Abstract
Bipolar disorder (BD) is a severe psychiatric illness characterized by recurrent manic and depressive episodes, without a characteristic neuropathology or clear etiology. Drugs effective in BD target many key signaling pathways in animal and cell studies. However, their mode of action in the BD brain remains elusive. In the rat brain, some of the mood stabilizers effective in treating mania (lithium, carbamazepine, valproate) or depression (lamotrigine) in BD are reported to decrease transcription of cytosolic phospholipase A2 and cyclooxygenase-2 and to reduce levels of AP-2 and NF-κB, transcription factors of the two enzymes. The anti-manic drugs also decrease arachidonic acid (AA) turnover in brain phospholipids when given chronically to rats. Thus, drugs effective in BD commonly target AA cascade kinetics as well as AA cascade enzymes and their transcription factors in the rat brain. These studies suggest that BD is associated with increased AA signaling in the brain. Developing therapeutic agents that suppress brain AA signaling could lead to additional treatments for BD. In this review, we discuss the mechanisms of action of mood stabilizers and the effects of docosahexaenoic acid on AA cascade enzymes in relation to BD.
Keywords: bipolar disorder, cPLA2, sPLA2, COX-2, AP-2, NF-κB, arachidonic acid, mood stabilizers
INTRODUCTION
Bipolar disorder (BD) is a complex psychiatric disorder, characterized by recurrent depressive and manic episodes. Epidemiological studies show that BD afflicts 1.5 % of the United States population [1] and that BD patients have a 5-to-17 fold increased risk of suicide relative to the general population [2]. However, BD has no characteristic neuropathology and an unknown etiology. Several hypotheses have been proposed to explain BD based on alterations in signal transduction pathways [3]: reduced levels of neurosurvival factors [4], atrophy in brain regions [4–8] and involvement of many genes[9, 10]. Recent studies have suggested excitotoxicity [11–14] and neuroinflammation in BD with elevated pro-inflammatory cytokines [15, 16]. Some of these pathological processes change arachidonic acid (AA: 20:4n-6) metabolism [17–19] and neuronal plasticity. Excessive AA release could promote apoptosis [20]. A number of medications are employed to treat BD, including lithium, antiepileptics, antidepressants and antipsychotic drugs. However, they differ in structure and modes of action. In this review, we discuss the modes of action of different types of mood stabilizers that share common targets in the rat brain and their use in treating BD.
AA is a nutritionally essential polyunsaturated fatty acid predominantly found in the stereospecifically numbered-2 (sn-2) position of membrane phospholipids. AA can be hydrolyzed from membrane phospholipids by calcium-dependent AA-selective cytosolic phospholipase A2 (cPLA2 IVa) or secretory PLA2 (sPLA2 IIa) [21]. In addition, a Ca++-independent phospholipase A2 (iPLA2) is thought to be selective for docosahexaenoic acid (DHA, 22:6n-3). The PLA2 enzymes differ in their calcium requirement, phosphorylation, and substrate specificities [22–26].
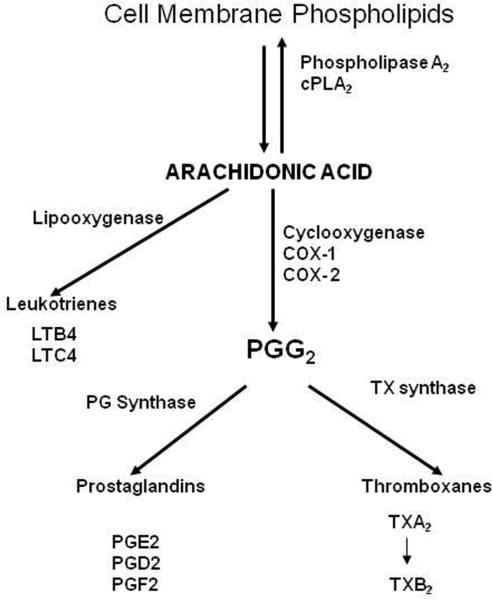
A portion of the AA released by cPLA2 is metabolized to bioactive eicosanoids by cyclooxygenase (COX-1 or COX-2), lipoxygenase, or cytochrome P450 epoxygenase enzymes [27]. Of the two COX isoenzymes, COX-1 is usually constitutively expressed, whereas COX-2 is constitutively expressed but also is inducible by various brain insults [28, 29]. cPLA2 and COX-2 genes are regulated by many transcription factors including activator protein-1(AP-1), AP-2, nuclear factor kappa B (NF-κB), polyoma enhancer activator 3 (PEA3), cyclic AMP response element binding protein (CREB) and glucocorticoid response element (GRE) [30, 31]. Released AA and its metabolites can modulate signal transduction, transcription, neuronal activity, apoptosis, and many other processes within the brain [32–34] (Figure-1).
Figure-1.
Arachidonic acid (AA) is released from membrane phospholipids at the sn-2 position by the catalytic action of Ca++-dependent cytosolic phospholipase A2. Released AA directly mediates various cellular actions or is converted into many bioactive metabolites by cyclooxygenases and other enzymes.
Does abnormal AA metabolism play a role in bipolar disorder?
A number of clinical studies have indicated an alteration in AA metabolism in BD patients, with increased hydrolysis of serum phospholipids [35–37] and increased levels of prostaglandins, a product of AA, in saliva [38], cerebrospinal fluid [39] and serum [36]. Genetic studies also indicate a variant in the sPLA2 gene in BD patients [40]. Postmortem studies in BD have demonstrated increased expression of cPLA2, sPLA2, COX-2, and their transcription factors AP-2 and NF-κB in the frontal cortex (Rao et al Unpublished data) [41]. In agreement, a rat model of BD-like behavioral symptoms [42] showed increased AA signaling in the frontal cortex [19]. These findings suggest the upregulation of AA cascade in BD.
MOOD STABILIZERS EFFECTS ON BRAIN ARACHIDONIC ACID CASCADE ENZYMES
Lithium
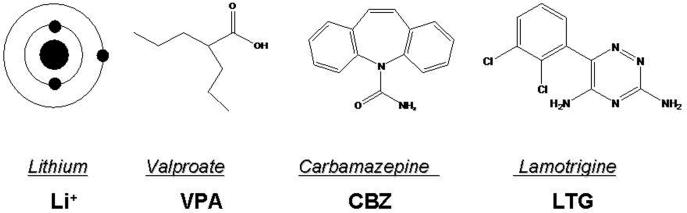
Lithium has been employed in treating BD for more than five decades, but its mode of action remains unclear. Lithium is a monovalent cation (Figure-2) and is known to inhibit inositol monophosphatase [43], G-proteins [44–46], cyclic adenosine monophosphate (cAMP) [47, 48], glycogen synthetase kinase-3 beta (GSK-3β) [49], protein kinase A (PKA) [50], protein kinase C (PKC) [51–54] and its substrate myristoylated alanine-rich C kinase substrate (MARCKS) [55, 56]. Six weeks of chronic lithium administration to rats that produced therapeutically relevant concentrations in brain and blood reduced AA turnover but not DHA turnover in brain phospholipids by reducing transcription of cPLA2 group IVA [57–59]. The decrease in cPLA2 mRNA was ascribed to a selective decrease in AP-2 transcription factor activity and protein levels of the AP-2α and β subunits [60]. AP-2 is recognized on the promoter region of the cPLA2 gene [31]. Chronic lithium had no effect on other cPLA2 - regulating transcription factors (NF-κB, PEA3, GRE) or on expression of iPLA2 group VIA or sPLA2 group IIA [60].
Figure-2.
Chemical structures of mood stabilizers approved for treating bipolar disorder.
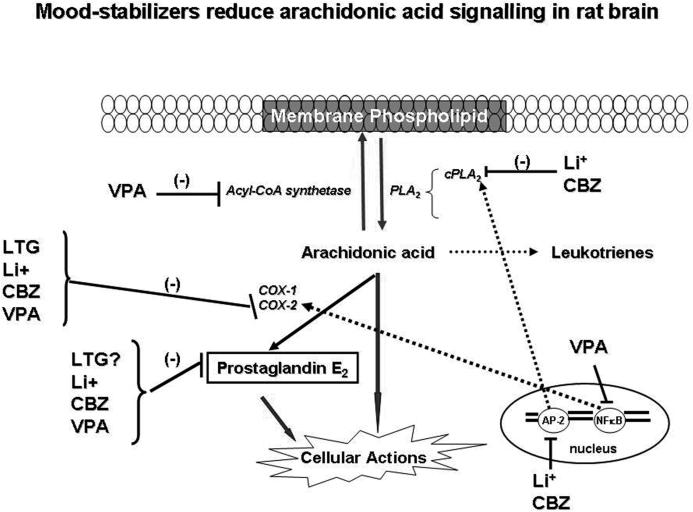
Activation of AP-2 requires phosphorylation by PKA or PKCε [61]. Phosphorylated AP-2 subunits translocate to the nucleus, where they recognize a specific AP-2 binding sequence on chromatin so as to initiate transcription. Chronic lithium treatment decreased PKAα and PKCε protein levels as well as AA-dependent PKC activity in rat brain [60]. The decreased phosphorylation of AP-2 subunits may be responsible for the decreased AP-2 activity [60]. Decreased AP-2 thus likely accounts for the reduced cPLA2 mRNA after chronic lithium administration. The decrease in AA signaling by chronic lithium in turn reduces downstream AA metabolism. Chronic lithium administration decreased activities of COX-1 and COX-2 and the concentration of one of their products prostaglandin E2 (PGE2) in rat brain [62] (Figure-3).
Figure-3.
Chronic mood stabilizer administration to rats reduces AA turnover in membrane phospholipids by either inhibiting acyl-CoA synthethase or transcription of cPLA2. Conversion of AA into eicosanoids is reduced by reduced cyclooxygenase-2 activity.
Carbamazepine
Carbamazepine (5H-Dibenz[b,f]azepine-5-carboxamide; Tegretol) (Figure-2) is an anticonvulsant also effective in treating bipolar disorder [63]. Chronic carbamazepine treatment protects against NMDA-mediated toxicity [64], inhibits adenylyl cyclase and the synthesis of cAMP [51], reduces expression of Go and Gs proteins in neostriatum, increases Gβ protein expression in rat frontal cortex [65], and increases brain phosphorylation of MARCKS. Chronic (30 days) carbamazepine administration in rats, which produced therapeutically relevant plasma levels (53.6 μM) [66], decreased the turnover of AA but not DHA in brain phospholipids [67], and decreased brain mRNA, protein, and activity of cPLA2 group IVA but had no effect on sPLA2 group IIA or iPLA2 group VIA expression or activity, similar to lithium [66]. Chronic carbamazepine, like lithium, decreased brain COX-2 activity and PGE2 concentration [62, 66] without altering 5-lipoxygenase or cytochrome p450 protein levels or leukotriene B4 or thromboxane B2 concentrations [66]. Carbamazepine decreased the cPLA2 gene transcription factor AP-2 (Figure-3) but not other cPLA2 gene regulating transcription factors (AP-1, NF-κB, GRE or PEA3) [41]. Carbamazepine decreased AP-2 binding activity by decreasing cAMP dependent PKA activity, a known activator of AP-2 [61] (Figure-3), and phosphorylated AP-2 and protein levels of the AP-2α subunit. Unlike lithium, chronic carbamazepine had no effect on PKCα or PKCε protein levels in rat frontal cortex [19].
Valproic acid
Valproic acid (VPA, 2-propylpentanoic acid) is a branched-chain carboxylic acid (Figure-2) used in treating acute mania and mixed episodes in BD [44, 68]. VPA shares some biochemical and cellular targets with lithium, including inhibiting the activities of glycogen synthase kinase-3 β [54, 69] and PKC [70, 71], and increasing AP-1 DNA binding [72, 73]. Studies also indicate that VPA directly inhibits histone deacetylase [74]. Chronic (30 days) administration of VPA, to produce therapeutically relevant plasma levels (0.2 mM) [44, 75], was shown to decrease the turnover rate of AA but not DHA in brain phospholipids of unanesthetized rats [67, 75]. Like lithium and carbamazepine, chronic VPA decreased rat brain COX activity and PGE2 concentration [76], without altering 5-lipoxygenase or cytochrome p450 protein levels or leukotriene B4 or thromboxane B2 concentrations [76]. Two weeks of VPA administration to rats also decreased the ex vivo production of COX metabolites from isolated platelets and brain capillaries [77]. VPA decreased rat frontal cortex COX-2 mRNA levels and the binding activity of NF-κB, a transcription factor for COX-2 [19]. It decreased the p50 protein component of NF-κB, without changing the rat frontal cortex protein level of p65. Unlike lithium and carbamazepine, VPA did not change expression or activity of cPLA2 group IVA, nor did it alter sPLA2 group IIA or iPLA2 group VIA expression, or AP-2 binding activity [19]. Because of this difference, we studied the effects of VPA on other enzymes regulating AA turnover within brain phospholipids, namely microsomal acyl-CoA synthetase. VPA was found to act as an ordered noncompetitive inhibitor of microsomal acyl-CoA synthetase in vitro (Figure 3), and its Ki for inhibiting arachidonoyl-CoA formation was lower than that for inhibiting formation of docosahexaenoyl- CoA or palmitoyl-CoA [67]. This likely explains why VPA decreased the turnover of AA but not of DHA within brain phospholipids of the unanesthetized rat.
Lamotrigine
Lamotrigine [Lamictal; 6-(2,3-Dichlorophenyl)-1,2,4-triazine-3,5-diamine)] (Figure-2) is a novel anticonvulsant that has been proven effective in the treatment of bipolar depression [78] and rapid cycling BD [79]. Studies in rodents have revealed that lamotrigine increases brain gamma amino butyric acid (GABA) turnover [80] and hippocampal serotonin (5-HT) and dopamine levels [81], but decreases brain glutamate [82]. Chronic administration of lamotrigine decreased COX-2 protein and mRNA in rat frontal cortex without changing protein levels of COX-1 or of PLA2 subtypes. Lamotrigine's therapeutic action in bipolar disorder may be related to reductions in AA signaling via COX-2 and the formation of COX-2 derived PGE2 and other eicosanoids.
Lamotrigine [83] decreased locomotor hyperactivity in amphetamine models of mania, and decreased incorporation of AA into brain phospholipids of unanesthetized rats [41]. Lamotrigine does not delay the onset of mania in patients with bipolar disorder, although it does delay the onset of depressive symptoms [84] and is effective in rapid-cycling bipolar disorder [85]. The mood stabilizers for bipolar also reduce NMDA induced AA incorporation in rat brain [41, 86].
Antidepressants
To test the increased AA signaling hypothesis for bipolar mania, we examined the effects of fluoxetine and imipramine which increase switching to mania in bipolar depressed patients. In awake rats, chronic fluoxetine or imipramine increased AA turnover and cPLA2 expression in rat brain without changing expression of sPLA2 or iPLA2 or COX isoforms [41, 87]. In contrast, chronic bupropion, an antidepressant that does not switch to manic symptoms in bipolar depressive patients, had no effect on AA turnover or cPLA2 in rat brain [87]. These studies imply that an upregulated AA cascade signaling is related to the manic symptoms in BD.
Topiramate
Phase I clinical trials suggested that topiramate was effective in BD [88] and it was shown to effective in quinpirole model of mania [89]. Despite achieving a therapeutically relevant plasma topiramate level of 18.1 μM after chronic treatment, chronic topiramate did not alter expression of cPLA2 or any of the measured enzymes in the AA cascade, nor did it alter AA or DHA turnover in brain phospholipids of the unanesthetized rat [67, 90]. Consistent with these negative findings, four recent double-blind placebo-controlled trials demonstrated that topiramate is not an effective antibipolar drug [91], a finding that was predicted by the AA model [67, 90].
Factors contributing to upregulation of AA cascade enzymes
Numerous conditions can influence expression of AA cascade enzymes: neuroinflammation, excitotoxicity, long-term treatment with fluoxetine, dietary deprivation of n-3 polyunsaturated fatty acids, lipopolysaccharide infusion, chronic NMDA administration and genetic factors. Some of these conditions may be implicated in the pathophysiology of BD.
Mood stabilizers effective in the treatment of BD can attenuate inflammation-induced and excitotoxicity-induced AA signaling in rat brain [17, 41]. Chronic NMDA administration decreased NMDA receptor (NMDAR) (NR-1 and NR-3A) subunits and increased AA turnover [17] in rat brain, possibly by upregulating cPLA2 group IVA protein and mRNA expression as well as AP-2 DNA binding activity and AP-2α and AP-2β protein levels [41]. Altered NMDA function, an elevated brain glutamate/glutamine ratio, and decreased NR-1 and NR-3A levels have been reported in children and adult BD patients as well as in postmortem brain from BD patients [11, 12, 92]. Gene variants of the NR1 and NR2 subunits of the NMDAR also have been linked to risk for BD [12, 93, 94]. In addition, NMDA receptor density and levels of NR1, NR2A and NR3A are decreased in the postmortem bipolar brain, as are densities of the NMDAR-associated post-synaptic proteins PSD-95 and SAP102 [14, 95]. The subunit variants can produce increased NMDAR function because NMDAR stimulation by glutamate or NMDA decreases NR-1 and NR-3A expression [41, 96]. In vitro studies indicate that the NR3A subunit co-assembles with other subunits (NR1, NR2A or NR2B) to form NMDARs with reduced activity and Ca2+ influx [97, 98], and mice lacking the NR3A subunit have increased NMDAR activity [99]. These observations suggest that increased NMDA function leads to increased AA signaling. In contrast, mood stabilizers attenuate NMDA induced AA incorporation in rat brain [17, 41]. A recent study showed that rats exposed to chronic NMDA had increased brain protein and mRNA levels of neuroinflammatory markers, such as interleukin 1 beta (IL-1β), tumor necrosis factor alpha (TNFα), glial fibrillary acidic protein (GFAP) and inducible nitric oxide synthase (iNOS) [100]. This suggests cross-talk between excitotoxicity and neuroinflammation.
In addition to NMDA excitotoxicity, lipopolysaccharide exposure induced cPLA2 protein expression in an NF-κB and AP-2 dependent manner in rat astrocyte cultures [67, 101] and increased AA incorporation and cPLA2 Bactivity in rat brain [41, 102]. Excessive release of glutamate may trigger neuroinflammatory reactions, since neuroinflammatory cytokine genes were upregulated with chronic NMDA administration to rats [103]. A combination of excitotoxicity and neuroinflammation could lead to activation of many transcription factors and thereby induce expression of many genes, including those related to the AA cascade. A clinical study reported increased neuroinflammation in BD patients associated with an increase in pro-inflammatory cytokines and attenuated by mood stabilizers [15]. Animal studies have reported that bacterial endotoxin infusion produced pro-inflammatory cytokines (IL-2, TNF α) and a variety of behavioral changes including aggression [104, 105]. Clinical reports also suggest a link between increased cytokine levels and aggressive behavior [106, 107]. Taken together these studies indicate that inflammation could play a role in BD.
Clinical studies suggest that dietary supplementation of DHA is beneficial in patients with BD [108]. DHA is a polyunsaturated fatty acid (PUFA) that is highly enriched in the brain [109]. It is not synthesized de novo in vertebrates but is obtained directly from the diet or synthesized in the liver by the desaturation/elongation of its dietary precursor, α-linolenic (18:3n-3) acid [110]. Dietary deprivation of DHA in rats causes BD-like behavioral symptoms [42] and is associated with increased expression of cPLA2 group IVA, sPLA2 group IIA and COX-2 in frontal cortex [19]. These changes are opposite in direction to the effects of chronic mood stabilizer administration in rat brain, suggesting that dietary supplementation of n-3 PUFAs could attenuate AA signaling in rat brain, in a manner comparable to the action of mood stabilizers. Such supplementation may be beneficial in patients with BD [108], but further testing is required to validate its efficacy.
BD is complex, heterogenous disease that involves multiple genes, and has no appropriate animal model. Consequently, development of a specific drug based on pathology has not occurred. However, available FDA approved drugs are known to target AA cascade markers particularly cPLA2 and COX-2 enzyme expression/or activity. Mood-stabilizers are also reported to attenuate the NMDA and lipopolysaccacharide induced AA signaling in rat brain [41, 86]. Increased AA cascade signaling will arise from either excess glutamate or inflammation. Further evaluation of agents such as cPLA2 inhibitors, NMDA antagonists, COX 2 inhibitors is warranted for pre-clinical studies as well as studies in BD patients. Glutamatergic modulating agents are also promising based on pre-clinical and clinical studies. These agents include Riluzole (2-amino-6-trifluoromethoxy benzothiazole), memantine, Ceftriaxone and felbamate [111]. The effects of these drugs have not been studied on AA cascade markers in animal studies.
In conclusion, mood stabilizers share common effects by downregulating the AA cascade in rat brain. Conversely, a pathological upregulation of the AA cascade may play a role in BD symptoms.
Acknowledgements
This work was entirely supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health. We thank the National Cancer Institute (NCI), Center for Cancer Research (CCR) Fellows Editorial Board, for proofreading the manuscript. The authors have no conflict of interest.
Glossary
Abbreviations
- AA
arachidonic acid
- AP-2
activator protein-2
- BD
bipolar disorder
- cPLA2
cytosolic phospholipase AB2B
- COX
cyclooxygenase
- DHA
docosahexaenoic acid
- iPLA2
calcium-independent phospholipase A2
- NF-κB
nuclear factor kappa B
- sPLA2
secretory phospholipase A2
- NMDA
N-methyl-D-aspartate
- PGE2
prostaglandin E2
- PKA
protein kinase A
- MARKS
myristoylated alanine-rich C kinase substrate
- NMDA R
N-methyl-D aspartate receptor
References
- 1.Judd LL, Akiskal HS. The prevalence and disability of bipolar spectrum disorders in the US population: re-analysis of the ECA database taking into account subthreshold cases. J. Affect. Disord. 2003;73:123–131. doi: 10.1016/s0165-0327(02)00332-4. [DOI] [PubMed] [Google Scholar]
- 2.Bostwick JM, Pankratz VS. Affective disorders and suicide risk: a reexamination. Am. J. Psychiatry. 2000;157:1925–1932. doi: 10.1176/appi.ajp.157.12.1925. [DOI] [PubMed] [Google Scholar]
- 3.Chang A, Li PP, Warsh JJ. Altered cAMP-dependent protein kinase subunit immunolabeling in post-mortem brain from patients with bipolar affective disorder. J. Neurochem. 2003;84:781–791. doi: 10.1046/j.1471-4159.2003.01605.x. [DOI] [PubMed] [Google Scholar]
- 4.Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol. Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- 5.Brauch RA, Adnan El-Masri M., Parker JC, Jr., El-Mallakh RS. Glial cell number and neuron/glial cell ratios in postmortem brains of bipolar individuals. J. Affect. Disord. 2006;91:87–90. doi: 10.1016/j.jad.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Lyoo IK, Kim MJ, Stoll AL, Demopulos CM, Parow AM, Dager SR, Friedman SD, Dunner DL, Renshaw PF. Frontal lobe gray matter density decreases in bipolar I disorder. Biol. Psychiatry. 2004;55:648–651. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Lyoo IK, Sung YH, Dager SR, Friedman SD, Lee JY, Kim SJ, Kim N, Dunner DL, Renshaw PF. Regional cerebral cortical thinning in bipolar disorder. Bipolar Disord. 2006;8:65–74. doi: 10.1111/j.1399-5618.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- 8.Rajkowska G. Cell pathology in bipolar disorder. Bipolar Disord. 2002;4:105–116. doi: 10.1034/j.1399-5618.2002.01149.x. [DOI] [PubMed] [Google Scholar]
- 9.Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B, Schulze TG, Cichon S, Rietschel M, Nothen MM, Georgi A, Schumacher J, Schwarz M, Hofels S, Propping P, Satagopan J, Detera-Wadleigh SD, Hardy J, McMahon FJ. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol. Psychiatry. 2008;13:197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manji HK, Duman RS. Impairments of neuroplasticity and cellular resilience in severe mood disorders: implications for the development of novel therapeutics. Psychopharmacol. Bull. 2001;35:5–49. [PubMed] [Google Scholar]
- 11.Mueller HT, Meador-Woodruff JH. NR3A NMDA receptor subunit mRNA expression in schizophrenia, depression and bipolar disorder. Schizophr. Res. 2004;71:361–370. doi: 10.1016/j.schres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Mundo E, Tharmalingham S, Neves-Pereira M, Dalton EJ, Macciardi F, Parikh SV, Bolonna A, Kerwin RW, Arranz MJ, Makoff AJ, Kennedy JL. Evidence that the N-methyl-D-aspartate subunit 1 receptor gene (GRIN1) confers susceptibility to bipolar disorder. Mol. Psychiatry. 2003;8:241–245. doi: 10.1038/sj.mp.4001218. [DOI] [PubMed] [Google Scholar]
- 13.Nudmamud-Thanoi S, Reynolds GP. The NR1 subunit of the glutamate/NMDA receptor in the superior temporal cortex in schizophrenia and affective disorders. Neurosci. Lett. 2004;372:173–177. doi: 10.1016/j.neulet.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 14.Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32:1888–1902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- 15.Kim YK, Jung HG, Myint AM, Kim H, Park SH. Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. J. Affect. Disord. 2007;104:91–95. doi: 10.1016/j.jad.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien SM, Scully P, Scott LV, Dinan TG. Cytokine profiles in bipolar affective disorder: focus on acutely ill patients. J. Affect. Disord. 2006;90:263–267. doi: 10.1016/j.jad.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Basselin M, Chang L, Bell JM, Rapoport SI. Chronic Lithium Chloride Administration Attenuates Brain NMDA Receptor-Initiated Signaling via Arachidonic Acid in Unanesthetized Rats. Neuropsychopharmacology. 2005;30:1064–1075. doi: 10.1038/sj.npp.1300671. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto Y, Yamaguchi T, Watanabe S, Yamamoto T. Involvement of arachidonic acid cascade in working memory impairment induced by interleukin-1 beta. Neuropharmacology. 2004;46:1195–1200. doi: 10.1016/j.neuropharm.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Lee HJ, Rao JS, Chang L, Rapoport SI, Bazinet RP. Chronic N-methyl-D-aspartate administration increases the turnover of arachidonic acid within brain phospholipids of the unanesthetized rat. J. Lipid Res. 2008;49:162–168. doi: 10.1194/jlr.M700406-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Garrido R, Springer JE, Hennig B, Toborek M. Apoptosis of spinal cord neurons by preventing depletion nicotine attenuates arachidonic acid-induced of neurotrophic factors. J. Neurotrauma. 2003;20:1201–1213. doi: 10.1089/089771503322584628. [DOI] [PubMed] [Google Scholar]
- 21.Ong WY, Sandhya TL, Horrocks LA, Farooqui AA. Distribution of cytoplasmic phospholipase A2 in the normal rat brain. J. Hirnforsch. 1999;39:391–400. [PubMed] [Google Scholar]
- 22.Akiba S, Mizunaga S, Kume K, Hayama M, Sato T. Involvement of group VI Ca2+-independent phospholipase A2 in protein kinase C-dependent arachidonic acid liberation in zymosan-stimulated macrophage-like P388D1 cells. J. Biol. Chem. 1999;274:19906–19912. doi: 10.1074/jbc.274.28.19906. [DOI] [PubMed] [Google Scholar]
- 23.Murakami M, Kambe T, Shimbara S, Kudo I. Functional coupling between various phospholipase A2s and cyclooxygenases in immediate and delayed prostanoid biosynthetic pathways. J. Biol. Chem. 1999;274:3103–3115. doi: 10.1074/jbc.274.5.3103. [DOI] [PubMed] [Google Scholar]
- 24.Murakami M, Kudo I. Phospholipase A2. J. Biochem. (Tokyo) 2002;131:285–292. doi: 10.1093/oxfordjournals.jbchem.a003101. [DOI] [PubMed] [Google Scholar]
- 25.Murakami M, Shimbara S, Kambe T, Kuwata H, Winstead MV, Tischfield JA, Kudo I. The functions of five distinct mammalian phospholipase A2S in regulating arachidonic acid release. Type IIa and type V secretory phospholipase A2S are functionally redundant and act in concert with cytosolic phospholipase A2. J. Biol. Chem. 1998;273:14411–14423. doi: 10.1074/jbc.273.23.14411. [DOI] [PubMed] [Google Scholar]
- 26.Yang HC, Mosior M, Johnson CA, Chen Y, Dennis EA. Group-specific assays that distinguish between the four major types of mammalian phospholipase A2. Anal. Biochem. 1999;269:278–288. doi: 10.1006/abio.1999.4053. [DOI] [PubMed] [Google Scholar]
- 27.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 28.Seibert K, Masferrer J, Zhang Y, Gregory S, Olson G, Hauser S, Leahy K, Perkins W, Isakson P. Mediation of inflammation by cyclooxygenase-2. Agents Actions. Suppl. 1995;46:41–50. doi: 10.1007/978-3-0348-7276-8_5. [DOI] [PubMed] [Google Scholar]
- 29.Pepicelli O, Fedele E, Bonanno G, Raiteri M, Ajmone-Cat MA, Greco A, Levi G, Minghetti L. In vivo activation of N-methyl-D-aspartate receptors in the rat hippocampus increases prostaglandin E(2) extracellular levels and triggers lipid peroxidation through cyclooxygenase-mediated mechanisms. J. Neurochem. 2002;81:1028–1034. doi: 10.1046/j.1471-4159.2002.00897.x. [DOI] [PubMed] [Google Scholar]
- 30.Appleby SB, Ristimaki A, Neilson K, Narko K, Hla T. Structure of the human cyclo-oxygenase-2 gene. Biochem. J. 1994;302(Pt 3):723–727. doi: 10.1042/bj3020723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morri H, Ozaki M, Watanabe Y. 5'-flanking region surrounding a human cytosolic phospholipase A2 gene. Biochem. Biophys. Res. Commun. 1994;205:6–11. doi: 10.1006/bbrc.1994.2621. [DOI] [PubMed] [Google Scholar]
- 32.Kam PC, See AU. Cyclo-oxygenase isoenzymes: physiological and pharmacological role. Anaesthesia. 2000;55:442–449. doi: 10.1046/j.1365-2044.2000.01271.x. [DOI] [PubMed] [Google Scholar]
- 33.Leslie JB, Watkins WD. Eicosanoids in the central nervous system. J. Neurosurg. 1985;63:659–668. doi: 10.3171/jns.1985.63.5.0659. [DOI] [PubMed] [Google Scholar]
- 34.O'Banion MK. Cyclooxygenase-2: molecular biology, pharmacology, and neurobiology. Crit. Rev. Neurobiol. 1999;13:45–82. doi: 10.1615/critrevneurobiol.v13.i1.30. [DOI] [PubMed] [Google Scholar]
- 35.Hibbeln JR, Palmer JW, Davis JM. Are disturbances in lipid-protein interactions by phospholipase-A2 a predisposing factor in affective illness? Biol. Psychiatry. 1989;25:945–961. doi: 10.1016/0006-3223(89)90274-6. [DOI] [PubMed] [Google Scholar]
- 36.Lieb J, Karmali R, Horrobin D. Elevated levels of prostaglandin E2 and thromboxane B2 in depression. Prostaglandins. Leukot. Med. 1983;10:361–367. doi: 10.1016/0262-1746(83)90048-3. [DOI] [PubMed] [Google Scholar]
- 37.Sublette ME, Russ MJ, Smith GS. Evidence for a role of the arachidonic acid cascade in affective disorders: a review. Bipolar Disord. 2004;6:95–105. doi: 10.1046/j.1399-5618.2003.00094.x. [DOI] [PubMed] [Google Scholar]
- 38.Nishino S, Ueno R, Ohishi K, Sakai T, Hayaishi O. Salivary prostaglandin concentrations: possible state indicators for major depression. Am. J. Psychiatry. 1989;146:365–368. doi: 10.1176/ajp.146.3.365. [DOI] [PubMed] [Google Scholar]
- 39.Linnoila M, Whorton AR, Rubinow DR, Cowdry RW, Ninan PT, Waters RN. CSF prostaglandin levels in depressed and schizophrenic patients. Arch. Gen. Psychiatry. 1983;40:405–406. doi: 10.1001/archpsyc.1983.01790040059008. [DOI] [PubMed] [Google Scholar]
- 40.Jacobsen NJ, Franks EK, Owen MJ, Craddock NJ. Mutational analysis of phospholipase A2A: a positional candidate susceptibility gene for bipolar disorder. Mol. Psychiatry. 1999;4:274–279. doi: 10.1038/sj.mp.4000476. [DOI] [PubMed] [Google Scholar]
- 41.Basselin M, Villacreses NE, Chen M, Bell JM, Rapoport SI. Chronic carbamazepine administration reduces N-methyl-D-aspartate receptor-initiated signaling via arachidonic acid in rat brain. Biol. Psychiatry. 2007;62:934–943. doi: 10.1016/j.biopsych.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao JS, Ertley RN, DeMar JC, Jr., Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol. Psychiatry. 2007;12:151–157. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- 43.Sherman WR, Leavitt AL, Honchar MP, Hallcher LM, Phillips BE. Evidence that lithium alters phosphoinositide metabolism: chronic administration elevates primarily D-myo-inositol-1-phosphate in cerebral cortex of the rat. J. Neurochem. 1981;36:1947–1951. doi: 10.1111/j.1471-4159.1981.tb10819.x. [DOI] [PubMed] [Google Scholar]
- 44.Jacobsen FM. Low-dose valproate: a new treatment for cyclothymia, mild rapid cycling disorders, and premenstrual syndrome. J. Clin. Psychiatry. 1993;54:229–234. [PubMed] [Google Scholar]
- 45.Wang HY, Friedman E. Effects of lithium on receptor-mediated activation of G proteins in rat brain cortical membranes. Neuropharmacology. 1999;38:403–414. doi: 10.1016/s0028-3908(98)00197-x. [DOI] [PubMed] [Google Scholar]
- 46.Miki M, Hamamura T, Ujike H, Lee Y, Habara T, Kodama M, Ohashi K, Tanabe Y, Kuroda S. Effects of subchronic lithium chloride treatment on G-protein subunits (Golf, Ggamma7) and adenylyl cyclase expressed specifically in the rat striatum. Eur. J. Pharmacol. 2001;428:303–309. doi: 10.1016/s0014-2999(01)01343-7. [DOI] [PubMed] [Google Scholar]
- 47.Mork A. Actions of lithium on the cyclic AMP signalling system in various regions of the brain--possible relations to its psychotropic actions. A study on the adenylate cyclase in rat cerebral cortex, corpus striatum and hippocampus. Pharmacol. Toxicol. 1993;73(Suppl 3):1–47. doi: 10.1111/j.1600-0773.1993.tb01704.x. [DOI] [PubMed] [Google Scholar]
- 48.Mork A, Geisler A. Effects of chronic lithium treatment on agonist-enhanced extracellular concentrations of cyclic AMP in the dorsal hippocampus of freely moving rats. J. Neurochem. 1995;65:134–139. doi: 10.1046/j.1471-4159.1995.65010134.x. [DOI] [PubMed] [Google Scholar]
- 49.De Sarno P, Li X, Jope RS. Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002;43:1158–1164. doi: 10.1016/s0028-3908(02)00215-0. [DOI] [PubMed] [Google Scholar]
- 50.Mori S, Tardito D, Dorigo A, Zanardi R, Smeraldi E, Racagni G, Perez J. Effects of lithium on cAMP-dependent protein kinase in rat brain. Neuropsychopharmacology. 1998;19:233–240. doi: 10.1016/S0893-133X(98)00018-9. [DOI] [PubMed] [Google Scholar]
- 51.Chen G, Pan B, Hawver DB, Wright CB, Potter WZ, Manji HK. Attenuation of cyclic AMP production by carbamazepine. J. Neurochem. 1996;67:2079–2086. doi: 10.1046/j.1471-4159.1996.67052079.x. [DOI] [PubMed] [Google Scholar]
- 52.Manji HK, Etcheberrigaray R, Chen G, Olds JL. Lithium decreases membrane-associated protein kinase C in hippocampus: selectivity for the alpha isozyme. J. Neurochem. 1993;61:2303–2310. doi: 10.1111/j.1471-4159.1993.tb07474.x. [DOI] [PubMed] [Google Scholar]
- 53.Soares JC, Chen G, Dippold CS, Wells KF, Frank E, Kupfer DJ, Manji HK, Mallinger AG. Concurrent measures of protein kinase C and phosphoinositides in lithium-treated bipolar patients and healthy individuals: a preliminary study. Psychiatry Res. 2000;95:109–118. doi: 10.1016/s0165-1781(00)00175-x. [DOI] [PubMed] [Google Scholar]
- 54.Chen G, Huang LD, Jiang YM, Manji HK. The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. J. Neurochem. 1999;72:1327–1330. doi: 10.1046/j.1471-4159.2000.0721327.x. [DOI] [PubMed] [Google Scholar]
- 55.Lenox RH, Watson DG, Patel J, Ellis J. Chronic lithium administration alters a prominent PKC substrate in rat hippocampus. Brain Res. 1992;570:333–340. doi: 10.1016/0006-8993(92)90598-4. [DOI] [PubMed] [Google Scholar]
- 56.Watson DG, Lenox RH. Chronic lithium-induced down-regulation of MARCKS in immortalized hippocampal cells: potentiation by muscarinic receptor activation. J. Neurochem. 1996;67:767–777. doi: 10.1046/j.1471-4159.1996.67020767.x. [DOI] [PubMed] [Google Scholar]
- 57.Chang MC, Grange E, Rabin O, Bell JM, Allen DD, Rapoport SI. Lithium decreases turnover of arachidonate in several brain phospholipids. Neurosci. Lett. 1996;220:171–174. doi: 10.1016/s0304-3940(96)13264-x. [DOI] [PubMed] [Google Scholar]
- 58.Rintala J, Seemann R, Chandrasekaran K, Rosenberger TA, Chang L, Contreras MA, Rapoport SI, Chang MC. 85 kDa cytosolic phospholipase A2 is a target for chronic lithium in rat brain. Neuroreport. 1999;10:3887–3890. doi: 10.1097/00001756-199912160-00030. [DOI] [PubMed] [Google Scholar]
- 59.Chang MC, Bell JM, Purdon AD, Chikhale EG, Grange E. Dynamics of docosahexaenoic acid metabolism in the central nervous system: lack of effect of chronic lithium treatment. Neurochem. Res. 1999;24:399–406. doi: 10.1023/a:1020989701330. [DOI] [PubMed] [Google Scholar]
- 60.Bazinet RP, Rao JS, Chang L, Rapoport SI, Lee HJ. Chronic valproate does not alter the kinetics of docosahexaenoic acid within brain phospholipids of the unanesthetized rat. Psychopharmacology (Berl) 2005;182:180–185. doi: 10.1007/s00213-005-0059-7. [DOI] [PubMed] [Google Scholar]
- 61.Imagawa M, Chiu R, Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987;51:251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- 62.Bosetti F, Rintala J, Seemann R, Rosenberger TA, Contreras MA, Rapoport SI, Chang MC. Chronic lithium downregulates cyclooxygenase-2 activity and prostaglandin E(2) concentration in rat brain. Mol. Psychiatry. 2002a;7:845–850. doi: 10.1038/sj.mp.4001111. [DOI] [PubMed] [Google Scholar]
- 63.Belmaker RH. Bipolar disorder. N. Engl. J. Med. 2004;351:476–486. doi: 10.1056/NEJMra035354. [DOI] [PubMed] [Google Scholar]
- 64.Bown CD, Wang JF, Young LT. Attenuation of N-methyl-D-aspartate-mediated cytoplasmic vacuolization in primary rat hippocampal neurons by mood stabilizers. Neuroscience. 2003;117:949–955. doi: 10.1016/s0306-4522(02)00743-1. [DOI] [PubMed] [Google Scholar]
- 65.Jakobsen SN, Wiborg O. Selective effects of long-term lithium and carbamazepine administration on G-protein subunit expression in rat brain. Brain Res. 1998;780:46–55. doi: 10.1016/s0006-8993(97)01181-5. [DOI] [PubMed] [Google Scholar]
- 66.Ghelardoni S, Tomita YA, Bell JM, Rapoport SI, Bosetti F. Chronic carbamazepine selectively downregulates cytosolic phospholipase A2 expression and cyclooxygenase activity in rat brain. Biol. Psychiatry. 2004;56:248–254. doi: 10.1016/j.biopsych.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 67.Bazinet RP, Rao JS, Chang L, Rapoport SI, Lee HJ. Chronic carbamazepine decreases the incorporation rate and turnover of arachidonic acid but not docosahexaenoic acid in brain phospholipids of the unanesthetized rat: relevance to bipolar disorder. Biol. Psychiatry. 2006;59:401–407. doi: 10.1016/j.biopsych.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 68.McElroy SL, Keck PE, Jr., Pope HG, Jr., Hudson JI. Valproate in the treatment of bipolar disorder: literature review and clinical guidelines. J. Clin. Psychopharmacol. 1992;12:42S–52S. doi: 10.1097/00004714-199202001-00007. [DOI] [PubMed] [Google Scholar]
- 69.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. U. S. A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen G, Manji HK, Hawver DB, Wright CB, Potter WZ. Chronic sodium valproate selectively decreases protein kinase C alpha and epsilon in vitro. J. Neurochem. 1994;63:2361–2364. doi: 10.1046/j.1471-4159.1994.63062361.x. [DOI] [PubMed] [Google Scholar]
- 71.Manji HK, Chen G. PKC, MAP kinases and the bcl-2 family of proteins as long-term targets for mood stabilizers. Mol. Psychiatry. 2002;7(Suppl 1):S46–56. doi: 10.1038/sj.mp.4001018. [DOI] [PubMed] [Google Scholar]
- 72.Chen G, Yuan P, Hawver DB, Potter WZ, Manji HK. Increase in AP-1 transcription factor DNA binding activity by valproic acid. Neuropsychopharmacology. 1997;16:238–245. doi: 10.1016/S0893-133X(96)00239-4. [DOI] [PubMed] [Google Scholar]
- 73.Yuan PX, Huang LD, Jiang YM, Gutkind JS, Manji HK, Chen G. The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. J. Biol. Chem. 2001;276:31674–31683. doi: 10.1074/jbc.M104309200. [DOI] [PubMed] [Google Scholar]
- 74.Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- 75.Chang MC, Contreras MA, Rosenberger TA, Rintala JJ, Bell JM, Rapoport SI. Chronic valproate treatment decreases the in vivo turnover of arachidonic acid in brain phospholipids: a possible common effect of mood stabilizers. J. Neurochem. 2001;77:796–803. doi: 10.1046/j.1471-4159.2001.00311.x. [DOI] [PubMed] [Google Scholar]
- 76.Bosetti F, Weerasinghe GR, Rosenberger TA, Rapoport SI. Valproic acid down-regulates the conversion of arachidonic acid to eicosanoids via cyclooxygenase-1 and -2 in rat brain. J. Neurochem. 2003;85:690–696. doi: 10.1046/j.1471-4159.2003.01701.x. [DOI] [PubMed] [Google Scholar]
- 77.Szupera Z, Mezei Z, Kis B, Gecse A, Vecsei L, Telegdy G. The effects of valproate on the arachidonic acid metabolism of rat brain microvessels and of platelets. Eur. J. Pharmacol. 2000;387:205–210. doi: 10.1016/s0014-2999(99)00764-5. [DOI] [PubMed] [Google Scholar]
- 78.Calabrese JR, Bowden CL, Sachs GS, Ascher JA, Monaghan E, Rudd GD. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J. Clin. Psychiatry. 1999;60:79–88. doi: 10.4088/jcp.v60n0203. [DOI] [PubMed] [Google Scholar]
- 79.Calabrese JR, Rapport DJ, Youngstrom EA, Jackson K, Bilali S, Findling RL. New data on the use of lithium, divalproate, and lamotrigine in rapid cycling bipolar disorder. Eur Psychiatry. 2005;20:92–95. doi: 10.1016/j.eurpsy.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 80.Hassel B, Tauboll E, Gjerstad L. Chronic lamotrigine treatment increases rat hippocampal GABA shunt activity and elevates cerebral taurine levels. Epilepsy Res. 2001;43:153–163. doi: 10.1016/s0920-1211(00)00196-0. [DOI] [PubMed] [Google Scholar]
- 81.Ahmad S, Fowler LJ, Whitton PS. Effect of acute and chronic lamotrigine on basal and stimulated extracellular 5-hydroxytryptamine and dopamine in the hippocampus of the freely moving rat. Br. J. Pharmacol. 2004;142:136–142. doi: 10.1038/sj.bjp.0705737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ahmad S, Fowler LJ, Whitton PS. Effects of combined lamotrigine and valproate on basal and stimulated extracellular amino acids and monoamines in the hippocampus of freely moving rats. Naunyn. Schmiedebergs Arch. Pharmacol. 2005;371:1–8. doi: 10.1007/s00210-004-1008-4. [DOI] [PubMed] [Google Scholar]
- 83.Arban R, Maraia G, Brackenborough K, Winyard L, Wilson A, Gerrard P, Large C. Evaluation of the effects of lamotrigine, valproate and carbamazepine in a rodent model of mania. Behav. Brain Res. 2005;158:123–132. doi: 10.1016/j.bbr.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 84.Bowden CL, Calabrese JR, Sachs G, Yatham LN, Asghar SA, Hompland M, Montgomery P, Earl N, Smoot TM, DeVeaugh-Geiss J. A placebo- controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch. Gen. Psychiatry. 2003;60:392–400. doi: 10.1001/archpsyc.60.4.392. [DOI] [PubMed] [Google Scholar]
- 85.Ross BM, Hughes B, Kish SJ, Warsh JJ. Serum calcium-independent phospholipase A2 activity in bipolar affective disorder. Bipolar Disord. 2006;8:265–270. doi: 10.1111/j.1399-5618.2006.00299.x. [DOI] [PubMed] [Google Scholar]
- 86.Basselin M, Chang L, Bell JM, Rapoport SI. Chronic lithium chloride administration attenuates brain NMDA receptor-initiated signaling via arachidonic acid in unanesthetized rats. Neuropsychopharmacology. 2006;31:1659–1674. doi: 10.1038/sj.npp.1300920. [DOI] [PubMed] [Google Scholar]
- 87.Carta MG, Hardoy MC, Pilu A, Sorba M, Floris AL, Mannu FA, Baum A, Cappai A, Velluti C, Salvi M. Improving physical quality of life with group physical activity in the adjunctive treatment of major depressive disorder. Clin Pract Epidemol Ment Health. 2008;4:1. doi: 10.1186/1745-0179-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Calabrese JR, Keck PE, Jr., McElroy SL, Shelton MD. A pilot study of topiramate as monotherapy in the treatment of acute mania. J. Clin. Psychopharmacol. 2001;21:340–342. doi: 10.1097/00004714-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 89.Shaldubina A, Einat H, Szechtman H, Shimon H, Belmaker RH. Preliminary evaluation of oral anticonvulsant treatment in the quinpirole model of bipolar disorder. J. Neural Transm. 2002;109:433–440. doi: 10.1007/s007020200035. [DOI] [PubMed] [Google Scholar]
- 90.Ghelardoni S, Bazinet RP, Rapoport SI, Bosetti F. Topiramate does not alter expression in rat brain of enzymes of arachidonic acid metabolism. Psychopharmacology (Berl) 2005;180:523–529. doi: 10.1007/s00213-005-2189-3. [DOI] [PubMed] [Google Scholar]
- 91.Kushner SF, Khan A, Lane R, Olson WH. Topiramate monotherapy in the management of acute mania: results of four double-blind placebo-controlled trials. Bipolar Disord. 2006;8:15–27. doi: 10.1111/j.1399-5618.2006.00276.x. [DOI] [PubMed] [Google Scholar]
- 92.Hashimoto K, Sawa A, Iyo M. Increased Levels of Glutamate in Brains from Patients with Mood Disorders. Biol. Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 93.Itokawa M, Yamada K, Iwayama-Shigeno Y, Ishitsuka Y, Detera-Wadleigh S, Yoshikawa T. Genetic analysis of a functional GRIN2A promoter (GT)n repeat in bipolar disorder pedigrees in humans. Neurosci. Lett. 2003;345:53–56. doi: 10.1016/s0304-3940(03)00501-9. [DOI] [PubMed] [Google Scholar]
- 94.Martucci L, Wong AH, De Luca V, Likhodi O, Wong GW, King N, Kennedy JL. N-methyl-D-aspartate receptor NR2B subunit gene GRIN2B in schizophrenia and bipolar disorder: Polymorphisms and mRNA levels. Schizophr. Res. 2006;84:214–221. doi: 10.1016/j.schres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 95.Scarr E, Pavey G, Sundram S, MacKinnon A, Dean B. Decreased hippocampal NMDA, but not kainate or AMPA receptors in bipolar disorder. Bipolar Disord. 2003;5:257–264. doi: 10.1034/j.1399-5618.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- 96.Gascon S, Deogracias R, Sobrado M, Roda JM, Renart J, Rodriguez-Pena A, Diaz-Guerra M. Transcription of the NR1 subunit of the N-methyl-D-aspartate receptor is down-regulated by excitotoxic stimulation and cerebral ischemia. J. Biol. Chem. 2005;280:35018–35027. doi: 10.1074/jbc.M504108200. [DOI] [PubMed] [Google Scholar]
- 97.Ciabarra AM, Sullivan JM, Gahn LG, Pecht G, Heinemann S, Sevarino KA. Cloning and characterization of chi-1: a developmentally regulated member of a novel class of the ionotropic glutamate receptor family. J. Neurosci. 1995;15:6498–6508. doi: 10.1523/JNEUROSCI.15-10-06498.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sucher NJ, Akbarian S, Chi CL, Leclerc CL, Awobuluyi M, Deitcher DL, Wu MK, Yuan JP, Jones EG, Lipton SA. Developmental and regional expression pattern of a novel NMDA receptor-like subunit (NMDAR-L) in the rodent brain. J. Neurosci. 1995;15:6509–6520. doi: 10.1523/JNEUROSCI.15-10-06509.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Das S, Sasaki YF, Rothe T, Premkumar LS, Takasu M, Crandall JE, Dikkes P, Conner DA, Rayudu PV, Cheung W, Chen HS, Lipton SA, Nakanishi N. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature. 1998;393:377–381. doi: 10.1038/30748. [DOI] [PubMed] [Google Scholar]
- 100.Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate- modulating drug riluzole. Mol. Psychiatry. 2008 doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou HR, Islam Z, Pestka JJ. Kinetics of lipopolysaccharide-induced transcription factor activation/inactivation and relation to proinflammatory gene expression in the murine spleen. Toxicol. Appl. Pharmacol. 2003;187:147–161. doi: 10.1016/s0041-008x(02)00077-7. [DOI] [PubMed] [Google Scholar]
- 102.Rosenberger TA, Villacreses NE, Hovda JT, Bosetti F, Weerasinghe G, Wine RN, Harry GJ, Rapoport SI. Rat brain arachidonic acid metabolism is increased by a 6-day intracerebral ventricular infusion of bacterial lipopolysaccharide. J. Neurochem. 2004;88:1168–1178. doi: 10.1046/j.1471-4159.2003.02246.x. [DOI] [PubMed] [Google Scholar]
- 103.Chang YC, Kim HW, Rapoport SI, Rao JS. Chronic NMDA administration increases neuroinflammatory markers in rat frontal cortex: cross-talk between excitotoxicity and neuroinflammation. Neurochem. Res. 2008;33:2318–2323. doi: 10.1007/s11064-008-9731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Petitto JM, Lysle DT, Gariepy JL, Lewis MH. Association of genetic differences in social behavior and cellular immune responsiveness: effects of social experience. Brain. Behav. Immun. 1994;8:111–122. doi: 10.1006/brbi.1994.1011. [DOI] [PubMed] [Google Scholar]
- 105.Gasparotto OC, Carobrez SG, Bohus BG. Effects of LPS on the behavioural stress response of genetically selected aggressive and nonaggressive wild house mice. Behav. Brain Res. 2007;183:52–59. doi: 10.1016/j.bbr.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 106.Zalcman SS, Siegel A. The neurobiology of aggression and rage: role of cytokines. Brain. Behav. Immun. 2006;20:507–514. doi: 10.1016/j.bbi.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 107.Siegel A, Bhatt S, Bhatt R, Zalcman SS. The neurobiological bases for development of pharmacological treatments of aggressive disorders. Curr. Neuropharmacol. 2007;5:135–147. doi: 10.2174/157015907780866929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stoll AL, Severus WE, Freeman MP, Rueter S, Zboyan HA, Diamond E, Cress KK, Marangell LB. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch. Gen. Psychiatry. 1999;56:407–412. doi: 10.1001/archpsyc.56.5.407. [DOI] [PubMed] [Google Scholar]
- 109.Diau GY, Hsieh AT, Sarkadi-Nagy EA, Wijendran V, Nathanielsz PW, Brenna JT. The influence of long chain polyunsaturate supplementation on docosahexaenoic acid and arachidonic acid in baboon neonate central nervous system. BMC Med. 2005;3:11. doi: 10.1186/1741-7015-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Salem N, Jr., Wegher B, Mena P, Uauy R. Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc. Natl. Acad. Sci. U. S. A. 1996;93:49–54. doi: 10.1073/pnas.93.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zarate CA, Jr., Quiroz JA, Singh JB, Denicoff KD, De Jesus G, Luckenbaugh DA, Charney DS, Manji HK. An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol. Psychiatry. 2005;57:430–432. doi: 10.1016/j.biopsych.2004.11.023. [DOI] [PubMed] [Google Scholar]