Abstract
It was demonstrated previously that low dietary potassium (K) intake stimulates Src family protein tyrosine kinase (PTK) expression via a superoxide-dependent signaling. This study explored the role of mitogen-activated protein kinase (MAPK) in mediating the effect of superoxide anions on PTK expression and ROMK (Kir 1.1) channel activity. Western blot analysis demonstrated that low K intake significantly increased the phosphorylation of P38 MAPK (P38) and extracellular signal–regulated kinase (ERK) but had no effect on phosphorylation of c-JUN N-terminus kinase in renal cortex and outer medulla. The stimulatory effect of low K intake on P38 and ERK was abolished by treatment of rats with tempol. The possibility that increases in superoxide and related products that are induced by low K intake were responsible for stimulating phosphorylation of P38 and ERK also was supported by the finding that application of H2O2 increased the phosphorylation of ERK and P38 in the cultured mouse collecting duct cells. Simultaneous blocking of ERK and P38 completely abolished the effect of H2O2 on c-Src expression in mouse collecting duct cells. For determination of the role of P38 and ERK in the regulation of ROMK-like small-conductance K (SK) channels, the patch-clamp technique was used to study the effect of inhibiting P38 and ERK on SK channels in the cortical collecting duct from rats that were on a control K diet (1.1%) and on a K-deficient diet for 1 d. Inhibition of ERK, c-JUN N-terminus kinase, or P38 alone had no effect on SK channels. In contrast, simultaneous inhibition of P38 and ERK significantly increased channel activity. The effect of inhibiting MAPK on SK channels was not affected in the presence of herbimycin A, a PTK inhibitor, and was larger in rats that were on a K-deficient diet than in rats that were on a normal-K diet. However, the stimulatory effect of inhibiting ERK and P38 on SK was absent in the cortical collecting duct that was treated with colchicine. It is concluded that low K intake–induced increases in superoxide levels are responsible for stimulation of P38 and ERK and that MAPK inhibit the SK channels by stimulating PTK expression and via a PTK-independent mechanism.
The kidney plays a key role in maintaining potassium (K) homeostasis, which is essential for the function of a variety of cells, including neurons, cardiac myocytes, and skeletal muscles (1). It is well established that increases in K intake stimulate whereas decreases in K intake suppress renal K excretion (1). Low K intake–induced suppression of K excretion is achieved by stimulation of K absorption in intercalated cells (2,3) and inhibition of K secretion in principal cells (4) in the connecting tubule and the cortical collecting duct (CCD). Inhibition of K secretion in principal cells is partially achieved by decreasing apical K channel expression (4,5). We previously demonstrated that low K intake decreases the apical small-conductance K (SK) channel activity (6). The effect of low K intake on the SK channels is mediated by a protein tyrosine kinase (PTK)-dependent pathway (7,8) because inhibition of PTK increases the SK channel activity in the CCD (9,10). Moreover, we have shown that low K intake increases superoxide levels which mediate the effect of low K intake on PTK expression (6) and that suppression of superoxide production with tempol diminishes the effect of low K intake on c-Src expression (6).
The role of superoxide in the regulation of SK channels is demonstrated best by findings that the SK channel activity in the CCD from the tempol-treated rats was higher than that without tempol. We hypothesized that low K intake stimulates superoxide levels in the kidney and increases the expression of Src family PTK, which enhances the tyrosine phosphorylation of ROMK (Kir 1.1) channels in the CCD (8). As a consequence of tyrosine phosphorylation, SK channels were internalized. However, the mechanism by which superoxide stimulates PTK expression is not understood. Also, the finding that increases in PTK expression were not observed until 2 to 3 d after K restriction whereas decreases in urinary K excretion took place several hours after K restriction suggests that signal molecules other than PTK regulate the SK channel activity in the early stage of K restriction. Increases in superoxide levels have been shown to activate extracellular signal–regulated kinase (ERK), P38, and c-JUN N-terminus kinase (JNK) (11–13). Moreover, stimulation of mitogen-activated protein kinase (MAPK) is known to increase the phosphorylation of transcription factors such as c-JUN (14). Therefore, it is conceivable that MAPK may be a member of signaling molecules that are required for the effect of low K intake on the Src family PTK and SK channel activity. Also, MAPK has been demonstrated to regulate a variety of ion channels (15–17). The goal of our study was to determine whether low K intake also stimulates MAPK and to examine the role of MAPK in the regulation of SK channel activity in the CCD.
Materials and Methods
Tissue Preparation
Sprague-Dawley rats (6 to 8 wk, either sex) were purchased from Taconic Farms (Germantown, NY). Rats were divided into three groups: (1) Control group, in which animals were kept on a normal K (1.1%) diet; (2) low K group, in which rats were maintained on a K-deficient (KD) diet (Harlan Teklad, Madison, WI) for 7 d and had a daily injection of vehicle; and (3) tempol-treated group, in which rats also were fed a KD diet and had a daily intraperitoneal injection of tempol (15 mg/kg) for 1 wk. Rats were killed by cervical dislocation, and kidneys were removed immediately. The renal cortex and the outer medulla were separated under a dissecting microscope and suspended in RIPA buffer solution (1:8 ratio, wt/vol) that contained 1× PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% SDS. A total of 10 µl of PMSF (10 mg/ml stock solution in isopropanol) and 10 µl of a cocktail of protease inhibitors (Sigma, St. Louis, MO) were added per milliliter of buffer at the time of lysis. The samples were homogenized on ice for 15 min with a mortar and pestle. The suspension was incubated at 4°C for 1 h in the presence of DNAse (5 µg/ml) followed by centrifugation at 1800 rpm for 10 min, and the resultant supernatant was collected. Protein concentrations were measured in duplicate using a Bio-Rad Dc protein assay kit (Hercules, CA).
Metabolic Cage
Rats were housed in metabolic cages for 1 d to study the urinary K excretion. After 3 d of training in the cage, rats were divided into a control group, in which rats were kept on a normal K diet, and the low K group, in which rats were maintained on a KD diet. Data regarding the 24-h food intake, body weight, and urine output were recorded. Urinary K concentrations were measured by a flame photometer, and daily K excretion were calculated as mEq/24 h.
Measurement of Superoxide Anion
Renal cortex and outer medulla were isolated from rats that were on a normal or KD diet for 1 d. The tissue (100 mg) was cut into a small piece with a sharp blade and suspended in air-equilibrated MOPS-sucrose buffer (pH 7.4) that contained 5 µM lucigenin. The chemiluminescence that was elicited in the presence of lucigenin was measured in a liquid scintillation counter with a single active photomultiplier tube positioned in out-of-coincidence mode. Blanks were subtracted from the average level of chemiluminescence signal.
Preparation of Mouse Collecting Duct Cells
Mouse collecting duct (M-1) cells, a mouse CCD line, were purchased from the American Type Culture Collection (Manassas, VA) and maintained in RPMI 1640 medium supplemented with 10% FBS. Before H2O2 treatment, the cells were cultured in medium that contained 1% FBS for 16 h, followed by incubation for an additional 30 min in a solution that contained 22 mM HEPES (pH 7.4), 124 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.5 mM CaCl2, 0.16 mM HPO4, 0.4 mM H2PO4, 5 mM NaHCO3, and 5.6 mM glucose. H2O2 (200 µM) was added directly to the cells in HEPES buffer for 60 to 120 min. The viability of M-1 cells that were treated with H2O2 as determined by Trypan Blue dye exclusion method was approximately 90% of the corresponding control cells. To study the phosphorylation of MAPK, we washed the cells with ice-cold PBS twice after treatment of H2O2 and incubated them for 30 min in RIPA lysis buffer. To study the expression of c-Src, we incubated cells for an additional 2 h in the control medium after removal of H2O2.
Immunoprecipitation and Western Blot
The corresponding antibody was added to the protein samples (500 µg) that were harvested from cell cultures at a ratio of 5 µl/ml solution. The mixture was rotated gently at 4°C overnight, followed by incubation with 25 µl of protein A/G agarose (Santa Cruz, CA) for an additional 2 h at 4°C. The tube that contained the mixture was centrifuged at 3000 rpm and washed twice with PBS that contained 10 µl/ml PMSF and 10 µl/ml protease inhibitor cocktail. The agarose pellet was resuspended in 25 µl of 2× SDS sample buffer that contained 4% SDS, 100 mM Tris-HCl (pH 6.8), 20% glycerol, 200 mM dithiothreitol, and 0.2% bromophenol blue. After the sample was boiled for 5 min, proteins were separated by electrophoresis on 10% SDS–polyacrylamide gels and transferred to Immuno-Blot PVDF membrane (Bio-Rad). The membrane was blocked with 5% nonfat dry milk in Tris-buffered saline and incubated overnight with the primary antibody at 4°C. The membrane was washed three times for 15 min with Tris-buffered saline that contained 0.05% Tween 20 followed by incubation for 30 min with respective second antibody horseradish peroxidase conjugate. ECL plus (Amersham Pharmacia Biotech, Piscataway, NJ) was used to detect the protein bands, and the intensity of the bands of interest was determined using Alpha DigiDoc 1000 (Alpha Innotech, San Leandro, CA).
Preparation of CCD for Patch-Clamping
We used rats that were on either a normal K diet or a KD diet for 1 d for the patch-clamp experiments. Single CCD were isolated, placed on a 5 × 5-mm coverglass coated with polylysine, and transferred to a chamber (1000 µl) mounted on an inverted Nikon microscope. The CCD were superfused with HEPES buffered NaCl solution that contained (in mM) 140 NaCl, 5 KCl, 1.8 CaCl2, 1.8 MgCl2, and 5 HEPES (pH 7.4). The pipette solution was composed of (in mM) 140 KCl, 1.8 MgCl2, and 5 HEPES (pH 7.4). The temperature of the chamber was maintained at 37 ± 1°C by circulation of warm water around the chamber. The CCD was cut open with a sharpened micropipette to expose the apical membrane. An Axon 200A patch-clamp amplifier was used to record channel current. The current was low-pass filtered at 1 KHz by an eight-pole Bessel filter (902LPF; Frequency Devices, Haverhill, MA) and digitized with Axon interface (Digidata 1200). Data were analyzed using the pClamp software system 6.04 (Axon Instruments, Burlingame, CA). Channel activity was defined as NPo, which was calculated from data samples of 60-s duration in the steady state as follows:
where ti is the fractional open time spent at each of the observed current levels.
Experimental Materials
Antibodies to phospho-P38, P38, phospho-ERK, ERK, phospho-JNK, JNK, and c-Src were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Herbimycin A, SB202190, SP600125, and PD098059 were purchased from Biomol (Plymouth Meeting, PA).
Statistical Analyses
The data are presented as mean ± SEM. We used paired t test to determine the statistical significance. P < 0.05 was considered to be significant.
Results
To determine the role of MAPK in mediating the effect of low K intake on the SK channels, we first examined the effect of low K intake on the expression and phosphorylation of P38, ERK, and JNK in the renal cortex and outer medulla. Figure 1A is a representative Western blot demonstrating the effect of low K intake on the expression of total P38 (middle) and the phosphorylation of P38 (top). From inspection of Figure 1A, it is apparent that K restriction for 7 d stimulates the phosphorylation of P38 by 155 ± 20% (P < 0.001; n = 5 rats). We also examined the effect of low K intake on the expression and phosphorylation of ERK in the renal cortex and outer medulla. From inspection of Figure 1B, it is apparent that low K intake also stimulated the phosphorylation of ERK by 150 ± 20% (P < 0.001; n = 5; top) but had no effect on the expression of total ERK (middle). However, low K intake did not significantly alter either phosphorylation or expression of JNK (Figure 1C). Therefore, our data demonstrate that low K intake increases the activity of ERK and P38 but not JNK in renal cortex and outer medulla.
Figure 1.
Effect of potassium (K) depletion on the phosphorylation of P38 mitogen-activated protein kinase (P38 MAPK; A), extracellular signal–regulated kinase (ERK; B), and c-JUN N-terminus kinase (JNK; C) in rats that received tempol treatment or vehicle. (Top) Phosphorylated P38, ERK, and JNK, respectively. (Middle) Corresponding total amount of P38, ERK, and JNK. Data are summarized as a bar graph at the bottom of each panel. Changes of the band intensity are determined by calculation of the ratio between phosphorylation band intensity (Exp)/control value times the ratio between total protein intensity (normal)/Exp. *P < 0.01, control versus experimental group.
We then explored the possibility that superoxide anions or related products may mediate the effect of low K intake on MAPK. This notion was based on the previous finding that low K intake increases the superoxide production and that superoxide anions and related products mediate the effect of low K intake on PTK expression (6). We examined the effect of H2O2 on MAPK phosphorylation in M-1 cells, a mouse CCD line. Previously, we demonstrated that treatment of M-1 cells with H2O2 mimicked the effect of low K on c-Src expression (6). Figure 2A is a typical Western blot showing the effect of H2O2 on the phosphorylation and expression of P38 in M-1 cells that were treated with 200 µM H2O2 for 60, 90, and 120 min. Treatment of M-1 cells with H2O2 for 60, 90, and 120 min increased phosphorylation of P38 by 30 ± 10, 90 ± 30 (P < 0.01; n = 5), and 130 ± 40% (P < 0.001), respectively. We also examined the effect of H2O2 on the phosphorylation and expression of ERK in M-1 cells. Figure 2B is a Western blot showing that treatment of M-1 cells with 200 µM H2O2 for 60, 90, and 120 min increased phosphorylation of ERK by 30 ± 15, 70 ± 20 (P < 0.01; n = 5), and 110 ± 30% (P < 0.001), respectively. Also, treatment of M-1 cells with H2O2 did not significantly stimulate the phosphorylation of JNK in M-1 cells. Therefore, the effect of H2O2 on P38 and ERK in M-1 cells was similar to those observed in tissue, suggesting that superoxide or related products may be responsible for mediating the effect of low K intake on P38 and ERK in the kidney.
Figure 2.
Effect of H2O2 on the phosphorylation of P38 MAPK (A), ERK (B), and JNK (C) in mouse collecting duct (M-1) cells. (Top and middle) Phosphorylated MAPK and the total MAPK, respectively. Data are summarized as a bar graph at the bottom of each panel. *P < 0.01, control versus experimental group.
The notion that superoxide anions and related products may be responsible for the effect of low K on MAPK phosphorylation is established further by experiments in which the effect of low K intake for 7 d on MAPK phosphorylation was examined in rats that were treated with tempol for 7 d. We previously showed that tempol treatment reduced the level of superoxide anions that are induced by low K intake (6). From inspection of Figure 1, it is apparent that tempol treatment abolished the effect of low K intake on the phosphorylation of P38 and ERK. The effect of tempol must be related specifically to the reduction of superoxide because tempol treatment did not significantly change the phosphorylation of P38 and ERK in rats that were on a normal K diet, which has a low superoxide level. Also, tempol treatment did not significantly affect the phosphorylation level of JNK. This suggests that low K intake–induced superoxide did not regulate the phosphorylation of JNK in renal cortex and outer medulla in our experimental settings. Therefore, our study strongly suggests that low K intake–induced increase in superoxide anions is responsible for the stimulation of phosphorylation of P38 and ERK during K restriction.
We then explored the physiologic role of P38 and ERK in mediating the effect of low K on the expression of c-Src, which serves as a representative member of Src family PTK. We previously showed that low K intake stimulates the expression of c-Src (6,9) and that the effect of low K intake on c-Src expression is mediated by superoxide anions. Therefore, we used M-1 cells to examine whether inhibiting MAPK could abolish the effect of H2O2 on c-Src expression. Figure 3 is a Western blot demonstrating the effect of H2O2 on c-Src expression in the absence (control) and presence of SB202190 (5 µM), PD098059 (50 µM), SP600125 (10 µM), SB202190+PD098059, or three inhibitors. We confirmed the previous report that H2O2 increased the expression of c-Src by 200 ± 20% (P < 0.001; n = 5). However, inhibition of ERK with PD098059 (18) or JNK with SP600125 (19) or P38 with SB202190 (20) only attenuated the effect of H2O2 on c-Src expression and still increased the expression of c-Src by 80 ± 25 (P < 0.01; n = 5), 90 ± 20 (P < 0.01; n = 5), and 60 ± 10% (P < 0.01; n = 5), respectively. When P38 and ERK MAPK were inhibited simultaneously, the effect of H2O2 on the expression of c-Src was completely abolished. Also, application of triple inhibitors did not cause further inhibition of the effect of H2O2 on c-Src expression. In considering that low K intake did not significantly increase the JNK phosphorylation in the rat kidney and that H2O2 did not affect the phosphorylation of JNK in M-1 cells, it is possible that the suppressing effect of SP600125 may be the result of inhibiting MAPK other than JNK, such as P38 or ERK. This view is also supported by the Western blot analysis that is shown in Figure 3B, in which application of SP+PD had a similar effect of SB+PD, whereas SP+SB did not completely abolish the effect of H2O2 on c-Src expression in M-1 cells (n = 3). Thus, P38 and ERK MAPK are involved mainly in mediating the effect of low K or superoxide on the expression of c-Src. However, considering the possible cross-action of PD098059 or SB202190, we could not completely exclude the role of JNK in mediating the effect of H2O2 on c-Src expression.
Figure 3.
(A) Effect of H2O2 on the expression of c-Src in M-1 cells in the absence of MAPK inhibitors and in the presence of PD098059 (50 µM), SP600125 (10 µM), SB202190 (5 µM), PD+SB, or three inhibitors. Data are summarized and presented as bar graph at the bottom of the figure. **P < 0.001 and *P < 0.01, control versus experimental group. IP, immunoprecipitation; IB, immunoblot. (B) A Western blot showing the effect of H2O2 on the expression of c-Src in M-1 cells in the presence of SP600125+SB202190, SP600125+PD098059, and SB202190+PD098059.
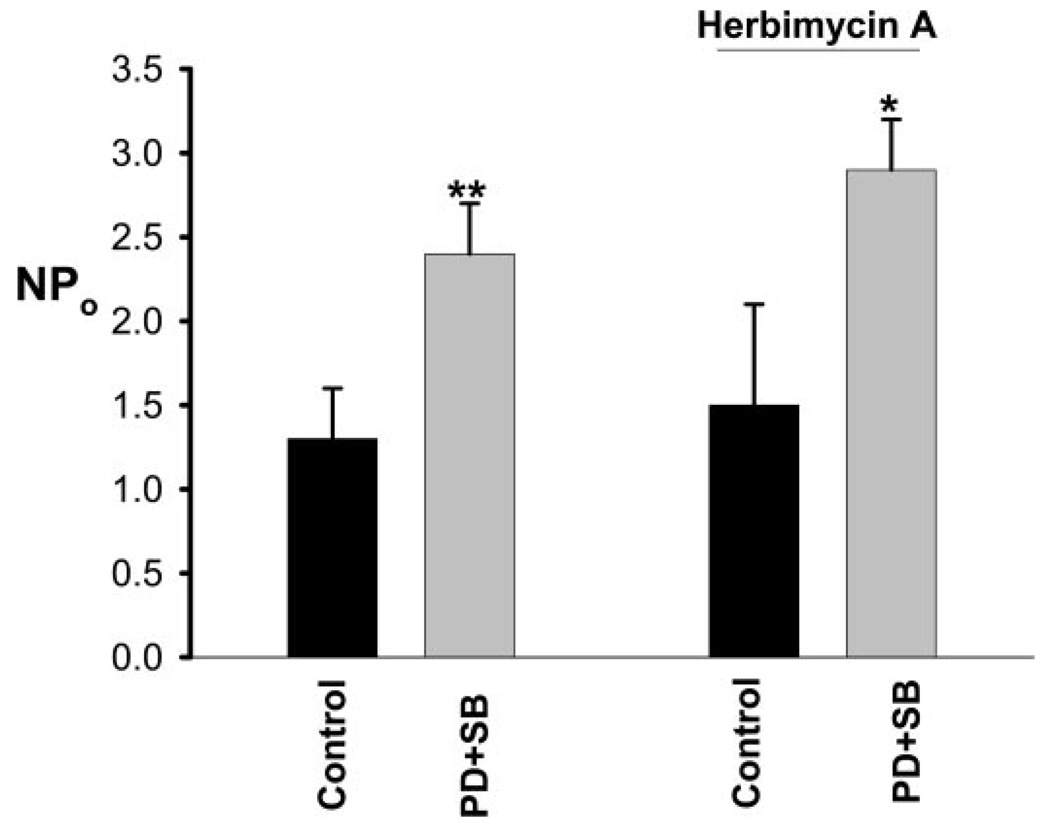
Because PTK has been shown to phosphorylate ROMK channels and facilitate the internalization (6,21), activation of P38 and ERK was expected to inhibit the ROMK-like SK channels via a PTK-dependent pathway. Therefore, it was conceivable that P38 and ERK are two molecules of the PTK-dependent pathway, which regulates the SK channels in the CCD. Moreover, MAPK have been shown to regulate ion channels by a PTK-independent pathway (17,22,23). Therefore, we carried out the patch-clamp experiments to determine whether inhibition of P38, ERK, or JNK could increase the SK channel activity in the CCD. Inhibition of ERK (n = 12) or blocking JNK (n = 8) had no significant effect on the SK channels (control 1.5 ± 0.2; 50 µM PD098059 1.5 ± 0.2; 10 µM SP600125 1.55 ± 0.2). Also, suppression of P38 with 5 µM SB202190 slightly but insignificantly increased SK channel activity from 1.4 ± 0.2 to 1.6 ± 0.2 (n = 12), but when both ERK and P38 were inhibited simultaneously by PD098059 and SB202190, the SK channel activity increased significantly. Figure 4 is a channel recording showing that application of SB202190+PD098059 stimulates the activity of SK channels and increased NPo from 0 to close to 2.8 in this particular patch of the CCD from rats that were on a control K diet. Data that are summarized in Figure 5 show that application of PD098059+SB202190 increased SK channel activity from 1.3 ± 0.3 to 2.4 ± 0.3 (n = 10; P < 0.01) in the CCD from rats that were on a control K diet. From inspection of Figure 4, it also is apparent that the stimulatory effect of SB202190+PD98059 on the SK channels occurs within 5 min. In 10 experiments, the onset time of the effect of MAPK inhibitors on SK channels took place in <10 min. In contrast, the stimulatory effect of inhibiting PTK on the SK channels was observed within 20 to 30 min (7). Therefore, it is unlikely that the effect of inhibiting MAPK on the SK channels was the result of suppression of PTK. This speculation also was supported by experiments in which the effect of SB202190+PD098059 on SK channels was examined in the presence of herbimycin A to block PTK. From inspection of Figure 6, it is apparent that inhibition of MAPK still can stimulate the SK channel activity after inhibition of PTK because NPo increased from 0 to 1.9 in this patch. Figure 5 summarized data showing that inhibiting MAPK increased channel activity from 1.5 ± 0.6 to 2.9 ± 0.3 (n = 5; P < 0.05). This suggests that the inhibitory effect of P38 and ERK on SK channels did not require the involvement of PTK. To determine the mechanism by which inhibiting MAPK stimulates the SK channel activity, we examined the effect of PD098059+SB202190 on SK channels in the CCD that were treated with colchicine. We previously showed that colchicine treatment blocked the effect of inhibiting PTK on SK channels (24), suggesting the role of microtubule in mediating the insertion of SK channels induced by inhibition of PTK. In 10 experiments, inhibition of microtubule completely abolished the effect of PD098059 and SB202190 on ROMK channel activity (control, NPo = 1.1 ± 0.2; PD+SB, NPo = 1.2 ± 0.25). This suggests that microtubule is required for the effect of MAPK on SK channels in the CCD.
Figure 4.
A channel recording showing that simultaneous inhibition of ERK with PD098059 (PD) and P38 MAPK with SB202190 (SB) activates the small-conductance K (SK) channels in the cortical collecting duct (CCD) from a rat that was on a normal K diet. The experiments were performed in cell-attached patches, and the holding potential was 30 mV. The top trace is the time course of the experiment, whereas three parts of trace, indicated by numbers, are extended to show the fast time resolution. The channel close level is indicated by “C.” The arrow indicates the addition of MAPK inhibitors that were present throughout the experiments. Also, the electric noise after addition of PD+SB was artifact.
Figure 5.
Effect of PD098059+SB202190 on the SK channels in the presence and absence of herbimycin A in the CCD from rats that were on a normal K diet. The experiments were performed in cell-attached patches. Herbimycin A (1 µM) was used to treated the CCD for 15 min and present throughout the experiment. *P < 0.05 and **P < 0.01 versus control.
Figure 6.
A channel recording showing that simultaneous inhibition of ERK and P38 MAPK activates the SK channels in the presence of herbimycin A. The experiments were performed in cell-attached patches, and the holding potential was 30 mV. The top trace is the time course of the experiment, whereas three parts of trace, indicated by numbers, are extended to show the fast time resolution. The channel close level is indicated by “C,” and the gap of the top trace is 180 s. The arrow indicates the addition of MAPK inhibitors, which were present throughout the experiments.
Because low K intake–induced increase in the expression of Src family PTK was not observed until 2 to 3 d after K restriction (9), we speculated that activation of MAPK may serve as an early signal molecule during K depletion and that it suppresses apical SK channels and renal K excretion. This notion is supported by experiments in which superoxide levels and phosphorylation of ERK and P38 were examined in renal cortex and outer medulla from rats that were on a KD diet for 24 h. Results that are summarized in Figure 7A demonstrate that superoxide levels were significantly higher (190 ± 30% of the control value in the kidney from rats that were on a KD diet for 1 d than those that were on a control K diet). Moreover, K restriction for 1 d increased the phosphorylation of P38 and ERK by 100 ± 10% (n = 3) but had no significant effect on the expression of c-Src (Figure 7B). We also examined the effect of P38 and ERK on the SK channel activity (Figure 7C) in the CCD and used the metabolic cage to examine the urinary K excretion in rats that were on a KD diet for 1 d. Low K intake for 1 d reduced channel activity to 0.7 ± 0.1 (n = 18), and inhibition of ERK and P38 with PD098059 and SB202190 significantly increased channel activity to 2.1 ± 0.3 (n = 11). Therefore, the effect of inhibiting ERK and P38 on the SK channels was larger (200% increase) in the CCD from rats that were on a KD diet for 1 d than that from rats that were on control K diet (changes in NPo from 1.3 to 2.4, an 85% increase). Also, metabolic cage study shows that urinary K secretion decreased from 3.5 ± 0.3 to 0.41 ± 0.02 mEq/L (n = 6) when rats were maintained on a KD diet for 24 h (data not shown).
Figure 7.
(A) A bar graph shows the effect of K restriction for 1 d on superoxide levels (n = 3). *Significant difference. (B) Western blots demonstrate the effect of 24-h K restriction on the phosphorylation of P38 and ERK and the expression of c-Src. (C) Effect of K restriction for 24 h on the SK channel activity in the presence or absence of inhibitors of MAPK (PD098059+ SB202190). *Significant different between control and K-deficient (KD) group; #significant difference between KD and KD+MAPK inhibitor groups. The experimental number is indicated under each bar.
Discussion
The main findings of our study are that low K intake activates the ERK and P38 and that the MAPK regulate ROMK channel activity in the CCD. Our data suggest that the SK channel activity is suppressed by P38 and ERK under physiologic conditions. Therefore, inhibition of MAPK relieves the inhibition of SK channels that is induced by P38 and ERK. Three lines of evidence indicate that superoxide is a mediator for the effect of low K intake on the expression of ERK and P38: (1) Low K intake increased the superoxide production in the renal cortex and outer medulla, (2) treatment of M-1 cells with H2O2 mimicked the effect of low K and increased the expression of ERK and P38, and (3) suppression of superoxide production with tempol abolished the effect of low K on the phosphorylation of MAPK. We previously showed that low K intake stimulates the expression of Src-family PTK (6,9), which in turn increases the tyrosine phosphorylation of ROMK channels and facilitates the internalization (6,8,25,26). We also showed that superoxide anion and the related products mediate the effect of low K intake on PTK activity, because decreasing superoxide levels abolished the effect of low K intake on PTK expression (6). Now we have demonstrated that superoxide anions and related products also mediate the effect of low K intake on ERK and P38.
Superoxide and the related products initially were identified to be involved in the regulation of immunoresponse and cell death. However, a large body of evidence supports the notion that superoxide anions play an important role in mediating a variety of cell functions (27). Superoxide anions have been demonstrated to modulate the activity of various protein kinases and phosphatases (12,28–32). H2O2 has been shown to inhibit protein tyrosine phosphatase (30,33) and activate several members of Src family PTK, such as Lck and Fyn (31,32). Superoxide anions have been shown to mediate the effect of nerve growth factor in neuronal cells (34) and EGF in human epidermoid carcinoma cells (35). Moreover, elimination of H2O2 by catalase has been demonstrated to inhibit EGF and nerve growth factor receptors. Stimulation of insulin receptors has been shown to augment the formation of superoxide (36), and low concentrations of H2O2 can potentiate the insulin effect in insulin-responsive tissues (37). Furthermore, high concentrations of H2O2 can induce insulin-like effects in the absence of insulin via stimulation of the insulin-independent tyrosine phosphorylation of the insulin receptor (38). H2O2 mediates the stimulatory effect of angiotensin II on nitric oxide production in endothelial cells (39). Also, H2O2 stimulates cGMP generation and causes the transient relaxation of calf coronary arteries (40).
Numerous experiments have demonstrated that superoxide and the related products play an important role in the regulation of MAPK (11,12,14,28). The activity of MAPK is regulated by phosphorylation or dephosphorylation on serine or tyrosine residues (13,14). H2O2 has been shown to increase the serine phosphorylation of JNK, P38, and ERK1/2 (13). In our study, low K intake stimulates significantly the phosphorylation of P38 and ERK but does not significantly alter the phosphorylation of JNK in the renal cortex and outer medulla. Although we cannot completely exclude the role of JNK in mediating the effect of low K intake on PTK activity and SK channel, the observation that inhibition of P38 and ERK abolished the effect of H2O2 on c-Src expression in M-1 cells suggests that P38 and ERK are responsible mainly for mediating the effect of low K intake. Although inhibition of JNK attenuated the H2O2-induced increases in c-Src expression, the effect may be the results of inhibition of ERK or P38. This notion is supported by two lines of evidence: (1) H2O2 did not significantly alter the phosphorylation of JNK in M-1 cells, and (2) inhibition of JNK did not have additive effect in comparison with that with inhibitors of P38 and ERK. Therefore, it is likely that ERK and P38 are two molecules that are involved in mediating the effect of low K on the expression of c-Src. We propose that low K intake increases superoxide anions and the related products that stimulate the phosphorylation of ERK and P38. Activation of MAPK increased the activity of some transcription factors and, accordingly, the expression of Src family PTK. Indeed, it has been reported that H2O2-induced activation of MAPK plays a key role in the stimulation of c-Jun activity (41).
In addition to stimulation of PTK expression by which MAPK are involved in regulating SK channels, MAPK regulate the SK channels in the CCD by a PTK-independent mechanism. This view is strongly supported by the finding that inhibition of MAPK stimulated SK channels in the presence of the inhibitor of PTK. The MAPK-induced inhibition of the SK channel activity should have a significant effect in response to K restriction. We have observed that K secretion decreases by 90% 1 d after maintaining rats on a KD diet, whereas PTK expression is not significantly altered. Therefore, it is possible that a PTK-independent mechanism may be responsible for inhibiting the SK channels and decreasing K secretion in response to low K intake before PTK is fully effective. MAPK-induced inhibition could serve as a PTK-independent mechanism by which the SK channels are blocked in response to K restriction. This notion is supported by two lines of evidence: (1) K restriction for 1 d significantly increased superoxide levels and MAPK phosphorylation in renal cortex and outer medulla, and (2) inhibition of P38 and ERK significantly increased the SK channel activity in the CCD from rats that were on a KD diet for 1 d whereas the effect of inhibiting PTK on the SK channels was still modest (9). Figure 8 is a cell model illustrating the role of MAPK in the regulation of ROMK channel activity. We speculate that low K intake increases superoxide and the related products after K restriction, which in turn activates both ERK and P38. Activation of MAPK inhibits SK channels in the CCD, and this mechanism may contribute partially to the suppression of renal K excretion at the initial stage of K restriction. In addition, MAPK are involved in mediating the stimulatory effect of low K intake on PTK expression, which further decreases channel activity by the internalization.
Figure 8.
A cell model illustrating the role of MAPK in mediating the effect of low K intake on ROMK (Kir 1.1) channel activity in the CCD.
The mechanism by which P38 and ERK inhibit the SK channels is not clear. It is well established that ERK and P38 also can regulate membrane proteins, including receptors and ion channels, in addition to the traditional role of regulating signal molecules in the nucleus and cytosol (15–17,22,23). It has been shown that P38 inhibits epithelial Na channels in alveolar epithelial cells by changing membrane expression or trafficking (16). The observation that inhibition of microtubule blocked the stimulatory effect of PD098059 and SB202190 on SK channels in the CCD suggests that microtubule is required for the effect of MAPK on SK channels. We previously showed that colchicine treatment abolished the ROMK channel insertion that was induced by inhibition of PTK (24). The finding that colchicine also abolished the effect of inhibition of MAPK on SK channels suggests that blocking P38- and ERK-induced increase in channel activity may be the result of altering channel trafficking. We need further experiments to explore the mechanism by which MAPK inhibits the SK channels in the CCD.
Conclusion
Low K intake stimulates superoxide levels, which activate ERK and P38. Activation of ERK and P38 inhibits the SK channels through two possibilities: (1) ERK and P38 inhibit the SK channels by a PTK-independent mechanism; and (2) ERK and P38 are involved in stimulating the expression of Src-family PTK, which in turn enhances in tyrosine phosphorylation and internalization of the SK channels in the CCD. Furthermore, ERK and P38 may play a key role in suppressing SK channels and renal K secretion in the early phase after K restriction.
Acknowledgments
The work was supported by National Institutes of Health grants DK 47402 and DK 54983.
References
- 1.Giebisch G. Renal potassium transport: Mechanisms and regulation. Am J Physiol. 1998;274:F817–F833. doi: 10.1152/ajprenal.1998.274.5.F817. [DOI] [PubMed] [Google Scholar]
- 2.Wingo CS, Seldin DW, Kokko JP, Jacobson HR. Dietary modulation of active potassium secretion in the cortical collecting tubule of adrenalectomized rabbits. J Clin Invest. 1982;70:579–586. doi: 10.1172/JCI110650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DuBose TD, Jr, Gitomer J, Codina J. H+,K+-ATPase. Curr Opin Nephrol Hypertens. 1999;8:597–602. doi: 10.1097/00041552-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Wang WH. Regulation of renal K transport by dietary K intake. Annu Rev Physiol. 2004;66:547–569. doi: 10.1146/annurev.physiol.66.032102.112025. [DOI] [PubMed] [Google Scholar]
- 5.Chu PY, Quigley R, Babich V, Huang CL. Dietary potassium restriction stimulates endocytosis of ROMK channel in rat cortical collecting duct. Am J Physiol Renal Physiol. 2003;285:F1179–F1187. doi: 10.1152/ajprenal.00150.2003. [DOI] [PubMed] [Google Scholar]
- 6.Babilonia E, Wei Y, Sterling H, Kaminski P, Wolin MS. Superoxide anions are involved in mediating the effect of low K intake on c-Src expression and renal K secretion in the cortical collecting duct. J Biol Chem. 2005;280:10790–10796. doi: 10.1074/jbc.M414610200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang WH, Lerea KM, Chan M, Giebisch G. Protein tyrosine kinase regulates the number of renal secretory K channel. Am J Physiol Renal Physiol. 2000;278:F165–F171. doi: 10.1152/ajprenal.2000.278.1.F165. [DOI] [PubMed] [Google Scholar]
- 8.Lin DH, Sterling H, Lerea KM, Welling P, Jin L, Giebisch G, Wang WH. K depletion increases the protein tyrosine-mediated phosphorylation of ROMK. Am J Physiol Renal Physiol. 2002;283:F671–F677. doi: 10.1152/ajprenal.00160.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei Y, Bloom P, Lin DH, Gu RM, Wang WH. Effect of dietary K intake on the apical small-conductance K channel in the CCD: Role of protein tyrosine kinase. Am J Physiol Renal Physiol. 2001;281:F206–F212. doi: 10.1152/ajprenal.2001.281.2.F206. [DOI] [PubMed] [Google Scholar]
- 10.Wei Y, Bloom P, Gu RM, Wang WH. Protein-tyrosine phosphatase reduces the number of apical small conductance K channels in the rat cortical collecting duct. J Bio Chem. 2000;275:20502–20507. doi: 10.1074/jbc.M000783200. [DOI] [PubMed] [Google Scholar]
- 11.Ranganathan AC, Nelson KK, Rodriguez AM, Kim K-H, Tower GB, Rutter JL, Brinckerhoff CE, Huang T-T, Epstein CJ, Jeffrey JJ, Melendez JA. Manganese superoxide dismutase signals matrix metalloproteinase expression via H2O2-dependent ERK1/2 Activation. J Biol Chem. 2001;276:14264–14270. doi: 10.1074/jbc.M100199200. [DOI] [PubMed] [Google Scholar]
- 12.Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ. Activation of mitogen-activated protein kinase by H2O2. Role of cell survival following oxidant injury. J Biol Chem. 1996;271:4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- 13.Schmitz ML, Bacher S, Droge W. Molecular analysis of mitogen-activated protein kinase signaling pathways induced by reactive oxygen intermediates. Methods Enzymol. 2002;352:53–61. doi: 10.1016/s0076-6879(02)52006-1. [DOI] [PubMed] [Google Scholar]
- 14.Chen K, Vita JA, Berk BC, Keany JF., Jr c-Jun N-terminal kinase activation by hydrogen peroxide in endothelial cells involves Src-dependent epidermal growth factor receptor transactivation. J Biol Chem. 2001;276:16045–16050. doi: 10.1074/jbc.M011766200. [DOI] [PubMed] [Google Scholar]
- 15.Chae KS, Dryer SE. The P38 mitogen-activated protein kinase pathway negatively regulates Ca2+-activated K channel trafficking in developing parasympathetic neurons. J Neurochem. 2005;94:367–379. doi: 10.1111/j.1471-4159.2005.03201.x. [DOI] [PubMed] [Google Scholar]
- 16.Roux J, Kawakatsu H, Gartland B, Pespeni M, Sheppard D, Matthay MA, Canessa CM, Pittet JF. Interleukin-1 beta decreases expression of the epithelial sodium channel alpha-subunit in alveolar epithelial cells via p38 MAPK-dependent signaling pathway. J Biol Chem. 2005;280:18579–18589. doi: 10.1074/jbc.M410561200. [DOI] [PubMed] [Google Scholar]
- 17.Shen MR, Chou CY, Hsu KF, Ellory JC. Osmotic shrinkage of human cervical cancer cells induces an extracellular Cl-dependent nonselective cation channel, which requires P38 MAPK. J Biol Chem. 2002;277:45776–45784. doi: 10.1074/jbc.M207993200. [DOI] [PubMed] [Google Scholar]
- 18.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 19.Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson D. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 21.Lin DH, Sterling H, Yang B, Hebert SC, Giebisch G, Wang WH. Protein tyrosine kinase is expressed and regulates ROMK1 location in the cortical collecting duct. Am J Physiol Renal Physiol. 2004;286:F881–F892. doi: 10.1152/ajprenal.00301.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aimond F, Rauzier JM, Bony C, Vassort G. Simultaneous activation of P38 MAPK and p42/44 MAPK by ATP stimulates the K current ITREK in cardiomyocytes. J Biol Chem. 2000;275:39110–39116. doi: 10.1074/jbc.M008192200. [DOI] [PubMed] [Google Scholar]
- 23.Chattopadhyay N. Regulation of a Ca2+ activated K channel by calcium-sensing receptor involves P38 MAP kinase. J Neurosci Res. 2004;75:491–498. doi: 10.1002/jnr.10875. [DOI] [PubMed] [Google Scholar]
- 24.Wei Y, Wang WH. The role of cytoskeleton in mediating the effect of vasopressin and herbimycin A on the secretory K channels in the CCD. Am J Physiol Renal Physiol. 2001;282:F680–F686. doi: 10.1152/ajprenal.00229.2001. [DOI] [PubMed] [Google Scholar]
- 25.Sterling H, Lin DH, Gu RM, Dong K, Hebert SC, Wang WH. Inhibition of protein-tyrosine phosphatase stimulates the dynamin-dependent endocytosis of ROMK1. J Biol Chem. 2002;277:4317–4323. doi: 10.1074/jbc.M109739200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sterling H, Lin DH, Wei Y, Wang WH. Tetanus toxin abolishes exocytosis of ROMK1 induced by inhibition of protein tyrosine kinase. Am J Physiol Renal Physiol. 2003;284:F510–F517. doi: 10.1152/ajprenal.00309.2002. [DOI] [PubMed] [Google Scholar]
- 27.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 28.Lo YYC, Wong JMS, Cruz TF. Reactive oxygen species mediate cytokine activation of c-jun NH2-terminal kinase. J Biol Chem. 1996;271:15703–15707. doi: 10.1074/jbc.271.26.15703. [DOI] [PubMed] [Google Scholar]
- 29.Baas AS, Berk BC. Differential activation of mitogen-activated protein kinase by H2O2 and O2− in vascular smooth muscle cells. Circ Res. 1995;77:29–36. doi: 10.1161/01.res.77.1.29. [DOI] [PubMed] [Google Scholar]
- 30.Mahadev K, Zilbering A, Zhu L, Goldstein BJ. Insulin-stimulated hydrogen peroxide reversibly inhibits protein tyrosine phosphatase 1B in vivo and enhances the early insulin action cascade. J Biol Chem. 2001;276:21938–21942. doi: 10.1074/jbc.C100109200. [DOI] [PubMed] [Google Scholar]
- 31.Brumell JH, Burkhardt AL, Bolen JB, Grinstein S. Endogenous reactive oxygen intermediates activate tyrosine kinase in human neutrophils. J Biol Chem. 1996;271:1455–1461. doi: 10.1074/jbc.271.3.1455. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura H, Hori T, Sato N, Sugie K, Kawakami T, Yodoi J. Redox regulation of a Src family protein tyrosine kinase p56Lck in T cells. Oncogene. 1993;8:3133–3139. [PubMed] [Google Scholar]
- 33.Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: Evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- 34.Suzukawa K, Miura K, Mitsushita J, Resau J, Hirose K, Crystal R, Kamata T. Nerve growth factor-induced neuronal differentiation requires generation of Rac1-regulated reactive oxygen species. J Biol Chem. 2000;275:13175–13178. doi: 10.1074/jbc.275.18.13175. [DOI] [PubMed] [Google Scholar]
- 35.Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. J Biol Chem. 1997;272:217–221. [PubMed] [Google Scholar]
- 36.Mahadev K, Wu X, Zilbering A, Zhu L, Lawrence JTR, Goldstein BJ. Hydrogen peroxide generated during cellular insulin stimulation is integral to activation of the distal insulin signaling cascade in 3T3-L1 adipocytes. J Biol Chem. 2001;276:48662–48669. doi: 10.1074/jbc.M105061200. [DOI] [PubMed] [Google Scholar]
- 37.Schmid E, Hotz-Wagenblatt A, Hack V, Droege W. Phosphorylation of the insulin receptor kinase by phosphocreatine in combination with hydrogen peroxide. The structure basis of redox priming. FASEB J. 1999;13:1491–1500. doi: 10.1096/fasebj.13.12.1491. [DOI] [PubMed] [Google Scholar]
- 38.Hayes GR, Lockwood DH. Role of insulin receptor phosphorylation in the insulinomimetic effects of hydrogen peroxide. Proc Natl Acad Sci U S A. 1987;84:8115–8119. doi: 10.1073/pnas.84.22.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai H, Li Z, Dikalov S, Holland SM, Hwang J, Jo H, Dudley SC, Jr, Harrison DG. NAD(P)H oxidase-derived hydrogen peroxide mediates endothelial nitric oxide production in response to angiotensin II. J Biol Chem. 2002;277:48311–48317. doi: 10.1074/jbc.M208884200. [DOI] [PubMed] [Google Scholar]
- 40.Mohazzab-H KM, Kaminski PM, Fayngersh RP, Wolin MS. Oxygen-elicited responses in calf coronary arteries: Role of H2O2 production via NADH-derived superoxide. Am J Physiol. 1996;270:H1044–H1053. doi: 10.1152/ajpheart.1996.270.3.H1044. [DOI] [PubMed] [Google Scholar]
- 41.Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: A protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]