Preface
G protein-coupled receptors (GPCRs) mediate physiological responses to a large variety of different hormones, neurotransmitters, sensory stimuli and other ligands. The signaling and trafficking properties of GPCRs are often highly malleable depending on cellular context. Such fine-tuning of GPCR function can be attributed in many cases to receptor-interacting proteins that are differentially expressed in distinct cell types. In some cases, these GPCR-interacting partners directly mediate receptor signaling, whereas in other instances the interacting partners serve mainly as scaffolds to modulate G protein-mediated signaling. Furthermore, GPCR-interacting proteins can also exert profound regulation over receptor trafficking, localization and/or pharmacological properties.
Introduction
G protein-coupled receptors (GPCRs) comprise the largest family of transmembrane proteins in vertebrates and are the molecular targets for nearly half of the therapeutic drugs that are prescribed worldwide1. The approximately 1000 members of this family can be divided into three main subfamilies (termed A, B, and C) based on sequence similarity, with all members exhibiting a conserved seven-transmembrane domain topology. The canonical view of how GPCRs regulate cellular physiology is that the binding of ligands (such as hormones, neurotransmitters or sensory stimuli) induces conformational changes in the receptors’ transmembrane and intracellular domains, thereby allowing receptor interactions with heterotrimeric G proteins. Activated receptors act as guanine nucleotide exchange factors (GEFs) for the Gα subunits, catalyzing the release of GDP and the binding of GTP to activate the G proteins. The activated G protein subunits (Gα and Gβγ) can then associate with downstream effectors to modulate various aspects of cellular physiology.
In addition to interacting with G proteins, agonist-bound GPCRs also associate with G protein-coupled receptor kinases (GRKs), leading to receptor phosphorylation. GRKs are a seven-member family of related kinases (GRK1-7) that have differential patterns of distribution across bodily tissues and distinct preferences for certain receptors2-4. However, a common property of GPCR phosphorylation by GRKs is decreased receptor interactions with G proteins and enhanced receptor interactions with arrestins, which are members of a family of four closely-related scaffold proteins. GPCR interactions with arrestins serve to further turn off receptor signaling through G proteins while simultaneously turning on certain other signaling pathways that are initiated via arrestin-mediated recruitment of signaling proteins to activated receptors2. Furthermore, arrestins can directly link active receptors to clathrin-coated pits to facilitate receptor endocytosis, which is an important process controlling the desensitization and resensitization of GPCR activity5.
GPCR interactions with G proteins, GRKs and arrestins have been intensively studied for a large number of receptors and exhaustively reviewed elsewhere2-5. For this reason, these broadly-important interactions, which now represent a canonical model of GPCR regulation (Box 1), will not be further reviewed here. Similarly, the importance of homomeric and heteromeric interactions between GPCRs has also been thoroughly reviewed elsewhere6-8 and will not be addressed here. The focus of this review will be on recent advances in the characterization of receptor-selective GPCR associations with various proteins outside of the four previously-mentioned families of general GPCR-interacting proteins (G proteins, GRKs, arrestins and other receptors). These receptor-selective partners can mediate GPCR signaling, organize receptor signaling through G proteins, direct GPCR trafficking, anchor GPCRs in particular subcellular areas and/or influence GPCR pharmacology. Since many of these receptor-selective partners exhibit limited patterns of tissue expression, these interactions can help to explain many examples of cell-specific fine-tuning of GPCR functional activity.
Main Text
Mediation of GPCR Signaling
For a GPCR-interacting protein to be considered as a mediator of GPCR signaling, it would seem a requirement that the protein’s interaction with the receptor should be regulated by agonist stimulation, since agonist-induced changes are the essence of receptor-initiated signaling. For example, GPCR interactions with G proteins, GRKs and arrestins are all strongly enhanced by agonist stimulation1-5. Beyond G proteins, GRKs and arrestins, certain other GPCR-interacting proteins have been shown to interact with specific receptors in an agonist-promoted fashion to mediate particular aspects of receptor signaling, and these examples will be considered here in this section. In contrast, a number of other GPCR-interacting proteins associate with receptors in an agonist-independent manner. Such proteins can potentially modulate G protein-mediated signaling, as described in the next section, but they should not themselves be considered as mediators of receptor signaling if their interactions with receptors are not influenced by agonist stimulation. If a GPCR can be considered as analogous in some ways to a gun, ligand-dependent interactors that mediate signaling are analogous to bullets, whereas ligand-independent interactors that modulate signaling are analogous to silencers and scopes, which influence gun function but do not directly mediate the effects of guns on targets.
Agonist-promoted interactors that mediate signaling
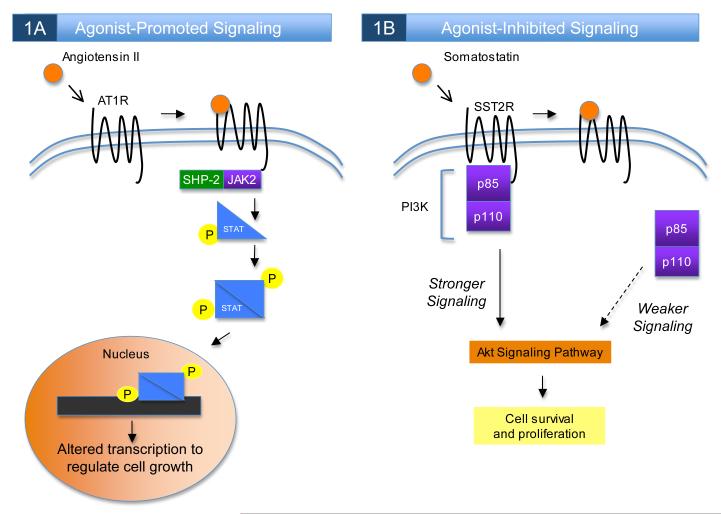
Several different GPCRs have been shown to initiate cellular signaling through agonist-promoted interactions with members of the Janus (Jak) family of non-receptor protein tyrosine kinases. For example, the agonist-dependent activation of the AT1 angiotensin receptor can recruit a complex of Jak2 and the tyrosine phosphatase SHP-2 to associate with the receptor’s C-terminus, facilitating Jak2 phosphorylation and activation9, 10. Activated Jak2 can then recruit and phosphorylate members of the STAT family of transcription factors, leading to STAT dissociation from Jak2 and translocation to the nucleus (Figure 1A). The agonist-promoted interaction between Jak2 and AT1 demonstrates an additional signaling avenue for AT1, beyond the receptor’s well-established coupling to Gαq, and can help to explain certain effects of AT1 stimulation on cellular physiology that are not explained by G protein-mediated signaling11. Interestingly, AT1 receptor coupling to Gαq can induce rises in intracellular calcium that further potentiate Jak/STAT signaling by the receptor12, 13, providing an example of how G protein-dependent and G protein-independent signaling mechanisms can in some cases work together synergistically. Another GPCR that can interact with Jak2 is the platelet activating factor (PAF) receptor, which associates with a Tyk2/Jak2 complex in an agonist-regulated manner14, 15. A mutant version of the PAF receptor that does not couple to G proteins but still interacts with the Tyk2/Jak2 complex is fully capable of activating downstream STATs, demonstrating the physiological importance of the receptor’s recruitment of Jak215.
Figure 1.
GPCR signaling can be mediated by receptor-interacting proteins. A) Certain GPCR-interacting proteins can act as mediators of agonist-induced receptor signaling, independent of G protein- or arrestin-mediated signaling pathways. An example of this phenomenon is the interaction of the non-receptor tyrosine kinase Jak2 with the angiotensin AT1 receptor. Association of Jak2 with the phosphatase SHP-2 and stimulation of AT1 with angiotensin II together promote Jak2 association with the AT1 receptor and initiation of Jak2-dependent signaling. Activated Jak2 can phosphorylate members of the STAT family of transcription factors, leading to STAT dimerization, translocation into the nucleus, and regulation of genes controlling cell growth. B) In other cases, the agonist-dependent dissociation of an interacting protein from a GPCR can alter the activity of an intracellular signaling pathway. For example, the interaction between the p85 regulatory subunit of phosphatidylinositol 3-kinase (PI3K) and the somatostatin type II receptor (SSTR2) is disrupted by agonist stimulation, leading to reduced PI3K-mediated Akt signaling and suppression of cell survival pathways.
GPCR interactions with proteins that possess PDZ domains can also in some cases mediate agonist-promoted GPCR signaling. PDZ domains, named for the first three proteins in which they were discovered (PSD-95, Discs-large and ZO-1), can mediate high-affinity interactions with specific motifs at the distal C-termini of target proteins16. For example, the PDZ protein Na+/H+ exchange regulatory factor 1 (NHERF-1; also known as EBP50) has been shown to associate in an agonist-promoted fashion with the C-terminus of the β2-adrenergic receptor (β2-AR)17, 18. The recruitment of NHERF-1 to the β2-AR disrupts the ability of NHERF-1 to inhibit the Na+/H+ exchanger type 3 (NHE3), providing a G protein-independent mechanism by which the β2-AR can activate Na+/H+ exchange in kidney cells17. The κ opioid receptor (κOPR) is another GPCR that can regulate Na+/H+ exchange via agonist-induced interactions with NHERF-119, 20. Interestingly, the studies on κOPR provide an example of how interaction with a protein such as NHERF-1 can confer cell-specific signaling to a given GPCR, as κOPR stimulation robustly activates NHE3 activity in cell lines expressing high levels of NHERF-1 but not in other cell lines that lack significant NHERF-1 expression20.
Agonist-disrupted interactors that mediate signaling
In addition to the aforementioned examples of agonist-promoted associations with receptor-interacting proteins mediating aspects of GPCR signaling, agonist-disrupted interactions between a GPCR and a cytoplasmic binding partner can also initiate cellular signaling. For example, it has been shown that agonist stimulation of the somatostatin receptor type 2 (SSTR2) disrupts its constitutive association with the phosphatidylinositol 3-kinase (PI3K) subunit p85 to negatively regulate PI3K signaling21 (Figure 1B). In the absence of agonist, association of p85 with the first intracellular loop of SSTR2 constitutively enhances PI3K activity to promote cell survival through the Akt pathway. However, following agonist stimulation of SSTR2, association of the receptor with p85 is disrupted, leading to decreased PI3K activity and sensitization of cells to stimuli that induce apoptosis21. Thus, even though the classical examples of proteins that mediate GPCR signaling (G proteins and arrestins) exhibit enhanced associations with receptors following agonist stimulation, it is evident that as long as a GPCR-interacting partner exhibits some type of change in its location and/or activity in response to agonist stimulation, this can be sufficient to lead to the initiation of cellular signaling.
Modulation of GPCR Signaling
Some GPCR-interacting partners increase the speed and efficiency of GPCR signaling by acting as scaffolds to tether downstream effectors in close proximity to the receptor. On the other hand, certain GPCR-interacting partners can decrease the intensity and/or time course of GPCR signaling by disrupting association with G proteins or, in some cases, recruiting negative regulators of GPCR signaling. By finely tuning the spatial and temporal resolution of signaling, certain GPCR interactors can dramatically affect the ability of GPCRs to transduce extracellular stimuli into changes in cellular physiology.
Interactors that enhance G protein-mediated signaling
The visual system of the fruit fly Drosophila melanogaster has long served as an important model system for studying fast and efficient G protein-mediated signaling. Visual signaling in Drosophila is mediated by light stimulation of rhodopsin, which couples to Gαq to activate phospholipase Cβ (PLCβ), leading to the generation of the second messengers inositol triphosphate (IP3) and diacylglycerol and the opening of transient receptor potential (TRP) calcium channels. The influx of calcium and production of diacylglycerol lead to activation of protein kinase C (PKC), which plays a key role in the termination of visual signaling. Interestingly, almost all of the components of the Drosophila visual signaling pathway are known to be tethered together by a large PDZ-domain containing scaffold protein known as InaD, which associates with rhodopsin, TRP channels, PLCβ and PKC22-28. By tethering these downstream effectors in close proximity to rhodopsin, InaD creates an efficient signaling complex that dramatically increases the speed and amplitude of physiological responses to light stimulation29.
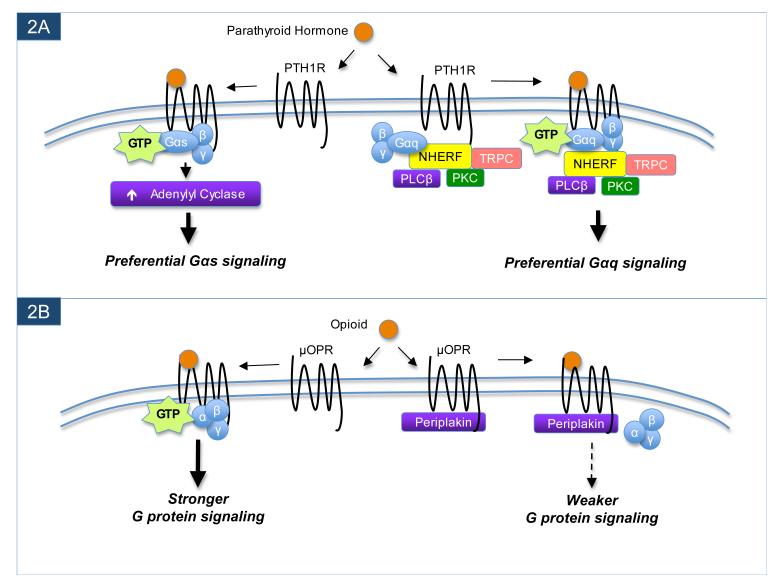
Analogous to Drosophila InaD, some mammalian PDZ scaffolds have been found to interact with GPCRs to enhance the efficiency of receptor-stimulated G protein signaling. For example, associations of NHERF-1 and/or the closely-related protein NHERF-2 with several different GPCRs, including the PTH1 parathyroid hormone receptor30-34, LPA2 lysophosphatidic acid receptor35, P2Y1 purinergic receptor36 and mGluR5 metabotropic glutamate receptor37 can enhance PLC-mediated signaling by these receptors. Unlike the aforementioned interactions of NHERF-1 with β2AR and κOPR, which are regulated by agonist, the associations of the NHERF proteins with PTH1-R, LPA2-R, P2Y1-R and mGluR5 are not altered by agonist stimulation and mainly seem to facilitate enhanced G protein-mediated signaling by the receptors. Interestingly, many known NHERF-binding partners (beyond GPCRs and NHE3) are downstream components of Gαq/PLC signaling pathways, including Gαq38, several TRP channels39,40, various isoforms of PLCβ41,36, PKC42 and protein kinase D (PKD)43. The interaction of NHERF-2 with PTH1-R provides a particularly compelling example as to how receptor-interacting scaffolds can help to explain cell-specific fine-tuning of receptor signaling. PTH1-R signals mainly through adenylyl cyclase (AC) regulation in ROS 17/2.8 cells, which do not express detectable levels of NHERF proteins, but mainly through PLC regulation in ECV304 cells, which contain high levels of both NHERF-1 and NHERF-230. PTH1-R has long been enigmatic for seeming to signal primarily through AC regulation in some cell types but PLC regulation in other cells, and it now seems that such cell-specific differences in the receptor’s signaling activity can be accounted for in many cases by differential cellular expression of the NHERF proteins44 (Fig. 2A).
Figure 2.
GPCR-interacting proteins can modulate G protein-mediated signaling. In the example shown in panel A, interaction between the NHERF scaffold proteins and the parathyroid hormone receptor (PTH1R) leads to a preferential enhancement of the downstream Gαq-mediated signaling by PTH1R. Through its various protein-protein interaction domains, NHERF not only binds to PTH1R, but also tethers multiple downstream signaling effectors in close proximity to the PTH1R, thereby creating an efficient complex for Gαq-mediated signaling. However, when PTH1R is in a different cell type or separate cellular compartment in which NHERF proteins are absent, PTH1R instead preferentially signals through Gαs to activate adenylyl cyclase. A contrasting example is shown in panel B. Periplakin can associate with the μ opioid receptor (μOPR) to attenuate G protein-mediated signaling. However, in cell types or cellular compartments where periplakin is not found, μOPR ligands can more robustly activate the receptor to stimulate signaling by G proteins.
In addition to the examples of the NHERF proteins and InaD, a variety of other PDZ scaffolds have been shown to associate with specific GPCRs to enhance certain signaling pathways. For example, associations of the multi-PDZ protein Mupp1 with GABAB receptors45 and MT1 melatonin receptors46 result in markedly enhanced Gαi-mediated signaling following receptor stimulation. Similarly, interactions of the PDZ scaffold MAGI-3 with Frizzled-447 and the LPA2 receptor48 enhance receptor-mediated activation of ERK/mitogen-activated protein (MAP) kinase pathways. Conversely, associations of the LPA2 receptor with two related PDZ scaffolds, PDZ-RhoGEF and LARG, do not result in enhanced downstream MAP kinase activation but rather potentiate LPA2-induced stimulation of Rho signaling to modify cytoskeleton dynamics49. Thus, the LPA2 receptor can preferentially couple to downstream PLC activation35, MAP kinase activation48 or Rho activation49 in a cell-specific manner depending upon which LPA2-interacting PDZ scaffold is expressed in a particular cell type.
Other prominent examples of GPCR-associated proteins that enhance the efficiency of G protein-mediated signaling are the Homer proteins, which associate with mGluR1 and mGluR550-53, and members of the A-kinase anchoring protein (AKAP) family, which interact with β-adrenergic receptors54-58. In addition to associating with mGluRs, the Homer proteins also interact with intracellular IP3 receptors, thereby serving to link the IP3 receptors, mGluRs and other components together to greatly increase the efficiency of mGluR-stimulated calcium signaling52, 59, 60. The AKAPs were originally named because of their associations with protein kinase A (PKA), and indeed the interactions of AKAP7955 and AKAP25054, 56, 57 with β2AR serve to tether PKA in the vicinity of the receptor and increase the efficiency of PKA-mediated phosphorylation of various substrates that are downstream of receptor activation, including the receptor itself. The consequences of the increased functional relationship between PKA and β2AR include enhanced efficiency of receptor resensitization54, 57 and more robust β2AR-mediated ERK signaling55.
Interactors that reduce G protein-mediated signaling
In contrast to the examples described above of GPCR-interacting proteins that increase the efficiency of certain GPCR-stimulated signaling pathways, some proteins that associate with GPCRs can decrease the efficiency of G protein-mediated signaling. The arrestins are perhaps the most general example of this phenomenon. A more receptor-specific example is spinophilin, which has been shown to interact with the third intracellular loops of a handful of different GPCRs, including members of the dopamine, adrenergic and muscarinic acetylcholine receptor families61-65. Spinophilin also binds to several members of the regulators of G-protein signaling (RGS) family of proteins, which tightly regulate the intensity and time course of GPCR signaling by accelerating the inherent GTPase activity of activated Gα subunits. Thus, spinophilin can tether RGS proteins in close proximity to receptors to attenuate receptor-stimulated G protein signaling63, 64. Interestingly, RGS proteins can also sometimes associate directly with the intracellular regions of certain GPCRs to inhibit receptor signaling and exert cell-specific regulation of receptor activity66-71. RGS proteins have been reviewed in detail elsewhere72, 73, and will therefore not be discussed further here.
Calmodulin is another protein that can interact with a variety of different GPCRs to modulate receptor functional properties. Calmodulin is a widely-expressed calcium-binding protein that can associate in a calcium-sensitive manner with metabotropic glutamate74-77, serotonin78-80, dopamine81, 82 and other83-85 receptors. The functional effects of calmodulin interaction vary depending on the receptor, but perhaps the most commonly-reported effect of calmodulin interaction with GPCRs is an attenuation of G protein coupling79, 81, 83, 85. Since stimulation of many GPCRs can result in downstream increases in cellular calcium levels, the calcium-dependent interactions of calmodulin with GPCRs can in some cases represent a form of feedback inhibition that restrains receptor-initiated G protein signaling. However, G protein-independent signaling pathways may actually be potentiated by receptor interactions with calmodulin, as for example calmodulin association with the serotonin 5-HT2C receptor strongly promotes arrestin-mediated signaling (but not G protein-mediated signaling) by the receptor in both transfected cells and cultured neurons80.
Two other proteins that can associate with a select number of GPCRs to tone down G protein-mediated signaling are periplakin and neurochondrin. Periplakin was first reported to associate with the C-terminus of the μ opioid receptor (μOPR)86 and the melanin-concentrating hormone receptor 1 (MCH1R)87, while neurochondrin was found to interact with the same region of the MCH1R C-terminus as periplakin88. Both periplakin and neurochondrin have recently been shown to interact with a handful of other GPCRs in addition to μOPR and MCH1R89. For all of the receptors that associate with periplakin and neurochondrin, the primary functional consequence is an attenuation of G protein-mediated signaling86-89 (Fig. 2B). Since periplakin and neurochondrin exhibit discrete patterns of distribution in the brain and other tissues89, it seems likely that they contribute to the cell-context-dependent sculpting of receptor signaling for the various receptors with which they interact. Considered together with the other GPCR partners discussed in this section, the emerging theme is that receptor-interacting proteins can exert bidirectional effects on the efficiency of G protein-mediated signaling to impart cell-specific fine-tuning of GPCR activity.
Regulation of GPCR Trafficking
GPCRs are typically trafficked to the plasma membrane to achieve functional activity. Following agonist stimulation, most receptors internalize into endosomes and may then either be targeted for lysosomal degradation or recycled back to the plasma membrane. A number of GPCR-interacting proteins have been shown to exert dramatic effects on both the biosynthetic trafficking and post-endocytic sorting of particular receptors.
Interactors that regulate biosynthetic trafficking
GPCRs must be properly folded after translation and in most cases transported to the plasma membrane to achieve functional activity. A number of GPCR-interacting proteins can regulate the folding, biosynthetic trafficking and surface expression of receptors in a cell-specific and receptor-specific manner. A classic example is the Drosophila melanogaster protein Nina A, which associates with rhodopsin to enhance the receptor’s folding and forward trafficking90, 91. Similarly, Ran-binding protein 2 is a vertebrate homolog of Nina A that associates with vertebrate opsins to enhance their biosynthetic trafficking92. Another protein that regulates rhodopsin trafficking is the dynein light chain component Tctex-1, which directly associates with vertebrate rhodopsin to promote the receptor’s surface trafficking93, 94. Other vertebrate proteins that have been found to act as chaperones to enhance GPCR surface expression in a receptor-specific manner include GEC195, 96, RACK197, DRIP7898, 99, ATBP50100, Usp4101, which are listed in Table 1. For each of these GPCR-interacting proteins, their expression level in a given cell type can strongly influence the level of functional receptor expression for the particular GPCRs with which they interact.
| Interactor* | Associated GPCR |
Location | Significance of Interaction | Ref. # |
|---|---|---|---|---|
| AKAP79 | β2-AR | CT | Tethers PKA near receptor | 55, 58 |
| AKAP250 | β2-AR | CT | Tethers PKA near receptor | 54, 56, 57 |
| ATBP50 | AT2R | CT | Enhances receptor surface expression | 100 |
| Calmodulin | 5-HT1A | i3L | Competes with PKC for receptor phosphorylation | 78 |
| Calmodulin | 5-HT2A | i2L, CT | Impairs G protein-coupling | 79 |
| Calmodulin | 5-HT2C | CT | Promotes arrestin-dependent ERK activation | 80 |
| Calmodulin | D2R | i3L | Modulates G protein signaling | 81, 82 |
| Calmodulin | mGluR5 | CT | Promotes receptor surface expression | 74, 75 |
| Calmodulin | mGluR7 | CT | Regulates receptor phosphorylation | 76, 77 |
| Calmodulin | PTH1R | CT | Inhibits receptor activity | 85 |
| Calmodulin | V2R | CT | Enhances receptor-induced calcium signaling | 84 |
| Calmodulin | μOPR | i3L | Inhibits G protein-coupling | 83 |
| DRIP78 | AT1R | CT | Promotes receptor surface expression | 99 |
| DRIP78 | D1R | CT | Promotes receptor surface expression | 98 |
| GASP1 | CB1R | CT | Targets receptor to lysosomes for degradation | 116, 117 |
| GASP1 | D2R | CT | Targets receptor to lysosomes for degradation | 115 |
| GASP1 | δOPR | CT | Targets receptor to lysosomes for degradation | 114 |
| GEC1 | EP3R | CT | Enhances receptor surface expression | 96 |
| GEC1 | κOPR | CT | Enhances receptor surface expression | 95, 96 |
| Homer | mGluR1 | CT | Regulates receptor signaling and localization | 51, 52, 127, 153 |
| Homer | mGluR5 | CT | Regulates receptor signaling and localization |
51, 52, 126, 128, 153, 154 |
| InaD | Rhodopsin | CT | Enhances speed and efficiency of receptor signaling | 22-29 |
| Jak2 | AT1 | CT | Promotes Jak/STAT signaling | 9, 10, 12, 13 |
| Jak2 | PAF | CT | Promotes Jak/STAT signaling | 14, 15 |
| LARG | LPA2 | CT | Facilitates receptor-mediated activation of Rho | 49 |
| M10 MHC | V2R | Enhances receptor surface expression | 108 | |
| MAGI-3 | Frizzled-4 | CT | Potentiates receptor-mediated ERK/MAPK activation | 47 |
| MAGI-3 | LPA2 | CT | Potentiates receptor-mediated ERK/MAPK activation | 48 |
| MPP3 | 5-HT2C | CT | Inhibits agonist-induced 5-HT2C internalization | 139 |
| MRAP | MC2R | NT, TM | Promotes forward trafficking of receptor | 109-113 |
| MRAPII | MC2R | NT, TM | Promotes forward trafficking of receptor | 110, 113 |
| Mupp1 | 5-HT2C | CT | Induces receptor clustering | 155 |
| Mupp1 | GABAB | CT | Increases receptor stability | 45 |
| Mupp1 | hSSTR3 | CT | Targets receptor to epithelial tight junctions | 144 |
| Mupp1 | MT1 | CT | Enhances receptor-mediated Gαi signaling | 46 |
| Neurochondrin | MCH1R | CT | Disrupts G protein-mediated signaling | 88 |
| NHERF-1 | PTH1R | CT | Enhances Gαq-mediated receptor signaling | 31-34 |
| NHERF-1 | β2-AR | CT | Mediates activation of Na+/H+ exchange, promotes receptor recycling |
17, 18, 122 |
| NHERF-1 | κOPR | CT | Mediates activation of Na+/H+ exchange, promotes receptor recycling |
19, 20 |
| NHERF-2 | LPA2 | CT | Enhances Gαq-mediated receptor signaling | 35 |
| NHERF-2 | mGluR5 | CT | Prolongs receptor-mediated calcium signaling | 37 |
| NHERF-2 | P2Y1 | CT | Prolongs receptor-mediated calcium signaling | 36 |
| NHERF-2 | PTH1R | CT | Enhances Gαq-mediated receptor signaling | 30 |
| Nina A | Rhodopsin | Promotes receptor biogenesis and trafficking | 90, 91 | |
| ODR-4 | ODR-10 | Facilitates receptor folding and surface expression | 103 | |
| p85 | SSTR2 | i1L, CT | Mediates survival signaling by receptor | 21 |
| PDZ-RhoGEF | LPA2 | CT | Facilitates receptor-mediated activation of Rho | 49 |
| Periplakin | MCH1R | CT | Impairs G protein-mediated signaling | 87 |
| Periplakin | μOPR | CT | Impairs G protein-mediated signaling | 86 |
| PICK1 | mGluR7a | CT | Facilitates receptor clustering in presynaptic active zones | 140, 141, 156 |
| PSD-95 | 5HT2A | CT | Impairs receptor internalization and directs localization | 134, 135 |
| PSD-95 | β1-AR | CT | Attenuates agonist-promoted receptor internalization | 136, 138 |
| RACK1 | TXA2R | i1L, CT | Promotes forward trafficking of receptor | 97 |
| RAMP1 | CRLR | NT, TM | Forms functional CGRP receptors | 146 |
| RAMP1 | CT | NT, TM | Forms functional amylin receptors | 149, 150 |
| RAMP2 | CRLR | NT, TM | Forms functional adrenomedullin receptors | 146 |
| RAMP3 | CRLR | NT, TM | Forms functional adrenomedullin receptors | 147, 148 |
| RAMP3 | CT | NT, TM | Forms functional amylin receptors | 149, 150 |
| RnBP2 | Opsin | Promotes forward trafficking of receptor | 92 | |
| REEP | OR | Promotes forward trafficking of receptors | 104 | |
| RTP | OR | Promotes forward trafficking of receptors | 104, 105 | |
| RTP/REEP | T2R | Promotes surface expression of receptors | 106 | |
| RTP4 | μ-δ OPR | CT | Promotes heterodimer surface expression | 107 |
| Shank | CL1 | CT | Promotes receptor clustering | 132 |
| Shank | mGluR1 | CT | Anchors receptor in mature dendritic spines | 129 |
| Shank | mGluR5 | CT | Anchors receptor in mature dendritic spines | 129 |
| SNX1 | PAR1 | CT | Facilitates agonist-promoted receptor degradation | 119, 120 |
| Spinophilin | D2R | i3L | Reduces G protein and arrestin-mediated signaling | 61 |
| Spinophilin | mAChR | i3L | Reduces receptor-mediated calcium signaling | 64 |
| Spinophilin | α2-AR | i3L | Reduces receptor-mediated calcium signaling | 62-65 |
| Syntrophin | α1D-AR | CT | Enhances receptor stability | 142, 143 |
| Tctex-1 | Rhodopsin | CT | Promotes apical delivery of receptor in polarized cells | 93, 94 |
| Usp4 | A2AR | CT | Enhances receptor surface expression | 101 |
A comprehensive list of interactors and their associated GPCRs. Interactors are organized alphabetically, followed by associated GPCR, location of interactor association on the receptor, functional significance of interaction, and references. Blanks in the table are because location of interaction is unknown. Not included on this list are GPCR interactions with G proteins, GRKs, arrestins, RGS proteins, or other receptors. Abbreviations used in the location column: CT, carboxyl-terminus; i3L, third intracellular loop; i2L, second intracellular loop; NT, amino-terminus; TM, transmembrane; i1L, first intracellular loop.
Olfactory receptors (ORs), the largest sub-family of GPCRs, have proven notoriously difficult to study in heterologous cells (e.g., cells other than olfactory sensory neurons) due to poor trafficking to the plasma membrane102. The severe trafficking deficits of ORs in heterologous cells suggest a key role for chaperone proteins in the cell-specific control of anterograde OR trafficking in olfactory sensory neurons. Indeed, the Caenorhabditis elegans protein ODR-4 is expressed exclusively in chemosensory neurons, where it regulates the forward trafficking of chemosensory receptors such as ODR-10103. In vertebrates, two unrelated families of transmembrane proteins have been shown to associate with vertebrate ORs to enhance receptor trafficking to the plasma membrane: the receptor transporting protein (RTPs) and receptor expression enhancing protein (REEPs)104, 105. Certain RTP and REEP isoforms are expressed exclusively in the olfactory epithelium, where they exert cell-specific enhancement of OR trafficking104. However, other RTP and REEP isoforms are more widely expressed and can interact with other GPCRs, including T2R bitter taste receptors106 and opioid receptors107, to enhance receptor trafficking to the plasma membrane.
Analogous to the RTPs and REEPs, two other types of single-transmembrane proteins have been shown to control the biosynthetic trafficking of particular GPCRs. V2R vomeronasal receptors had proven very difficult to study in heterologous cells until the observation that these receptors interact with M10 MHC molecules, which associate with β2-microglobulins to strongly promote the receptors’ surface expression in heterologous cells108. In a similar vein, the melanocortin 2 receptor (MC2R)-associated proteins, MRAP and MRAP2, can directly interact with MC2R and dramatically enhance the receptor’s surface expression109-113. The physiological importance of MC2R-MRAP interactions in vivo has been well-established by studies revealing that naturally-occurring MRAP mutations cause defects in MC2R trafficking and functionality, resulting in an inherited disease known as familial glucocorticoid deficiency type 2 109.
Interactors that influence post-endocytic trafficking
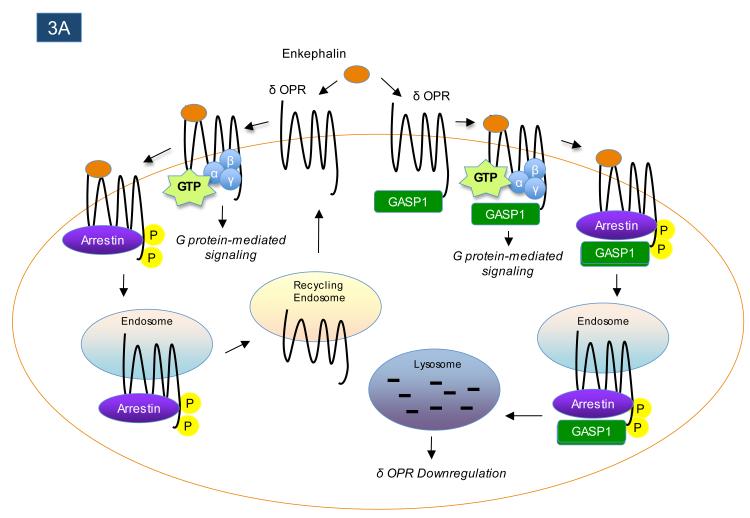
Most GPCRs undergo significant endocytosis from the plasma membrane in response to agonist stimulation. In some cases, the receptors are recycled back to the plasma membrane, but in other cases the receptors are targeted to lysosomes for degradation5. GPCR internalization is heavily influenced by two of the canonical families of GPCR-interacting proteins, the GRKs and arrestins2. However, certain other GPCR-interacting proteins can also regulate endocytic trafficking of GPCRs in a more receptor-selective fashion. For example, the GPCR-associated sorting proteins (GASPs) comprise a family of 10 proteins, with the founding member GASP1 originally identified in a yeast two-hybrid screen for proteins interacting with the C-terminus of the δ opioid receptor (δOPR)114. Association of δOPR with GASP1 strongly promotes receptor trafficking to lysosomes following agonist-stimulated endocytosis114 (Figure 3A), and has similar effects on a handful of other GPCRs, including D2 dopamine115 and CB1 cannabinoid receptors116, 117. Most of the GASP family members are preferentially expressed in the central nervous system (CNS) 118, suggesting that they may act in a cell-specific manner to control the post-endocytic fate (recycling versus degradation) of certain CNS-enriched GPCRs.
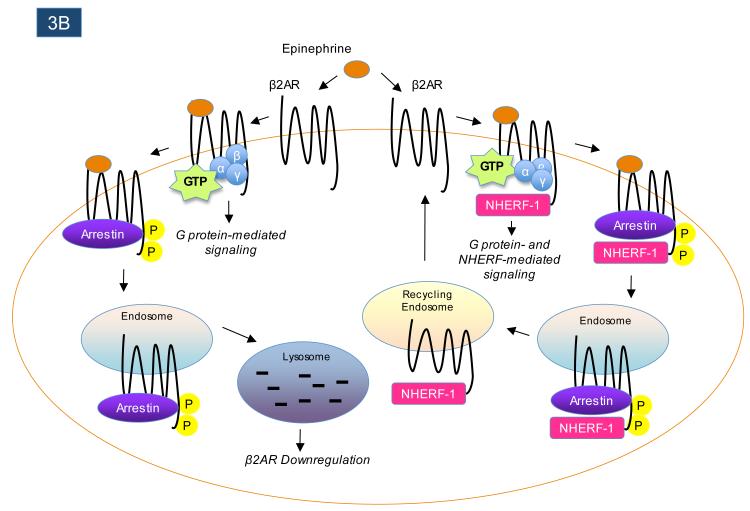
Figure 3.
GPCR-interacting proteins can regulate the post-endocytic trafficking of GPCRs. Following agonist-induced receptor endocytosis, some GPCRs are targeted for proteolytic and/or lysosomal degradation, while other GPCRs rapidly recycle back to the plasma membrane. As shown in panel A, the interaction between the GPCR-interacting protein GASP1 and the δ-opioid receptor (δOPR) promotes the endocytic targeting of agonist-internalized δ-opioid receptors to lysosomes, where the receptors are degraded. In contrast, as shown in panel B, the interaction between the GPCR-interacting protein NHERF-1 and the β2-adrenergic receptor (β2-AR) promotes the rapid recycling of receptors following agonist-promoted internalization.
Beyond the members of the GASP family, a handful of other GPCR-interacting proteins have also been shown to influence the post-endocytic sorting of particular receptors. For example, sorting nexin 1 (SNX1) directly associates with the protease-activated receptor 1 (PAR1) to promote PAR1 post-endocytic trafficking to lysosomes119, 120. SNX1 can also bind to the C-termini of several other GPCRs121, but how this influences the trafficking of those receptors is still unknown. In contrast to the effects of GASPs and SNX1, which associate with GPCRs to decrease receptor recycling to the plasma membrane, GPCR interactions with NHERF-1 have been found to promote receptor recycling following endocytosis19, 122-124. As mentioned earlier, β2AR and κOPR interactions with NHERF-1 mediate certain aspects of receptor signaling, while NHERF interactions with other GPCRs can modulate signaling by G proteins. Interestingly, mutant versions of β2AR that cannot associate with NHERF-1 are targeted much more robustly than wild-type receptors to lysosomes after agonist stimulation122, revealing that their interaction with NHERF-1 promotes receptor recycling (Figure 3B). Association of κOPR with NHERF-1 also promotes receptor recycling over targeting to lysosomes19, and interaction of PTH1-R with NHERF-1 increases the plasma membrane retention of the receptor125. Moreover, transplantation of the NHERF-1 binding motif onto the C-termini of receptors that do not normally interact with NHERF-1, such as δOPR, dramatically enhances the efficiency of receptor recycling back to the plasma membrane following endocytosis123, 124. These findings provide an example of how certain GPCR-interacting proteins, such as NHERF-1, can act as signaling intermediates, regulators of G protein signaling, and regulators of receptor trafficking, analogous to the multiple roles played by arrestins in the signaling and trafficking of many GPCRs2.
Control of GPCR Targeting
In addition to the effects of many of the aforementioned GPCR-interacting proteins on receptor signaling and trafficking, some of these interactions also control receptor anchoring to discrete regions of the plasma membrane. For example, Homer proteins not only enhance the efficiency of mGluR1/5-mediated calcium signaling (as described above), they also facilitate the clustering and anchoring of mGluR1/5 in post-synaptic dendritic spines126-128. Consequently, mGluR1/5 receptors can be selectively localized near the post-synaptic density, positioning them to respond to glutamate that is released into the synaptic cleft. Members of the Shank family of PDZ scaffolds interact with both Homer proteins and mGluRs to further strengthen the anchoring of mGluRs to post-synaptic regions129-131. The Shank family of scaffolding proteins also induce clustering of the latrotoxin-binding GPCR latrophilin-1 (also known as CL1 and CIRL-1) in heterologous cells and co-localize with latrophilin-1 at synapses in native brain tissue132, 133.
A variety of PDZ scaffolds beyond the Shank proteins have also been shown to regulate GPCR clustering and anchoring in receptor-specific ways. For example, interactions of the post-synaptic density protein PSD-95 with the serotonin 5-HT2A receptor induce clustering of 5-HT2A in heterologous cells134 and facilitate targeting of 5-HT2A to post-synaptic dendritic compartments in cultured cortical pyramidal neurons135. PSD-95 also associates with the C-terminus of the β1-AR to facilitate receptor clustering with other components of the post-synaptic density such as NMDA receptors136. For both 5-HT2A and β1AR, the anchoring to the plasma membrane induced by association with PSD-95, which is known to be palmitoylated and therefore tightly membrane-associated137, greatly reduces agonist-promoted internalization of the receptors134, 136, 138. In contrast, PSD-95 association with the serotonin 5-HT2C receptor actually facilitates agonist-dependent internalization, while another PDZ scaffold protein, MPP3, associates with 5-HT2C to prevent agonist-dependent internalization and stabilize the receptor at the cell surface in primary cortical neurons139. As for other examples, interaction of the mGluR7a C-terminus with the PDZ scaffold PICK1 results in specific clustering of the receptor at pre-synaptic sites140, 141, the α1D-adrenergic receptor interacts with syntrophins to achieve linkage to the dystrophin-utrophin complex in smooth muscle cells142, 143, and the SSTR3 somatostatin receptor interacts with the PDZ protein Mupp1 to be targeted to tight junctions in epithelial cells144. These examples illustrate how certain GPCR-interacting partners can selectively target GPCRs to specialized cellular compartments to promote the receptors’ physiological activity.
Regulation of Ligand Binding
The vast majority of GPCR-interacting proteins described above associate with intracellular regions of the receptors and therefore have no direct effects on the pharmacological properties of the receptors, which are typically determined by the receptors’ extracellular and/or transmembrane domains. However, a handful of GPCR-interacting proteins have been shown to exert striking effects on the agonist selectivity of the receptors with which they interact. The most intensively-studied examples of GPCR-interacting partners that regulate receptor pharmacology are the receptor activity modifying proteins, RAMP1, −2 and −3 145. The RAMP proteins were initially identified in experiments designed to search for the receptor activated by calcitonin gene-related peptide (CGRP). Surprisingly, it was found that expression of a functional CGRP receptor required co-expression of an orphan GPCR, known as the calcitonin-receptor-like receptor (CRLR, also known as CL), with a receptor-interacting partner, the single-transmembrane protein RAMP1146. When CRLR was co-expressed with RAMP2, this was shown to result in the formation of receptors activated not by CGRP but rather by a related peptide known as adrenomedullin146. Subsequent work has revealed that CRLR can also interact with RAMP3 to form a distinct subtype of adrenomedullin receptor147, 148. Moreover, expression of the calcitonin (CT) receptor with any of the RAMPs results in the formation of receptors with unique pharmacological properties, including preferential activation of some RAMP/CT receptor combinations by a distinct peptide known as amylin149, 150. It is clear from work in this area that the pharmacological properties of the CT receptor and CRLR are heavily regulated in a cell-specific fashion depending on which of the RAMP proteins is expressed in a given cell type, and furthermore the RAMP proteins can also dramatically affect the surface expression levels of the CT receptor and CRLR145. Thus, a comprehensive understanding of RAMP/GPCR interactions is essential for developing therapeutics that might target the various RAMP-interacting receptors145.
Conclusions and Perspectives
The signaling and trafficking of most GPCRs involves receptor interactions with G proteins, GRKs, arrestins and other receptors. In addition to these widespread canonical GPCR associations, there are also many other types of GPCR-interacting proteins that can interact with particular receptors to fine-tune receptor activity. GPCRs are important drug targets, and since it is often desirable to achieve cell-specificity of drug action in order to minimize side effects, it can be clinically useful to consider the ways in which GPCRs can be differentially modulated by therapeutics depending on cellular context. Along these lines, there has been increasing interest over the past few years in studies on “biased agonists” that can preferentially activate G protein-mediated versus arrestin-mediated GPCR signaling pathways151, 152. It is worth keeping in mind that agonists are likely to be differentially-biased depending on the cellular context, which is determined largely by the set of GPCR-interacting proteins that regulate receptor signaling, trafficking and/or localization in a particular cell type. Thus, uncovering the full set of GPCR-interacting proteins for receptors of clinical interest can provide novel therapeutic insights by shedding light on the fundamental mechanisms controlling the fine-tuning of GPCR activity.
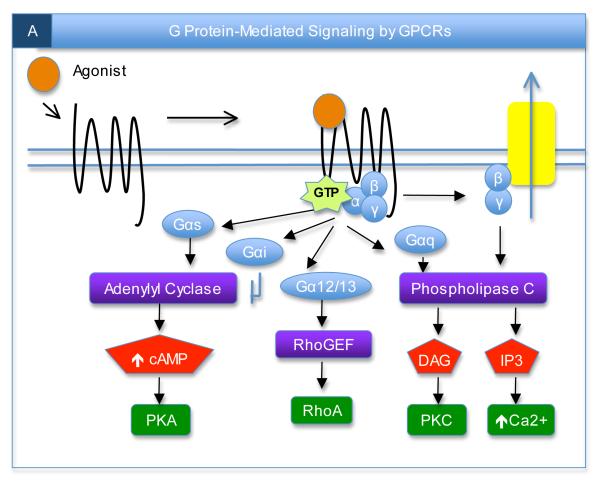
Box 1. Canonical mechanisms of GPCR signaling.
A) In the classical view of GPCR signaling, agonist binds to extracellular and/or transmembrane regions of the receptor, leading to interactions with heterotrimeric G proteins. The receptor acts as a guanine nucleotide exchange factor, catalyzing the exchange of GDP for GTP on the Gα subunit and inducing dissociation of the Gα and Gβγ subunits. Activated GTP-Gα subunits, of which there are multiple sub-types including Gαs, Gαi, Gα12/13, and Gαq, subsequently bind to and regulate the activity of effectors such as adenylyl cyclase, RhoGEF, or phospholipase C, thereby generating second messengers that modulate further downstream effectors. The Gβγ subunits can also bind to and regulate certain downstream effectors, such as ion channels and phospholipase C, following their liberation from the heterotrimeric complex.
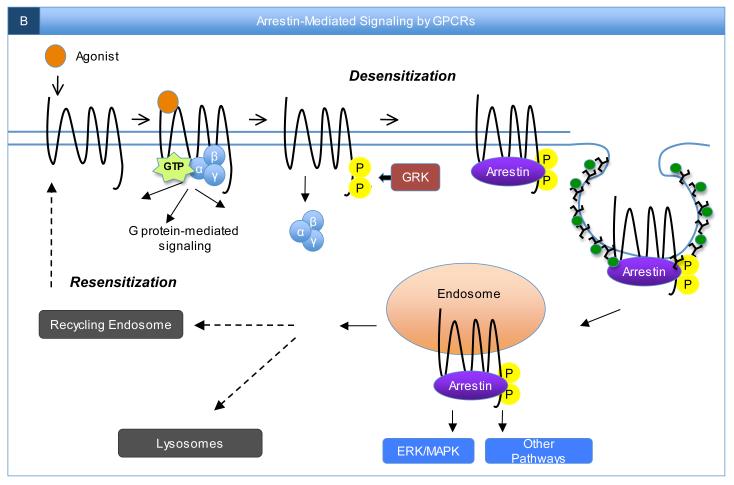
B) G protein-mediated signaling by agonist-activated receptors can be terminated via receptor phosphorylation by G protein-coupled receptor kinases (GRKs) and concomitant receptor association with arrestins, which interact with clathrin (black shapes) and the clathrin adaptor AP2 (green circles) to drive receptor internalization into endosomes2. This physiological process of receptor internalization regulates the functional process of receptor desensitization. However, in addition to attenuating G protein-mediated signaling and facilitating receptor internalization, recruitment of arrestins to activated GPCRs can also lead in many cases to the initiation of distinct arrestin-mediated signaling pathways. Following association with arrestins, GPCRs may then be trafficked to lysosomes, where they are ultimately degraded, or to recycling endosomes for recycling back to the cell surface in the functional process of resensitization. Interestingly, “biased” agonists have been recently characterized that specifically activate G protein-mediated signaling pathways over arrestin-mediated receptor signaling pathways or vice versa151, 152. This new concept illustrates the importance of characterizing all GPCR downstream signaling pathways in order to more fully exploit the therapeutic potential of clinically-important receptors.
References
- 1.Bridges TM, Lindsley CW. G-protein-coupled receptors: from classical modes of modulation to allosteric mechanisms. ACS Chem Biol. 2008;3:530–41. doi: 10.1021/cb800116f. [DOI] [PubMed] [Google Scholar]
- 2.Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17:159–65. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–82. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 4.Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol. 2007;69:511–34. doi: 10.1146/annurev.physiol.69.022405.154731. [DOI] [PubMed] [Google Scholar]
- 5.Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–68. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- 6.Bulenger S, Marullo S, Bouvier M. Emerging role of homo- and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol Sci. 2005;26:131–7. doi: 10.1016/j.tips.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Prinster SC, Hague C, Hall RA. Heterodimerization of g protein-coupled receptors: specificity and functional significance. Pharmacol Rev. 2005;57:289–98. doi: 10.1124/pr.57.3.1. [DOI] [PubMed] [Google Scholar]
- 8.Milligan G. G protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br J Pharmacol. 2009 doi: 10.1111/j.1476-5381.2009.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrero MB, Venema VJ, Ju H, Eaton DC, Venema RC. Regulation of angiotensin II-induced JAK2 tyrosine phosphorylation: roles of SHP-1 and SHP-2. Am J Physiol. 1998;275:C1216–23. doi: 10.1152/ajpcell.1998.275.5.C1216. [DOI] [PubMed] [Google Scholar]
- 10.Godeny MD, et al. The N-terminal SH2 domain of the tyrosine phosphatase, SHP-2, is essential for Jak2-dependent signaling via the angiotensin II type AT1 receptor. Cell Signal. 2007;19:600–9. doi: 10.1016/j.cellsig.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Aplin M, Christensen GL, Hansen JL. Pharmacologic perspectives of functional selectivity by the angiotensin II type 1 receptor. Trends Cardiovasc Med. 2008;18:305–12. doi: 10.1016/j.tcm.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Frank GD, et al. Requirement of Ca(2+) and PKCdelta for Janus kinase 2 activation by angiotensin II: involvement of PYK2. Mol Endocrinol. 2002;16:367–77. doi: 10.1210/mend.16.2.0768. [DOI] [PubMed] [Google Scholar]
- 13.Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol. 2006;20:953–70. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- 14.Lukashova V, Asselin C, Krolewski JJ, Rola-Pleszczynski M, Stankova J. G-protein-independent activation of Tyk2 by the platelet-activating factor receptor. J Biol Chem. 2001;276:24113–21. doi: 10.1074/jbc.M100720200. [DOI] [PubMed] [Google Scholar]
- 15.Lukashova V, Chen Z, Duhe RJ, Rola-Pleszczynski M, Stankova J. Janus kinase 2 activation by the platelet-activating factor receptor (PAFR): roles of Tyk2 and PAFR C terminus. J Immunol. 2003;171:3794–800. doi: 10.4049/jimmunol.171.7.3794. [DOI] [PubMed] [Google Scholar]
- 16.Dev KK. Making protein interactions druggable: targeting PDZ domains. Nat Rev Drug Discov. 2004;3:1047–56. doi: 10.1038/nrd1578. [DOI] [PubMed] [Google Scholar]
- 17.Hall RA, et al. The beta2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature. 1998;392:626–30. doi: 10.1038/33458. [DOI] [PubMed] [Google Scholar]
- 18.Hall RA, et al. A C-terminal motif found in the beta2-adrenergic receptor, P2Y1 receptor and cystic fibrosis transmembrane conductance regulator determines binding to the Na+/H+ exchanger regulatory factor family of PDZ proteins. Proc Natl Acad Sci USA. 1998;95:8496–501. doi: 10.1073/pnas.95.15.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li JG, Chen C, Liu-Chen LY. Ezrin-radixin-moesin-binding phosphoprotein-50/Na+/H+ exchanger regulatory factor (EBP50/NHERF) blocks U50,488H-induced down-regulation of the human kappa opioid receptor by enhancing its recycling rate. J Biol Chem. 2002;277:27545–52. doi: 10.1074/jbc.M200058200. [DOI] [PubMed] [Google Scholar]
- 20.Huang P, et al. kappa Opioid receptor interacts with Na(+)/H(+)-exchanger regulatory factor-1/Ezrin-radixin-moesin-binding phosphoprotein-50 (NHERF-1/EBP50) to stimulate Na(+)/H(+) exchange independent of G(i)/G(o) proteins. J Biol Chem. 2004;279:25002–9. doi: 10.1074/jbc.M313366200. [DOI] [PubMed] [Google Scholar]
- 21.Bousquet C, et al. Direct binding of p85 to sst2 somatostatin receptor reveals a novel mechanism for inhibiting PI3K pathway. EMBO J. 2006;25:3943–54. doi: 10.1038/sj.emboj.7601279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber A, et al. The transient receptor potential protein (Trp), a putative store-operated Ca2+ channel essential for phosphoinositide-mediated photoreception, forms a signaling complex with NorpA, InaC and InaD. EMBO J. 1996;15:7036–45. [PMC free article] [PubMed] [Google Scholar]
- 23.Shieh BH, Zhu MY. Regulation of the TRP Ca2+ channel by INAD in Drosophila photoreceptors. Neuron. 1996;16:991–8. doi: 10.1016/s0896-6273(00)80122-1. [DOI] [PubMed] [Google Scholar]
- 24.Chevesich J, Kreuz AJ, Montell C. Requirement for the PDZ domain protein, INAD, for localization of the TRP store-operated channel to a signaling complex. Neuron. 1997;18:95–105. doi: 10.1016/s0896-6273(01)80049-0. [DOI] [PubMed] [Google Scholar]
- 25.Tsunoda S, et al. A multivalent PDZ-domain protein assembles signalling complexes in a G-protein-coupled cascade. Nature. 1997;388:243–9. doi: 10.1038/40805. [DOI] [PubMed] [Google Scholar]
- 26.Xu XZ, Choudhury A, Li X, Montell C. Coordination of an array of signaling proteins through homo- and heteromeric interactions between PDZ domains and target proteins. J Cell Biol. 1998;142:545–55. doi: 10.1083/jcb.142.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popescu DC, Ham AJ, Shieh BH. Scaffolding protein INAD regulates deactivation of vision by promoting phosphorylation of transient receptor potential by eye protein kinase C in Drosophila. J Neurosci. 2006;26:8570–7. doi: 10.1523/JNEUROSCI.1478-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng L, Popescu DC, Wang N, Shieh BH. Anchoring TRP to the INAD macromolecular complex requires the last 14 residues in its carboxyl terminus. J Neurochem. 2008;104:1526–35. doi: 10.1111/j.1471-4159.2007.05096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott K, Zuker CS. Assembly of the Drosophila phototransduction cascade into a signalling complex shapes elementary responses. Nature. 1998;395:805–8. doi: 10.1038/27448. [DOI] [PubMed] [Google Scholar]
- 30.Mahon MJ, Donowitz M, Yun CC, Segre GV. Na(+)/H(+ ) exchanger regulatory factor 2 directs parathyroid hormone 1 receptor signalling. Nature. 2002;417:858–61. doi: 10.1038/nature00816. [DOI] [PubMed] [Google Scholar]
- 31.Sneddon WB, et al. Activation-independent parathyroid hormone receptor internalization is regulated by NHERF1 (EBP50) J Biol Chem. 2003;278:43787–96. doi: 10.1074/jbc.M306019200. [DOI] [PubMed] [Google Scholar]
- 32.Mahon MJ, Segre GV. Stimulation by parathyroid hormone of a NHERF-1-assembled complex consisting of the parathyroid hormone I receptor, phospholipase Cbeta, and actin increases intracellular calcium in opossum kidney cells. J Biol Chem. 2004;279:23550–8. doi: 10.1074/jbc.M313229200. [DOI] [PubMed] [Google Scholar]
- 33.Wheeler D, et al. Regulation of parathyroid hormone type 1 receptor dynamics, traffic, and signaling by the Na+/H+ exchanger regulatory factor-1 in rat osteosarcoma ROS 17/2.8 cells. Mol Endocrinol. 2008;22:1163–70. doi: 10.1210/me.2007-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang B, Yang Y, Abou-Samra AB, Friedman PA. NHERF1 regulates parathyroid hormone receptor desensitization: interference with beta-arrestin binding. Mol Pharmacol. 2009;75:1189–97. doi: 10.1124/mol.108.054486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh YS, et al. NHERF2 specifically interacts with LPA2 receptor and defines the specificity and efficiency of receptor-mediated phospholipase C-beta3 activation. Mol Cell Biol. 2004;24:5069–79. doi: 10.1128/MCB.24.11.5069-5079.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fam SR, et al. P2Y1 receptor signaling is controlled by interaction with the PDZ scaffold NHERF-2. Proc Natl Acad Sci USA. 2005;102:8042–7. doi: 10.1073/pnas.0408818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paquet M, et al. The PDZ scaffold NHERF-2 interacts with mGluR5 and regulates receptor activity. J Biol Chem. 2006;281:29949–61. doi: 10.1074/jbc.M602262200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rochdi MD, et al. Regulation of GTP-binding protein alpha q (Galpha q) signaling by the ezrin-radixin-moesin-binding phosphoprotein-50 (EBP50) J Biol Chem. 2002;277:40751–9. doi: 10.1074/jbc.M207910200. [DOI] [PubMed] [Google Scholar]
- 39.Tang Y, et al. Association of mammalian trp4 and phospholipase C isozymes with a PDZ domain-containing protein, NHERF. J Biol Chem. 2000;275:37559–64. doi: 10.1074/jbc.M006635200. [DOI] [PubMed] [Google Scholar]
- 40.Lee-Kwon W, Wade JB, Zhang Z, Pallone TL, Weinman EJ. Expression of TRPC4 channel protein that interacts with NHERF-2 in rat descending vasa recta. Am J Physiol Cell Physiol. 2005;288:C942–9. doi: 10.1152/ajpcell.00417.2004. [DOI] [PubMed] [Google Scholar]
- 41.Hwang JI, et al. Regulation of phospholipase C-beta 3 activity by Na+/H+ exchanger regulatory factor 2. J Biol Chem. 2000;275:16632–7. doi: 10.1074/jbc.M001410200. [DOI] [PubMed] [Google Scholar]
- 42.Lee-Kwon W, et al. Ca2+-dependent inhibition of NHE3 requires PKC alpha which binds to E3KARP to decrease surface NHE3 containing plasma membrane complexes. Am J Physiol Cell Physiol. 2003;285:C1527–36. doi: 10.1152/ajpcell.00017.2003. [DOI] [PubMed] [Google Scholar]
- 43.Kunkel MT, Garcia EL, Kajimoto T, Hall RA, Newton AC. The protein scaffold NHERF-1 controls the amplitude and duration of localized protein kinase D activity. J Biol Chem. 2009 doi: 10.1074/jbc.M109.024547. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinman EJ, Hall RA, Friedman PA, Liu-Chen LY, Shenolikar S. The association of NHERF adaptor proteins with g protein-coupled receptors and receptor tyrosine kinases. Annu Rev Physiol. 2006;68:491–505. doi: 10.1146/annurev.physiol.68.040104.131050. [DOI] [PubMed] [Google Scholar]
- 45.Balasubramanian S, Fam SR, Hall RA. GABAB receptor association with the PDZ scaffold Mupp1 alters receptor stability and function. J Biol Chem. 2007;282:4162–71. doi: 10.1074/jbc.M607695200. [DOI] [PubMed] [Google Scholar]
- 46.Guillaume JL, et al. The PDZ protein mupp1 promotes Gi coupling and signaling of the Mt1 melatonin receptor. J Biol Chem. 2008;283:16762–71. doi: 10.1074/jbc.M802069200. [DOI] [PubMed] [Google Scholar]
- 47.Yao R, Natsume Y, Noda T. MAGI-3 is involved in the regulation of the JNK signaling pathway as a scaffold protein for frizzled and Ltap. Oncogene. 2004;23:6023–30. doi: 10.1038/sj.onc.1207817. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Wang D, Sun H, Hall RA, Yun CC. MAGI-3 regulates LPA-induced activation of Erk and RhoA. Cell Signal. 2007;19:261–8. doi: 10.1016/j.cellsig.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamada T, Ohoka Y, Kogo M, Inagaki S. Physical and functional interactions of the lysophosphatidic acid receptors with PDZ domain-containing Rho guanine nucleotide exchange factors (RhoGEFs) J Biol Chem. 2005;280:19358–63. doi: 10.1074/jbc.M414561200. [DOI] [PubMed] [Google Scholar]
- 50.Brakeman PR, et al. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–8. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- 51.Xiao B, et al. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–16. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- 52.Tu JC, et al. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–26. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 53.Kato A, et al. Novel members of the Vesl/Homer family of PDZ proteins that bind metabotropic glutamate receptors. J Biol Chem. 1998;273:23969–75. doi: 10.1074/jbc.273.37.23969. [DOI] [PubMed] [Google Scholar]
- 54.Shih M, Lin F, Scott JD, Wang HY, Malbon CC. Dynamic complexes of beta2-adrenergic receptors with protein kinases and phosphatases and the role of gravin. J Biol Chem. 1999;274:1588–95. doi: 10.1074/jbc.274.3.1588. [DOI] [PubMed] [Google Scholar]
- 55.Fraser ID, et al. Assembly of an A kinase-anchoring protein-beta(2)-adrenergic receptor complex facilitates receptor phosphorylation and signaling. Curr Biol. 2000;10:409–12. doi: 10.1016/s0960-9822(00)00419-x. [DOI] [PubMed] [Google Scholar]
- 56.Fan G, Shumay E, Wang H, Malbon CC. The scaffold protein gravin (cAMP-dependent protein kinase-anchoring protein 250) binds the beta 2-adrenergic receptor via the receptor cytoplasmic Arg-329 to Leu-413 domain and provides a mobile scaffold during desensitization. J Biol Chem. 2001;276:24005–14. doi: 10.1074/jbc.M011199200. [DOI] [PubMed] [Google Scholar]
- 57.Tao J, Wang HY, Malbon CC. Protein kinase A regulates AKAP250 (gravin) scaffold binding to the beta2-adrenergic receptor. EMBO J. 2003;22:6419–29. doi: 10.1093/emboj/cdg628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gardner LA, Tavalin SJ, Goehring AS, Scott JD, Bahouth SW. AKAP79-mediated targeting of the cyclic AMP-dependent protein kinase to the beta1-adrenergic receptor promotes recycling and functional resensitization of the receptor. J Biol Chem. 2006;281:33537–53. doi: 10.1074/jbc.M601809200. [DOI] [PubMed] [Google Scholar]
- 59.Kammermeier PJ, Worley PF. Homer 1a uncouples metabotropic glutamate receptor 5 from postsynaptic effectors. Proc Natl Acad Sci USA. 2007;104:6055–60. doi: 10.1073/pnas.0608991104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kammermeier PJ. Endogenous homer proteins regulate metabotropic glutamate receptor signaling in neurons. J Neurosci. 2008;28:8560–7. doi: 10.1523/JNEUROSCI.1830-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith FD, Oxford GS, Milgram SL. Association of the D2 dopamine receptor third cytoplasmic loop with spinophilin, a protein phosphatase-1-interacting protein. J Biol Chem. 1999;274:19894–900. doi: 10.1074/jbc.274.28.19894. [DOI] [PubMed] [Google Scholar]
- 62.Richman JG, et al. Agonist-regulated Interaction between alpha2-adrenergic receptors and spinophilin. J Biol Chem. 2001;276:15003–8. doi: 10.1074/jbc.M011679200. [DOI] [PubMed] [Google Scholar]
- 63.Wang X, et al. Spinophilin regulates Ca2+ signalling by binding the N-terminal domain of RGS2 and the third intracellular loop of G-protein-coupled receptors. Nat Cell Biol. 2005;7:405–11. doi: 10.1038/ncb1237. [DOI] [PubMed] [Google Scholar]
- 64.Wang X, et al. Spinophilin/neurabin reciprocally regulate signaling intensity by G protein-coupled receptors. EMBO J. 2007;26:2768–76. doi: 10.1038/sj.emboj.7601701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Q, et al. Spinophilin blocks arrestin actions in vitro and in vivo at G protein-coupled receptors. Science. 2004;304:1940–4. doi: 10.1126/science.1098274. [DOI] [PubMed] [Google Scholar]
- 66.Snow BE, et al. GTPase activating specificity of RGS12 and binding specificity of an alternatively spliced PDZ (PSD-95/Dlg/ZO-1) domain. J Biol Chem. 1998;273:17749–55. doi: 10.1074/jbc.273.28.17749. [DOI] [PubMed] [Google Scholar]
- 67.Bernstein LS, et al. RGS2 binds directly and selectively to the M1 muscarinic acetylcholine receptor third intracellular loop to modulate Gq/11alpha signaling. J Biol Chem. 2004;279:21248–56. doi: 10.1074/jbc.M312407200. [DOI] [PubMed] [Google Scholar]
- 68.Hague C, et al. Selective inhibition of alpha1A-adrenergic receptor signaling by RGS2 association with the receptor third intracellular loop. J Biol Chem. 2005;280:27289–95. doi: 10.1074/jbc.M502365200. [DOI] [PubMed] [Google Scholar]
- 69.Georgoussi Z, et al. Selective interactions between G protein subunits and RGS4 with the C-terminal domains of the mu- and delta-opioid receptors regulate opioid receptor signaling. Cell Signal. 2006;18:771–82. doi: 10.1016/j.cellsig.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 70.Itoh M, Nagatomo K, Kubo Y, Saitoh O. Alternative splicing of RGS8 gene changes the binding property to the M1 muscarinic receptor to confer receptor type-specific Gq regulation. J Neurochem. 2006;99:1505–16. doi: 10.1111/j.1471-4159.2006.04220.x. [DOI] [PubMed] [Google Scholar]
- 71.Miyamoto-Matsubara M, Saitoh O, Maruyama K, Aizaki Y, Saito Y. Regulation of melanin-concentrating hormone receptor 1 signaling by RGS8 with the receptor third intracellular loop. Cell Signal. 2008;20:2084–94. doi: 10.1016/j.cellsig.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 72.Neitzel KL, Hepler JR. Cellular mechanisms that determine selective RGS protein regulation of G protein-coupled receptor signaling. Semin Cell Dev Biol. 2006;17:383–9. doi: 10.1016/j.semcdb.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 73.Xie GX, Palmer PP. How regulators of G protein signaling achieve selective regulation. J Mol Biol. 2007;366:349–65. doi: 10.1016/j.jmb.2006.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Minakami R, Jinnai N, Sugiyama H. Phosphorylation and calmodulin binding of the metabotropic glutamate receptor subtype 5 (mGluR5) are antagonistic in vitro. J Biol Chem. 1997;272:20291–8. doi: 10.1074/jbc.272.32.20291. [DOI] [PubMed] [Google Scholar]
- 75.Lee JH, et al. Calmodulin dynamically regulates the trafficking of the metabotropic glutamate receptor mGluR5. Proc Natl Acad Sci USA. 2008;105:12575–80. doi: 10.1073/pnas.0712033105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakajima Y, Yamamoto T, Nakayama T, Nakanishi S. A relationship between protein kinase C phosphorylation and calmodulin binding to the metabotropic glutamate receptor subtype 7. J Biol Chem. 1999;274:27573–7. doi: 10.1074/jbc.274.39.27573. [DOI] [PubMed] [Google Scholar]
- 77.Sorensen SD, Macek TA, Cai Z, Saugstad JA, Conn PJ. Dissociation of protein kinase-mediated regulation of metabotropic glutamate receptor 7 (mGluR7) interactions with calmodulin and regulation of mGluR7 function. Mol Pharmacol. 2002;61:1303–12. doi: 10.1124/mol.61.6.1303. [DOI] [PubMed] [Google Scholar]
- 78.Turner JH, Gelasco AK, Raymond JR. Calmodulin interacts with the third intracellular loop of the serotonin 5-hydroxytryptamine1A receptor at two distinct sites: putative role in receptor phosphorylation by protein kinase C. J Biol Chem. 2004;279:17027–37. doi: 10.1074/jbc.M313919200. [DOI] [PubMed] [Google Scholar]
- 79.Turner JH, Raymond JR. Interaction of calmodulin with the serotonin 5-hydroxytryptamine2A receptor. A putative regulator of G protein coupling and receptor phosphorylation by protein kinase C. J Biol Chem. 2005;280:30741–50. doi: 10.1074/jbc.M501696200. [DOI] [PubMed] [Google Scholar]
- 80.Labasque M, Reiter E, Becamel C, Bockaert J, Marin P. Physical interaction of calmodulin with the 5-hydroxytryptamine2C receptor C-terminus is essential for G protein-independent, arrestin-dependent receptor signaling. Mol Biol Cell. 2008;19:4640–50. doi: 10.1091/mbc.E08-04-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bofill-Cardona E, et al. Binding of calmodulin to the D2-dopamine receptor reduces receptor signaling by arresting the G protein activation switch. J Biol Chem. 2000;275:32672–80. doi: 10.1074/jbc.M002780200. [DOI] [PubMed] [Google Scholar]
- 82.Liu Y, Buck DC, Macey TA, Lan H, Neve KA. Evidence that calmodulin binding to the dopamine D2 receptor enhances receptor signaling. J Recept Signal Transduct Res. 2007;27:47–65. doi: 10.1080/10799890601094152. [DOI] [PubMed] [Google Scholar]
- 83.Wang D, Sadee W, Quillan JM. Calmodulin binding to G protein-coupling domain of opioid receptors. J Biol Chem. 1999;274:22081–8. doi: 10.1074/jbc.274.31.22081. [DOI] [PubMed] [Google Scholar]
- 84.Nickols HH, Shah VN, Chazin WJ, Limbird LE. Calmodulin interacts with the V2 vasopressin receptor: elimination of binding to the C terminus also eliminates arginine vasopressin-stimulated elevation of intracellular calcium. J Biol Chem. 2004;279:46969–80. doi: 10.1074/jbc.M407351200. [DOI] [PubMed] [Google Scholar]
- 85.Mahon MJ, Shimada M. Calmodulin interacts with the cytoplasmic tails of the parathyroid hormone 1 receptor and a sub-set of class b G-protein coupled receptors. FEBS Lett. 2005;579:803–7. doi: 10.1016/j.febslet.2004.12.056. [DOI] [PubMed] [Google Scholar]
- 86.Feng GJ, et al. Selective interactions between helix VIII of the human mu-opioid receptors and the C terminus of periplakin disrupt G protein activation. J Biol Chem. 2003;278:33400–7. doi: 10.1074/jbc.M305866200. [DOI] [PubMed] [Google Scholar]
- 87.Murdoch H, et al. Periplakin interferes with G protein activation by the melanin-concentrating hormone receptor-1 by binding to the proximal segment of the receptor C-terminal tail. J Biol Chem. 2005;280:8208–20. doi: 10.1074/jbc.M405215200. [DOI] [PubMed] [Google Scholar]
- 88.Francke F, et al. Interaction of neurochondrin with the melanin-concentrating hormone receptor 1 interferes with G protein-coupled signal transduction but not agonist-mediated internalization. J Biol Chem. 2006;281:32496–507. doi: 10.1074/jbc.M602889200. [DOI] [PubMed] [Google Scholar]
- 89.Ward RJ, Jenkins L, Milligan G. Selectivity and functional consequences of interactions of family A G protein-coupled receptors with neurochondrin and periplakin. J Neurochem. 2009;109:182–92. doi: 10.1111/j.1471-4159.2009.05918.x. [DOI] [PubMed] [Google Scholar]
- 90.Shieh BH, Stamnes MA, Seavello S, Harris GL, Zuker CS. The ninaA gene required for visual transduction in Drosophila encodes a homologue of cyclosporin A-binding protein. Nature. 1989;338:67–70. doi: 10.1038/338067a0. [DOI] [PubMed] [Google Scholar]
- 91.Baker EK, Colley NJ, Zuker CS. The cyclophilin homolog NinaA functions as a chaperone, forming a stable complex in vivo with its protein target rhodopsin. EMBO J. 1994;13:4886–95. doi: 10.1002/j.1460-2075.1994.tb06816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ferreira PA, Nakayama TA, Pak WL, Travis GH. Cyclophilin-related protein RanBP2 acts as chaperone for red/green opsin. Nature. 1996;383:637–40. doi: 10.1038/383637a0. [DOI] [PubMed] [Google Scholar]
- 93.Tai AW, Chuang JZ, Bode C, Wolfrum U, Sung CH. Rhodopsin’s carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex-1. Cell. 1999;97:877–87. doi: 10.1016/s0092-8674(00)80800-4. [DOI] [PubMed] [Google Scholar]
- 94.Yeh TY, Peretti D, Chuang JZ, Rodriguez-Boulan E, Sung CH. Regulatory dissociation of Tctex-1 light chain from dynein complex is essential for the apical delivery of rhodopsin. Traffic. 2006;7:1495–502. doi: 10.1111/j.1600-0854.2006.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen C, et al. GEC1 interacts with the kappa opioid receptor and enhances expression of the receptor. J Biol Chem. 2006;281:7983–93. doi: 10.1074/jbc.M509805200. [DOI] [PubMed] [Google Scholar]
- 96.Chen Y, et al. GEC1-kappa opioid receptor binding involves hydrophobic interactions: GEC1 has chaperone-like effect. J Biol Chem. 2009;284:1673–85. doi: 10.1074/jbc.M808303200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Parent A, Laroche G, Hamelin E, Parent JL. RACK1 regulates the cell surface expression of the G protein-coupled receptor for thromboxane A(2) Traffic. 2008;9:394–407. doi: 10.1111/j.1600-0854.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- 98.Bermak JC, Li M, Bullock C, Zhou QY. Regulation of transport of the dopamine D1 receptor by a new membrane-associated ER protein. Nat Cell Biol. 2001;3:492–8. doi: 10.1038/35074561. [DOI] [PubMed] [Google Scholar]
- 99.Leclerc PC, et al. A polyaromatic caveolin-binding-like motif in the cytoplasmic tail of the type 1 receptor for angiotensin II plays an important role in receptor trafficking and signaling. Endocrinology. 2002;143:4702–10. doi: 10.1210/en.2002-220679. [DOI] [PubMed] [Google Scholar]
- 100.Wruck CJ, et al. Regulation of transport of the angiotensin AT2 receptor by a novel membrane-associated Golgi protein. Arterioscler Thromb Vasc Biol. 2005;25:57–64. doi: 10.1161/01.ATV.0000150662.51436.14. [DOI] [PubMed] [Google Scholar]
- 101.Milojevic T, et al. The ubiquitin-specific protease Usp4 regulates the cell surface level of the A2A receptor. Mol Pharmacol. 2006;69:1083–94. doi: 10.1124/mol.105.015818. [DOI] [PubMed] [Google Scholar]
- 102.Bush CF, Hall RA. Olfactory receptor trafficking to the plasma membrane. Cell Mol Life Sci. 2008;65:2289–95. doi: 10.1007/s00018-008-8028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dwyer ND, Troemel ER, Sengupta P, Bargmann CI. Odorant receptor localization to olfactory cilia is mediated by ODR-4, a novel membrane-associated protein. Cell. 1998;93:455–66. doi: 10.1016/s0092-8674(00)81173-3. [DOI] [PubMed] [Google Scholar]
- 104.Saito H, Kubota M, Roberts RW, Chi Q, Matsunami H. RTP family members induce functional expression of mammalian odorant receptors. Cell. 2004;119:679–91. doi: 10.1016/j.cell.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 105.Zhuang H, Matsunami H. Synergism of accessory factors in functional expression of mammalian odorant receptors. J Biol Chem. 2007;282:15284–93. doi: 10.1074/jbc.M700386200. [DOI] [PubMed] [Google Scholar]
- 106.Behrens M, et al. Members of RTP and REEP gene families influence functional bitter taste receptor expression. J Biol Chem. 2006;281:20650–9. doi: 10.1074/jbc.M513637200. [DOI] [PubMed] [Google Scholar]
- 107.Decaillot FM, Rozenfeld R, Gupta A, Devi LA. Cell surface targeting of mu-delta opioid receptor heterodimers by RTP4. Proc Natl Acad Sci USA. 2008;105:16045–50. doi: 10.1073/pnas.0804106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Loconto J, et al. Functional expression of murine V2R pheromone receptors involves selective association with the M10 and M1 families of MHC class Ib molecules. Cell. 2003;112:607–18. doi: 10.1016/s0092-8674(03)00153-3. [DOI] [PubMed] [Google Scholar]
- 109.Metherell LA, et al. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat Genet. 2005;37:166–70. doi: 10.1038/ng1501. [DOI] [PubMed] [Google Scholar]
- 110.Roy S, Rached M, Gallo-Payet N. Differential regulation of the human adrenocorticotropin receptor [melanocortin-2 receptor (MC2R)] by human MC2R accessory protein isoforms alpha and beta in isogenic human embryonic kidney 293 cells. Mol Endocrinol. 2007;21:1656–69. doi: 10.1210/me.2007-0041. [DOI] [PubMed] [Google Scholar]
- 111.Sebag JA, Hinkle PM. Melanocortin-2 receptor accessory protein MRAP forms antiparallel homodimers. Proc Natl Acad Sci USA. 2007;104:20244–9. doi: 10.1073/pnas.0708916105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hinkle PM, Sebag JA. Structure and function of the melanocortin2 receptor accessory protein (MRAP) Mol Cell Endocrinol. 2009;300:25–31. doi: 10.1016/j.mce.2008.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sebag JA, Hinkle PM. Regions of melanocortin 2 (MC2) receptor accessory protein necessary for dual topology and MC2 receptor trafficking and signaling. J Biol Chem. 2009;284:610–8. doi: 10.1074/jbc.M804413200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Whistler JL, et al. Modulation of postendocytic sorting of G protein-coupled receptors. Science. 2002;297:615–20. doi: 10.1126/science.1073308. [DOI] [PubMed] [Google Scholar]
- 115.Bartlett SE, et al. Dopamine responsiveness is regulated by targeted sorting of D2 receptors. Proc Natl Acad Sci USA. 2005;102:11521–6. doi: 10.1073/pnas.0502418102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tappe-Theodor A, et al. A molecular basis of analgesic tolerance to cannabinoids. J Neurosci. 2007;27:4165–77. doi: 10.1523/JNEUROSCI.5648-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Martini L, et al. Ligand-induced down-regulation of the cannabinoid 1 receptor is mediated by the G-protein-coupled receptor-associated sorting protein GASP1. FASEB J. 2007;21:802–11. doi: 10.1096/fj.06-7132com. [DOI] [PubMed] [Google Scholar]
- 118.Simonin F, Karcher P, Boeuf JJ, Matifas A, Kieffer BL. Identification of a novel family of G protein-coupled receptor associated sorting proteins. J Neurochem. 2004;89:766–75. doi: 10.1111/j.1471-4159.2004.02411.x. [DOI] [PubMed] [Google Scholar]
- 119.Wang Y, Zhou Y, Szabo K, Haft CR, Trejo J. Down-regulation of protease-activated receptor-1 is regulated by sorting nexin 1. Mol Biol Cell. 2002;13:1965–76. doi: 10.1091/mbc.E01-11-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gullapalli A, Wolfe BL, Griffin CT, Magnuson T, Trejo J. An essential role for SNX1 in lysosomal sorting of protease-activated receptor-1: evidence for retromer-, Hrs-, and Tsg101-independent functions of sorting nexins. Mol Biol Cell. 2006;17:1228–38. doi: 10.1091/mbc.E05-09-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Heydorn A, et al. A library of 7TM receptor C-terminal tails. Interactions with the proposed post-endocytic sorting proteins ERM-binding phosphoprotein 50 (EBP50), N-ethylmaleimide-sensitive factor (NSF), sorting nexin 1 (SNX1), and G protein-coupled receptor-associated sorting protein (GASP) J Biol Chem. 2004;279:54291–303. doi: 10.1074/jbc.M406169200. [DOI] [PubMed] [Google Scholar]
- 122.Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature. 1999;401:286–90. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- 123.Gage RM, Kim KA, Cao TT, von Zastrow M. A transplantable sorting signal that is sufficient to mediate rapid recycling of G protein-coupled receptors. J Biol Chem. 2001;276:44712–20. doi: 10.1074/jbc.M107417200. [DOI] [PubMed] [Google Scholar]
- 124.Gage RM, Matveeva EA, Whiteheart SW, von Zastrow M. Type I PDZ ligands are sufficient to promote rapid recycling of G Protein-coupled receptors independent of binding to N-ethylmaleimide-sensitive factor. J Biol Chem. 2005;280:3305–13. doi: 10.1074/jbc.M406934200. [DOI] [PubMed] [Google Scholar]
- 125.Wang B, Bisello A, Yang Y, Romero GG, Friedman PA. NHERF1 regulates parathyroid hormone receptor membrane retention without affecting recycling. J Biol Chem. 2007;282:36214–22. doi: 10.1074/jbc.M707263200. [DOI] [PubMed] [Google Scholar]
- 126.Ango F, et al. Dendritic and axonal targeting of type 5 metabotropic glutamate receptor is regulated by homer1 proteins and neuronal excitation. J Neurosci. 2000;20:8710–6. doi: 10.1523/JNEUROSCI.20-23-08710.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Das SS, Banker GA. The role of protein interaction motifs in regulating the polarity and clustering of the metabotropic glutamate receptor mGluR1a. J Neurosci. 2006;26:8115–25. doi: 10.1523/JNEUROSCI.1015-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Serge A, Fourgeaud L, Hemar A, Choquet D. Receptor activation and homer differentially control the lateral mobility of metabotropic glutamate receptor 5 in the neuronal membrane. J Neurosci. 2002;22:3910–20. doi: 10.1523/JNEUROSCI.22-10-03910.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tu JC, et al. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–92. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- 130.Sala C, et al. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron. 2001;31:115–30. doi: 10.1016/s0896-6273(01)00339-7. [DOI] [PubMed] [Google Scholar]
- 131.Hayashi MK, et al. The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell. 2009;137:159–71. doi: 10.1016/j.cell.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tobaben S, Sudhof TC, Stahl B. The G protein-coupled receptor CL1 interacts directly with proteins of the Shank family. J Biol Chem. 2000;275:36204–10. doi: 10.1074/jbc.M006448200. [DOI] [PubMed] [Google Scholar]
- 133.Kreienkamp HJ, Zitzer H, Gundelfinger ED, Richter D, Bockers TM. The calcium-independent receptor for alpha-latrotoxin from human and rodent brains interacts with members of the ProSAP/SSTRIP/Shank family of multidomain proteins. J Biol Chem. 2000;275:32387–90. doi: 10.1074/jbc.C000490200. [DOI] [PubMed] [Google Scholar]
- 134.Xia Z, Gray JA, Compton-Toth BA, Roth BL. A direct interaction of PSD-95 with 5-HT2A serotonin receptors regulates receptor trafficking and signal transduction. J Biol Chem. 2003;278:21901–8. doi: 10.1074/jbc.M301905200. [DOI] [PubMed] [Google Scholar]
- 135.Xia Z, Hufeisen SJ, Gray JA, Roth BL. The PDZ-binding domain is essential for the dendritic targeting of 5-HT2A serotonin receptors in cortical pyramidal neurons in vitro. Neuroscience. 2003;122:907–20. doi: 10.1016/s0306-4522(03)00589-x. [DOI] [PubMed] [Google Scholar]
- 136.Hu LA, et al. beta 1-adrenergic receptor association with PSD-95. Inhibition of receptor internalization and facilitation of beta 1-adrenergic receptor interaction with N-methyl-D-aspartate receptors. J Biol Chem. 2000;275:38659–66. doi: 10.1074/jbc.M005938200. [DOI] [PubMed] [Google Scholar]
- 137.Topinka JR, Bredt DS. N-terminal palmitoylation of PSD-95 regulates association with cell membranes and interaction with K+ channel Kv1.4. Neuron. 1998;20:125–34. doi: 10.1016/s0896-6273(00)80440-7. [DOI] [PubMed] [Google Scholar]
- 138.Xu J, et al. beta 1-adrenergic receptor association with the synaptic scaffolding protein membrane-associated guanylate kinase inverted-2 (MAGI-2). Differential regulation of receptor internalization by MAGI-2 and PSD-95. J Biol Chem. 2001;276:41310–7. doi: 10.1074/jbc.M107480200. [DOI] [PubMed] [Google Scholar]
- 139.Gavarini S, et al. Opposite effects of PSD-95 and MPP3 PDZ proteins on serotonin 5-hydroxytryptamine2C receptor desensitization and membrane stability. Mol Biol Cell. 2006;17:4619–31. doi: 10.1091/mbc.E06-03-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Boudin H, et al. Presynaptic clustering of mGluR7a requires the PICK1 PDZ domain binding site. Neuron. 2000;28:485–97. doi: 10.1016/s0896-6273(00)00127-6. [DOI] [PubMed] [Google Scholar]
- 141.Boudin H, Craig AM. Molecular determinants for PICK1 synaptic aggregation and mGluR7a receptor coclustering: role of the PDZ, coiled-coil, and acidic domains. J Biol Chem. 2001;276:30270–6. doi: 10.1074/jbc.M102991200. [DOI] [PubMed] [Google Scholar]
- 142.Chen Z, Hague C, Hall RA, Minneman KP. Syntrophins regulate alpha1D-adrenergic receptors through a PDZ domain-mediated interaction. J Biol Chem. 2006;281:12414–20. doi: 10.1074/jbc.M508651200. [DOI] [PubMed] [Google Scholar]
- 143.Lyssand JS, et al. Blood pressure is regulated by an alpha1D-adrenergic receptor/dystrophin signalosome. J Biol Chem. 2008;283:18792–800. doi: 10.1074/jbc.M801860200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liew CW, et al. Interaction of the human somatostatin receptor 3 with the multiple PDZ domain protein MUPP1 enables somatostatin to control permeability of epithelial tight junctions. FEBS Lett. 2009;583:49–54. doi: 10.1016/j.febslet.2008.11.048. [DOI] [PubMed] [Google Scholar]
- 145.Hay DL, Poyner DR, Sexton PM. GPCR modulation by RAMPs. Pharmacol Ther. 2006;109:173–97. doi: 10.1016/j.pharmthera.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 146.McLatchie LM, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–9. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 147.Fraser NJ, et al. The amino terminus of receptor activity modifying proteins is a critical determinant of glycosylation state and ligand binding of calcitonin receptor-like receptor. Mol Pharmacol. 1999;55:1054–9. doi: 10.1124/mol.55.6.1054. [DOI] [PubMed] [Google Scholar]
- 148.Hilairet S, Foord SM, Marshall FH, Bouvier M. Protein-protein interaction and not glycosylation determines the binding selectivity of heterodimers between the calcitonin receptor-like receptor and the receptor activity-modifying proteins. J Biol Chem. 2001;276:29575–81. doi: 10.1074/jbc.M102722200. [DOI] [PubMed] [Google Scholar]
- 149.Christopoulos G, et al. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol Pharmacol. 1999;56:235–42. doi: 10.1124/mol.56.1.235. [DOI] [PubMed] [Google Scholar]
- 150.Tilakaratne N, Christopoulos G, Zumpe ET, Foord SM, Sexton PM. Amylin receptor phenotypes derived from human calcitonin receptor/RAMP coexpression exhibit pharmacological differences dependent on receptor isoform and host cell environment. J Pharmacol Exp Ther. 2000;294:61–72. [PubMed] [Google Scholar]
- 151.Urban JD, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 152.Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–22. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 153.Ango F, et al. Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature. 2001;411:962–5. doi: 10.1038/35082096. [DOI] [PubMed] [Google Scholar]
- 154.Ango F, et al. Homer-dependent cell surface expression of metabotropic glutamate receptor type 5 in neurons. Mol Cell Neurosci. 2002;20:323–9. doi: 10.1006/mcne.2002.1100. [DOI] [PubMed] [Google Scholar]
- 155.Becamel C, et al. Interaction of serotonin 5-hydroxytryptamine type 2C receptors with PDZ10 of the multi-PDZ domain protein MUPP1. J Biol Chem. 2001;276:12974–82. doi: 10.1074/jbc.M008089200. [DOI] [PubMed] [Google Scholar]
- 156.Zhang CS, et al. Knock-in mice lacking the PDZ-ligand motif of mGluR7a show impaired PKC-dependent autoinhibition of glutamate release, spatial working memory deficits, and increased susceptibility to pentylenetetrazol. J Neurosci. 2008;28:8604–14. doi: 10.1523/JNEUROSCI.0628-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]