Abstract
Dynamic changes in the posttranslational modification of proteins govern most cellular signaling pathways. Work over the past decade has connected many of these processes with the covalent attachment of the small ubiquitin-like modifier (SUMO) protein to target proteins, but a global view of the dynamics of SUMOylation was missing. A system-level proteomics approach has now been used to describe quantitative changes in protein modification with the SUMO-2 paralog during the response to heat shock. The SUMOylation status of more than 700 proteins was monitored in HeLa cells during the induction of hyperthermic stress and the recovery period. A massive redistribution of SUMO-2 was observed that affected many biological pathways that are important for the heat shock response, including cell cycle regulation, transcription, translation, protein folding, and DNA repair. Collectively, these data suggest a wide-ranging role for SUMOylation in the cellular response to hyperthermic stress. The strategies that were developed to provide this global view of SUMOylation should guide future approaches to probing quantitative changes in protein modification.
The small ubiquitin-like modifier (SUMO) protein is the most prominent member of a growing class of proteins that resemble their famous cousin ubiquitin and are covalently attached to other proteins (1). Similar to other members of the family of ubiquitin-like protein modifiers (UBLs), attachment of SUMO to target proteins requires several enzymatic steps. SUMOylation is initiated with the activation of SUMO by the E1 enzyme followed by the E2-catalyzed modification of the substrate, which in some cases is directed and stimulated by SUMO E3 ligases (2, 3). Several SUMO proteins are found in human cells. SUMO-1 shares about 50% sequence homology with SUMO-2 and SUMO-3, which are almost identical to each other. All three paralogs are conjugated by the same E2 enzyme, Ubc9, and there appears to be a substantial overlap in their substrates. A gene that encodes a fourth SUMO protein is expressed in some human cells, but it is unclear whether SUMO-4 is conjugated to other proteins (3).
Attachment of SUMO introduces a new binding surface to the modified protein that can alter protein-protein interactions and often results in changes in specific subcellular localization. This regulation is dynamic because it is a highly reversible process due to a number of SUMO-specific proteases (SENP proteins) that remove SUMO from targets (4). Given the dynamic nature of SUMOylation, it is well suited to controlling cellular responses to various signals. SUMOylation has indeed been implicated in many signaling pathways, primarily through recognition that particular signal transduction components are SUMOylated (3, 5–7); however, a proteome-wide view of changes in SUMOylation in response to specific cellular cues—preferably in a quantitative way—was what the field desired.
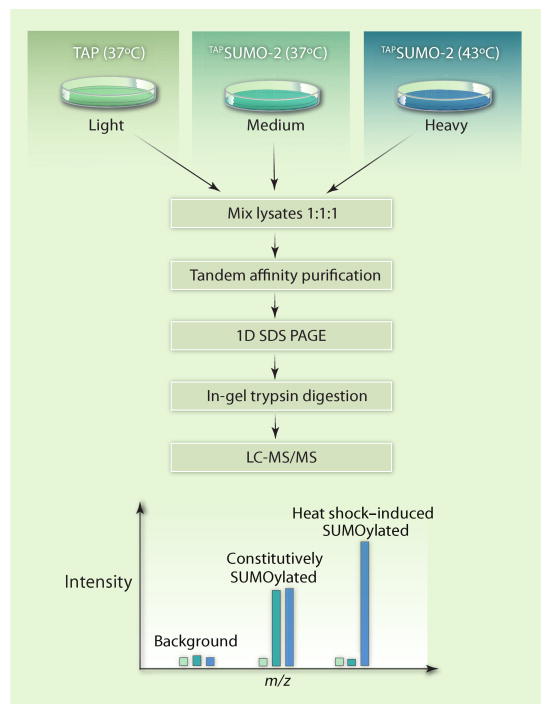
Golebiowski and colleagues developed such a strategy and applied it to describe quantitative changes in global SUMOylation profiles during the heat shock response (8). Hyperthermic stress as a test case for the developed mass spectrometric approach was well chosen, because previous experiments demonstrated a rapid and reversible increase in SUMO-2 and SUMO-3 conjugates when cells were shifted from the normal growth temperature of 37°C to the stressful 43°C (9). Furthermore, SUMOylation is important for the cellular defense against heat stress, because small interfering RNA (siRNA)–based knockdown of SUMO-2 and SUMO-3 substantially reduced cell survival after heat shock (8). To identify proteins modified with SUMO-2 and to follow the dynamics of the modification during heat stress and recovery, Golebiowski and colleagues combined purification of affinity-tagged SUMO-2 (TAPSUMO-2), stable isotope labeling (SILAC), and advanced mass spectrometry (MS) techniques (Fig. 1).
Fig. 1.
Affinity purification and quantitative MS to identify dynamic changes in the SUMO-2 proteome during heat shock. SUMO-2 fused to an affinity-tag (TAPSUMO-2) was expressed in HeLa cells, and proteins modified with TAPSUMO-2 were separated from all other cell components by binding to affinity resins (20). Purified fractions were separated by SDS–polyacrylamide gel electrophoresis (PAGE) to reduce sample complexity before the MS analysis. Quantitative comparison of the SUMO-2 proteome from different samples was achieved by a triple-SILAC strategy (16), which is based on the metabolic incorporation of stable (nonradioactive) isotope–labeled amino acids into the proteome (21–23). Three samples were compared: cells containing the TAP tag alone (for determination of purification background), cells containing TAPSUMO-2 that were incubated at 37°C, and cells containing TAPSUMO-2 that were incubated at 43°C for 2 hours. These different samples were cultured in “light,” “medium,” or “heavy” growth media, respectively. “Heavy” refers to the presence of Arg and Lys that were labeled with the stable isotopes 2H (deuterium), 13C, and 15N instead of the standard amino acids (1H, 12C, and 14N) in the “light” medium. The “medium” growth conditions contained Arg and Lys with an intermediate heavy isotope content. Heavy, medium, and light samples were analyzed together in the mass spectrometer, and identical peptides were detected as triplets because of the mass differences caused by the isotope-labeled amino acid residues. Relative quantitation was achieved by comparing the intensities of the peptide peaks. LC, liquid chromatography; m/z, mass/charge ratio.
Affinity-tagged ubiquitin and SUMO have been widely used to analyze global ubiquitination or SUMOylation (10, 11), but only a few studies have used quantitative MS methods (12–15). By adding novel techniques of data analysis, the work by Golebiowski et al. elevates such quantitative MS studies to the next level and provides a framework that can be easily adapted to the analysis of ubiquitin and other UBLs. A triple SILAC strategy was used to simultaneously compare three samples (16): cells containing the TAP tag (background control), cells containing TAPSUMO-2 that were grown at 37°C, and cells containing TAPSUMO-2 that were grown at 43°C (Fig. 1). The large data set generated by the MS analysis described abundance ratios for almost 1200 proteins; these data were presented in a comprehensible graphical format that the authors termed a triple-SILAC map (tsMap). Four clearly separated groups of proteins were revealed by the tsMap: “background” proteins, SUMOylated proteins unaffected by heat shock, and proteins that were either induced or reduced in SUMOylation state after heat stress.
Several interesting conclusions can be drawn from this analysis and from a related experiment that compared cells exposed to heat shock with cells that recovered from the heat stress. Western blotting analysis showed that the pool of free SUMO-2 was rapidly consumed as a result of the formation of new conjugates during heat shock (8, 9). What was not evident, but was revealed by the tsMap analyses, was that a substantial number of proteins were deSUMOylated during heat stress, which indicates a dynamic redistribution of both substrate-attached SUMO-2 molecules and free SUMO-2. Heat shock–induced SUMOylation of substrates was generally reversed by deSUMOylation during the recovery phase, but proteins that lost the SUMO-2 modification in response to heat shock were not readily remodified. The importance of this observation is not clear, but it might indicate that both global SUMOylation and deSUMOylation undergo different modes of regulation during the heat shock response.
Collectively, the study by Golebiowski and colleagues identified 766 putative substrates of SUMO-2. On average, these substrates contained two predicted SUMO consensus sites, but about 25% of the identified substrates lacked consensus sites (17). To further analyze this relatively complex data set, Golebiowski et al. used what they termed GOtsMaps (gene ontology triple-SILAC maps). GOtsMaps revealed the coordinated regulation of proteins belonging to certain functional categories. For example, transcription factors that form dimers—such as members of the Jun, Fos, and Maf families—were deSUMOylated during heat stress, whereas proteins involved in DNA repair and recombination were generally increased in SUMOylation status. A particularly interesting group of heat shock–induced SUMOylated proteins are components of the prereplication complex, including several minichromosome maintenance (MCM) and origin of replication complex (ORC) proteins (18), which might point to a heretofore unrecognized role of SUMOylation in the regulation of DNA replication.
A general complication with the monitoring of systemwide changes in protein modifications is to ensure that the observed dynamic changes, in this case SUMOylation, do not simply reflect changes in protein abundance. To address this problem, Golebiowski and co-workers identified abundance ratios for proteins in crude cell lysates. Of these measured proteins, 260 were also found in the TAP-purified samples, and the lack of correlation between the two abundance ratios showed that the heat shock–related changes generally reflected the dynamics of modification by SUMO-2 and not changes in the abundance of the proteins analyzed.
In addition to describing systemwide quantitative changes in SUMO-2 modification, the MS analyses identified a massive increase in polySUMO chains linked through Lys11 in SUMO-2 or SUMO-3. Poly-SUMO modification can serve as signal for ubiquitin-dependent proteolysis (19), but whether heat shock–stimulated polySUMOylation marks proteins for degradation remains to be tested. In general, it is unclear how the widespread increase in SUMOylation of proteins protects cells from heat stress. Important clues came from determining the functional categories to which the protein targets belonged, which showed heat stress–induced SUMOylation predominantly of proteins in pathways that are important for resistance to hyperthermic stress, such as cell cycle, DNA damage response, and protein folding and degradation. In addition, many factors directly involved in the heat stress response, such as heat shock factors and heat shock proteins, were modified with SUMO-2 during hyperthermic stress. These data link SUMOylation to processes that are important during heat shock, but it remains to be determined how SUMOylation affects these pathways and whether it changes the functions or activities of proteins or provides protection from thermal stress. Similarly, it will be of interest to determine the identity of the molecular signals leading to these systemwide changes in SUMOylation and whether these signals regulate SUMO conjugation reactions, deSUMOylation reactions, or both.
The work by Golebiowski and colleagues provides an unprecedented insight into the SUMO proteome and highlights the versatile role and dynamic character of this posttranslational modification. Beyond that, this study outlines a combination of affinity purification, quantitative MS, and strategies for data analysis that will advance system-level approaches to analyze the modification of proteins by other UBLs.
Acknowledgments
Supported by NIH grants GM066164 and CA113823.
References and Notes
- 1.Hochstrasser M. Origin and function of ubiquitin-like proteins. Nature. 2009;458:422–429. doi: 10.1038/nature07958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- 3.Geiss-Friedlander R, Melchior F. Concepts in sumoylation: A decade on. Nat Rev Mol Cell Biol. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- 4.Hay RT. SUMO-specific proteases: A twist in the tail. Trends Cell Biol. 2007;17:370–376. doi: 10.1016/j.tcb.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Hay RT. Role of ubiquitin-like proteins in transcriptional regulation. In: Berger SL, Nakanishi O, Haendler B, editors. The Histone Code and Beyond: New Approaches to Cancer Therapy. Springer; Berlin: 2006. pp. 173–192. [DOI] [PubMed] [Google Scholar]
- 6.Kirkin V, Dikic I. Role of ubiquitin- and Ubl-binding proteins in cell signaling. Curr Opin Cell Biol. 2007;19:199–205. doi: 10.1016/j.ceb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Bergink S, Jentsch S. Principles of ubiquitin and SUMO modifications in DNA repair. Nature. 2009;458:461–467. doi: 10.1038/nature07963. [DOI] [PubMed] [Google Scholar]
- 8.Golebiowski F, Matic I, Tatham MH, Cole C, Yin Y, Nakamura A, Cox J, Barton GJ, Mann M, Hay RT. System-wide changes to SUMO modifications in response to heat shock. Sci Signal. 2009;2:ra24. doi: 10.1126/scisignal.2000282. [DOI] [PubMed] [Google Scholar]
- 9.Saitoh H, Hinchey J. Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. J Biol Chem. 2000;275:6252–6258. doi: 10.1074/jbc.275.9.6252. [DOI] [PubMed] [Google Scholar]
- 10.Wilson VG, Heaton PR. Ubiquitin proteolytic system: Focus on SUMO. Expert Rev Proteomics. 2008;5:121–135. doi: 10.1586/14789450.5.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Guerrero C, Kaiser P, Huang L. Proteomics of proteasome complexes and ubiquitinated proteins. Expert Rev Proteomics. 2007;4:649–665. doi: 10.1586/14789450.4.5.649. [DOI] [PubMed] [Google Scholar]
- 12.Mayor T, Graumann J, Bryan J, MacCoss MJ, Deshaies RJ. Quantitative profiling of ubiquitylated proteins reveals proteasome substrates and the substrate repertoire influenced by the Rpn10 receptor pathway. Mol Cell Proteomics. 2007;6:1885–1895. doi: 10.1074/mcp.M700264-MCP200. [DOI] [PubMed] [Google Scholar]
- 13.Meierhofer D, Wang X, Huang L, Kaiser P. Quantitative analysis of global ubiquitination in HeLa cells by mass spectrometry. J Proteome Res. 2008;7:4566–4576. doi: 10.1021/pr800468j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu P, Duong DM, Seyfried NT, Cheng D, Xie Y, Robert J, Rush J, Hochstrasser M, Finley D, Peng J. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell. 2009;137:133–145. doi: 10.1016/j.cell.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vertegaal AC, Andersen JS, Ogg SC, Hay RT, Mann M, Lamond AI. Distinct and overlapping sets of SUMO-1 and SUMO-2 target proteins revealed by quantitative proteomics. Mol Cell Proteomics. 2006;5:2298–2310. doi: 10.1074/mcp.M600212-MCP200. [DOI] [PubMed] [Google Scholar]
- 16.Blagoev B, Ong SE, Kratchmarova I, Mann M. Temporal analysis of phosphotyrosine-dependent signaling networks by quantitative proteomics. Nat Biotechnol. 2004;22:1139–1145. doi: 10.1038/nbt1005. [DOI] [PubMed] [Google Scholar]
- 17.Xue Y, Zhou F, Fu C, Xu Y, Yao X. SUMOsp: A web server for sumoylation site prediction. Nucleic Acids Res. 2006;34:W254–W257. doi: 10.1093/nar/gkl207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem. 2002;71:333–374. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- 19.Tatham MH, Geoffroy MC, Shen L, Plechanovova A, Hattersley N, Jaffray EG, Palvimo JJ, Hay RT. RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation. Nat Cell Biol. 2008;10:538–546. doi: 10.1038/ncb1716. [DOI] [PubMed] [Google Scholar]
- 20.Rigaut G, Shevchenko A, Rutz B, Wilm M, Mann M, Seraphin B. A generic protein purification method for protein complex characterization and proteome exploration. Nat Biotechnol. 1999;17:1030–1032. doi: 10.1038/13732. [DOI] [PubMed] [Google Scholar]
- 21.Zhu H, Pan S, Gu S, Bradbury EM, Chen X. Amino acid residue specific stable isotope labeling for quantitative proteomics. Rapid Commun Mass Spectrom. 2002;16:2115–2123. doi: 10.1002/rcm.831. [DOI] [PubMed] [Google Scholar]
- 22.Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics. 2002;1:376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 23.Mann M. Functional and quantitative proteomics using SILAC. Nat Rev Mol Cell Biol. 2006;7:952–958. doi: 10.1038/nrm2067. [DOI] [PubMed] [Google Scholar]