol on L. major, L. aethiopica and L. tropica promastigotes. Parasitic death was calculated after 48 hours of exposure of Dasyscyphin C and Gymnemagenol (1000 μg/ml) of E. prostrata and G.sylvestre.
Dasyscyphin C. Jelly, 1H NMR (500 MHz in CDCl3), (OH) – 2.032 (m, 2H), 1-H -0.992 (t,2H), 2-H -0.889 (m,2H), 3-H -0.910 (t,2H), 5-H -1.316 (t,1H), 6-H -0.859 (t,2H), 7-H -0.876 (dd,2H), 9-H -1.301 (d,1H), 11-H -1.704 (t,2H), 12-H -5.197 (m,1H), 16-H -6.158 (s,1H), 18-H -1.629 (t,3H), 19,20-H -1.270 (s,6H), 21-H -2.310 (s,3H), 22-H -5.411 (m,2H), 22-H -5.411 (m,2H), 2′-H -3.683 (s,2H), 4′-H -4.069 (s,2H), 6′-H -1.149 (s,3H). ESI-m/z: 503 [M]−
Gymnemagenol. Crystalline needles, 1H NMR (500 MHz in CDCl3),δ: 5.182 (9H,m,H-18,H-19,H-24); 5.104(1H,t,H-6); 4.060(3H,dd,H-11,H-12); 3.505(4H,S,H-16,H-17);2.790(1H,t,H-1); 2.313 (3H,m,H-5,H-4);2.291(2H,t,H-2); 2.084(1H,d,H-7);2.029(4H,m,H-20,H-25,H-26,H-27); 1.619 (3H, m,H-14,H-15); 1.275 (10H,m,H-3,H-8,H-9,H-10,H-13); 1.235(3H,s,H-23); 0.876 (6H,m, H-21,H-22). ESI- MS m/z: 474 [M]+
Discussion
The leishmanicidal activity of dasyscyphin C and gymnemagenol, isolated from the medicinal plants E. prostrata and G. sylvestre, has been reported in this study. Saponins are the glycosides of triterpenoid or steroid aglycone as their backbone. The leishmanicidal activity of racemate type of compound derived from wood of Cordia fragrantissima has already been reported.[11] Leishmanicidal activity of a furostan-type of saponin from Brunfelsia grandiflora has been reported against L. major under in vitro conditions[12].
Recently, we reported that the isolated saponins from G. sylvestre and E. prostrata possess significant antibacterial and antifungal activity.[7] Further, these saponins have good cytotoxic activity on HeLa cells (unpublished data). However, the in vitro effects of these saponins on selected Leishmania promastigotes have not been reported till date. Thus, the present study was carried out to evaluate the leishmanicidal activity of these saponins on the growth of Leishmania promastigotes under in vitro conditions.
In our study, dasyscyphin C of E. prostrata was found to be more effective than gymnemagenol of G. sylvestre in inhibiting the growth of L. major promastigote. However, these saponins were not very effective against L. aethiopica and L. tropica promastigotes, even at higher concentrations tested.
Cardioquinol, isolated from the wood of Cordia fragrantissima, has been shown to contain very effective leishmanicidal activity at low concentrations (IC50 81.2 μg/ml).[11] As shown in Figure 1, L. major promastigote growth was significantly inhibited by the dasyscyphin C and gymnemagenol, with the concentrations of 500 and 1000 μg/ml. This value indicates a significant inhibitory activity against L. major promastigote.
Dasyscyphin C [Figure 2] and gymnemagenol [Figure 3] identity was proved by Thin layer chromatography, UV, FT-IR, 1H NMR and MS analysis. The presence of triterpenes in TLC plate was confirmed by Libermann-Burchard reaction and Carr-Price reagent. The UV spectrum showed the maximum absorption bands at 234, 238, 302 for dasyscyphin C and 223, 237, 274 for gymnemagenol, and the IR spectrum showed at 3435.80, 2921.82, 1635.05, 1245.75, 1050.66 for dasyscyphin C, and 3445.41, 2924.10, 1635.38, 1457.48 cm−1 for gymnemagenol. The FINNIGAN MAT 8230MS showed the [M] -ion at m/z 503 with the base peak at m/z 208 for dasyscyphin C and for gymnemagenol showed the [M] + ion at m/z 474 with the base peak at m/z 251. The chemical shift assignments obtained for dasyscyphin C and gymnemagenol from 1H-NMR correspond to the molecular formula C28H40O8[13] and C30H50O4.[14]
Figure 2.
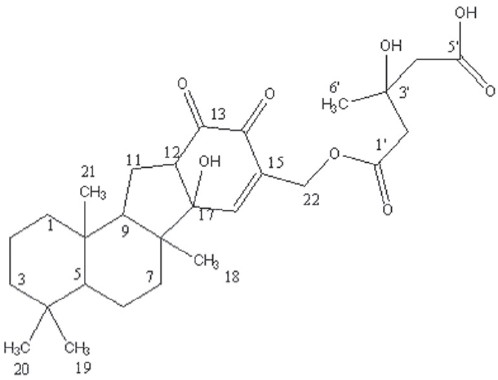
Structure of Dasyscyphin C
Figure 3.
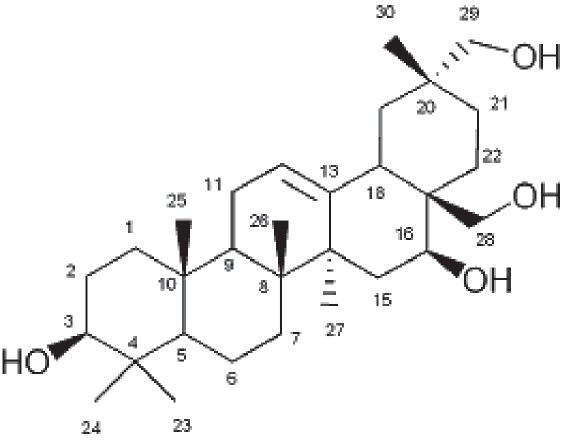
Structure of Gymnemagenol
Acknowledgments
The authors thank the management of VIT University, for their support in carrying out this study.
References
- 1.World Health Organisation. Leishmaniasis: The disease and its epidemiology. Available from: http://www.who.int/leishmaniasis/disease_epidemiology/en/index.html. [cited in 2007]
- 2.Davis AJ, Murray HW, Handman E. Drugs against leishmaniasis: A synergy of technology and partnerships. Trends Parasitol. 2004;20:73–6. doi: 10.1016/j.pt.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Berman JD. Current treatment approaches to leishmaniasis. Curr Opin Infect Dis. 2003;16:397–401. doi: 10.1097/00001432-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi M, Fuchino H, Sekita S, Satake M. In vitro leishmanicidal activity of some scarce natural products. Phytother Res. 2004;18:573–8. doi: 10.1002/ptr.1502. [DOI] [PubMed] [Google Scholar]
- 5.Reddy S, Gopal G, Sita G. In vitro multiplication of Gymnema sylvestre R Br: An important medicinal plant. Curr Sci. 2004;10:1–4. [Google Scholar]
- 6.Sandhya B, Thomas S, Isabel W, Shenbagarathai R. Ethnomedicinal plants used by the Valaiyan community of Piranmalai hills (reserved forest), Tamil Nadu, India: A pilot study. Afr J Traditional Compl Alter Med. 2006;3:101–14. [Google Scholar]
- 7.Gopiesh V, Kannabiran K. Larvicidal effect of Hemidesmus indicus, Gymnema sylvestre and Eclipta prostrata against Culex quinquifaciatus mosquito larvae. Afr J Biotechnol. 2007;3:307–11. [Google Scholar]
- 8.Yan W, Ohtani K, Kasai R, Yamasaki K. Steroidal saponin from fruits of Tribulus terrestris. Phytochem. 1996;42:1417–22. doi: 10.1016/0031-9422(96)00131-8. [DOI] [PubMed] [Google Scholar]
- 9.Favel A, Kemertelidze E, Benidze M, Fallague K, Regli P. Antifungal activity of steroidal glycosides from Yucca gloriosa L. Phytother Res. 2005;19:158–61. doi: 10.1002/ptr.1644. [DOI] [PubMed] [Google Scholar]
- 10.Ganguly S, Bandyopadhyay S, Bera A, Chatterjee M. Antipromastigote activity of an ethanolic extract of leaves of Artemisia indica. Indian J Pharmacol. 2006;38:64–5. [Google Scholar]
- 11.Mori K, Kawano M, Fuchino H, Ooi T, Satake M, Agatsuma Y, et al. Antileishmanial compounds from Cordia fragrantissima collected in Burma. J Nat Prod. 2007;71:18–21. doi: 10.1021/np070211i. [DOI] [PubMed] [Google Scholar]
- 12.Fuchino H, Sekita S, Mori K, Kawahara N, Satake M, Kiuchi F. A new leishmanicidal saponin from Brunfelsia grandiflora. Chem Pharm Bull. 2008;56:93–6. doi: 10.1248/cpb.56.93. [DOI] [PubMed] [Google Scholar]
- 13.Parra VR, Mierau V, Anke T, Sterner O. Cytotoxic terpenoids from Dasyscyphus niveus. Tetrahedron. 2006;62:1828–32. [Google Scholar]
- 14.Ye W, Liu X, Zhang Q, Che C, Zhao S. Antisweet saponins from Gymnema sylvestre. J Nat Prod. 2001;64:232–5. doi: 10.1021/np0004451. [DOI] [PubMed] [Google Scholar]


