Abstract
BACKGROUND:
In a cross-sectional study examining late effects of pediatric sarcoma therapy, long-term survivors were evaluated on their activities of daily living (ADL) performance.
PROCEDURE:
Thirty-two persons with Ewing sarcoma family of tumors, rhabdomyosarcoma, and non-rhabdomysarcoma-soft tissue sarcoma enrolled an average of 17 years after treatment. Participants were evaluated using the Assessment of Motor and Process Skills (AMPS) [1], a standardized observational evaluation of ADL task performance. Means and 95% confidence intervals for ADL motor and ADL process ability measures were calculated for four groups: 1) sarcoma survivors, 2) “well” adults matched for age and gender, 3) “well” adults matched for gender that were 10 years older; and 4) “well” adults matched for gender that were 20 years older.
RESULTS:
ADL motor ability was significantly lower for sarcoma survivors than for the age and gender matched comparison group (p<0.05). There was no significant difference between ADL motor ability of sarcoma survivors and the comparison group 10 years older, but sarcoma survivors had significantly better ADL motor ability (p<0.05) than the oldest comparison group (20 years older). Sarcoma survivors had significantly worse ADL process ability than the age matched group (p<0.05). There was no difference in ADL process ability between the sarcoma survivors and comparison groups that were 10 and 20 years older.
CONCLUSIONS:
This first report of a clinical evaluation of ADL limitation in pediatric sarcoma survivors treated with intensive multimodal cancer therapy suggests that influences on performance of daily life activities are more common than previously reported.
Keywords: late effects, pediatric sarcoma, pediatric sarcoma survivors, ADL
INTRODUCTION
While cancer is rare among those younger than 20 years of age, it is estimated that approximately 12,400 children younger than 20 years old were diagnosed with cancer in 1998 with 2,500 dying of the illness in 1998 [2]. Improved diagnosis and comprehensive treatment of childhood cancers have increased the overall survival rate in this population. For example, from 1991 to 2000, 79% of childhood cancer survivors were expected to be alive at 5 years and approximately 75% at 10 years, compared with 56% expected to live >_ 5 years after diagnosis during 1974-1976 [3].
As a growing number of long-term survivors reach adulthood [4], it has become increasingly evident that they may develop long-term effects from cancer treatment received as children [5-8]. Cancer researchers have followed their patients as long as 30 years after initial treatment to pinpoint medical, functional, and emotional problems that may occur as a result of treatment and to develop strategies for avoiding or addressing their treatment late effects [9]. Treatment side effects can involve several biological systems, with cardiopulmonary complications, endocrine effects, gonadal failure, neurocognitive deficits, and psychosocial impacts widely reported [10-14].
The impact of late effects on “function,” activities of daily living (ADL) ability, and quality of life has not been as clear, however. A review of the literature on ADL ability, including self-care or personal ADL and domestic or instrumental activities of daily living [1], shows that ADL ability/function is most commonly measured in the treatment phase. This is often for decision-making purposes, e.g., scheduling admission [15] or discharge [16], or to note patients' progress as they respond to chemotherapy [17]. In contrast, reports regarding long-term follow-up of late effects using explicit measures of ADL ability are rare.
Rather than using distinct measures of ADL, most late effects reports employ quality of life (QOL) and health-related quality of life (HRQOL) indicators, such as level of education achieved, insurability, employability, and marriageability, to represent “function.” This practice leads to conflicting results. For example, perceived health-related quality of life among cancer survivors has been reported as equal to or better than that of normal controls [18]. In contrast, Novakovic et al. [19] found that sarcoma survivors scored worse on functional status than controls, based on scores from the Karnofsky performance status scale [20]. In their review of quality of life studies, Langeveld et al. [21] noted that most did not even measure functioning with standardized, well-validated instruments. Another factor contributing to the confusion and contradiction around “functional” status are self-report instruments or those based on broad functional classifications, whose ambiguity results from many factors: known shortcomings of such instruments [22,23], potential for respondents to unintentionally inflate their own “functional” status (from lack of self-awareness, mild cognitive deficits, or short-term memory problems), and lack of specificity in scales employing broad categories of function to describe daily performance. Franklin (2007) observed that optimal functional assessment tools for cancer patients have yet to be identified [24]. The literature on late effects and “function” shows equivocal results with a range of problems from unaffected to some significant effects [10,21,25,26]. The inconsistency is plainly linked to methodological shortcomings, and specifically to the current state of “functional” outcome measurement in pediatric cancer survivors.
The purpose of our study was to examine the ADL functioning of a group of sarcoma survivors using a well standardized ADL performance-based assessment.
METHODS
Participants
The Pediatric Oncology Branch (POB) at the National Cancer Institute (NCI), National Institutes of Health (NIH) has treated pediatric sarcoma patients with multi-modality therapy over the past 35 years. Treatment late effects observed in this cohort have been reported in detail previously [27-29].
The present study, part of a larger multidisciplinary study at the NIH, was approved by the institutional review board of the NCI. All participants were enrolled after obtaining informed consent. Study participants included 32 persons (17 males, 15 females) ranging in age from 17 to 55 (mean age at time of treatment 16.2; range 7.1 to 34.2). All participants had been diagnosed with Ewing sarcoma family of tumors, rhabdomyosarcoma, and non-rhabdomysarcoma-soft tissue sarcoma and were enrolled an average of 17 years after treatment at the NIH. Table I provides a breakdown of gender, age at diagnosis, age at evaluation, time since treatment, site of lesion, diagnosis, and type of radiotherapy received.
Table I.
Patient Characteristics (n=32)
| Gender | |
| Male | 17 |
| Female | 15 |
| Age at Diagnosis | |
| 7-10 yrs | 4 |
| 11-20 yrs | 26 |
| 21-30 yrs | 1 |
| 31-35 yrs | 1 |
| Age at Evaluation | |
| 17-20 yrs | 2 |
| 21-30 yrs | 11 |
| 31-40 yrs | 8 |
| 41-50 yrs | 9 |
| 51-55 yrs | 2 |
| Time since Treatment | |
| 3-10 yrs | 7 |
| 11-20 yrs | 12 |
| 21-30 yrs | 10 |
| 31-33 yrs | 3 |
| Lesion Site | |
| Axial | 16 |
| Extremity without amputation | 12 |
| Extremity with amputation | 4 |
| Diagnosis | |
| Ewing's sarcoma family of tumors | 27 |
| Alveolar rhabdomyosarcoma | 3 |
| Primitive sarcoma of bone | 1 |
| Synovial sarcoma | 1 |
| Type of Irradiation | |
| Local therapy | 10 |
| Total body irradiation | 7 |
| Cranial irradiation | 6 |
| Whole lung irradiation | 5 |
| None | 4 |
For purposes of comparison, Assessment of Motor and Process Skills (AMPS) [1] ADL motor and ADL process ability measures were randomly drawn from three groups of well individuals without known medical or psychiatric illness, who are part of a standardization sample within the international AMPS database: one group matched for age and gender, and two groups matched for gender but 10 and 20 years older than study participants.
Instrumentation
The Assessment of Motor and Process Skills (AMPS) [1,30], a standardized objective measure, was used to evaluate the quality of ADL task performance. The AMPS evaluates 16 ADL motor (e.g., “reaches,” “walks,” “stabilizes,” “bends”) and 20 ADL process (e.g., “terminates,” “paces,” “continues,” “notices and responds”) skills that are the smallest observable units of ADL task performance. ADL motor skills are observable actions used to move oneself and task objects. ADL process skills are observable actions used to organize and adapt task actions to prevent or overcome problems. Eighty-five separate ADL tasks are standardized for use with the AMPS. During AMPS administration, an occupational therapist observes a client performing two culturally relevant ADL tasks with which he has familiarity and prior experience from the list of calibrated AMPS tasks, such as preparing breakfast or vacuuming a small room. The occupational therapist rates quality of performance on each of the 16 ADL motor and 20 ADL process skills using a four-point ordinal scale, ranging from deficient = 1 to competent = 4, according to very explicit, specific criteria.
The AMPS [1] has been standardized on over 125,000 clients worldwide. Studies support the reliability and validity of the AMPS across age groups and gender [31,32] and its sensitivity as an outcome measure [33-41].
Procedure
The AMPS [1] was administered to study subjects in an occupational therapy clinic mock apartment. Two occupational therapists trained in its use and calibrated with demonstrated rater reliability administered the AMPS interchangeably and per standardized procedure.
Data Analysis
AMPS computer-scoring software [42] was used to generate ADL motor and ADL process ability measures for each study participant. This program uses many-faceted Rasch analysis described elsewhere [1,43,44] to convert the raw, ordinal ADL motor and ADL process skill scores to linear ADL motor and ADL process ability measures expressed in equal-interval, log odds probability units, termed “logits.” Higher logits represent better ADL ability.
Means and 95% confidence intervals for ADL motor and ADL process ability measures were calculated for four groups of participants: 1) sarcoma survivors, 2) well individuals matched for age and gender, 3) well individuals matched for gender who were 10 years older than sarcoma survivors; and 4) well individuals matched for gender who were 20 years older than sarcoma survivors. Differences in mean ADL motor and ADL process ability measures for the four participant groups were determined using one-way analyses of variance with post-hoc Dunnett tests where sarcoma survivors were compared to each comparison group (p<0.05).
Additional analyses were performed to further describe sarcoma survivors. Pearson correlation coefficients were used to evaluate the relationships between ADL motor and ADL process ability measures versus 1) age at evaluation, 2) age at diagnosis, and 3) time since treatment among sarcoma survivors. An independent t-test was used to determine gender differences in ADL motor and process ability measures for sarcoma survivors. One-way analyses of variance with post-hoc Tukey tests were used to determine differences in ADL motor and process ability measures among three mutually exclusive sub-groups of sarcoma survivors by lesion site: axial lesions, extremity lesions without amputation, and extremity lesions with amputation. Sarcoma survivors were also classified into mutually exclusive sub-groups by the type of radiotherapy they received: total body irradiation, cranial irradiation, whole lung irradiation, local irradiation, or no irradiation (i.e., those who received surgery only) [27]. Two participants received both cranial and total body irradiation. Both were included in the sub-group with total body irradiation, because the long-term impact on function of the total body irradiation regimen is less widely reported than that of cranial irradiation [45,46]. Mean ADL motor and ADL process ability measures were compared between these treatment subgroups and the cohort of age and gender matched well adults using analyses of variance with post-hoc Dunnett tests (p<0.05).
RESULTS
Comparison of AMPS scores between sarcoma survivors and comparison groups
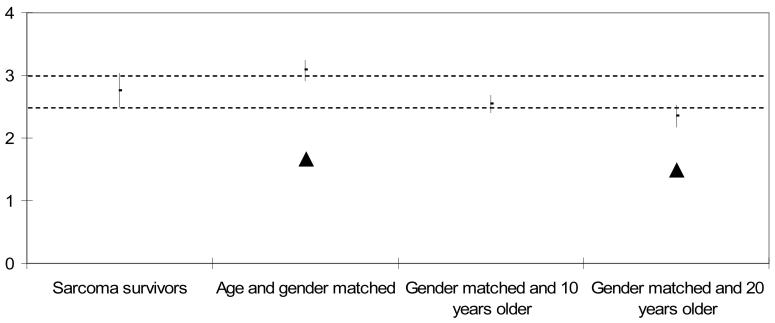
Means and 95% confidence intervals for ADL motor and ADL process ability measures for the sarcoma survivors and the three comparison groups are shown in Table II and Figures 1 and 2. Note that the age and gender matched well adults had the highest ability measures of any of the groups. It is also noteworthy that among the well comparison groups, ADL motor and ADL process ability tend to decline with increasing age, which is consistent with previous reports in the literature [47,48]. ADL motor ability was significantly lower for sarcoma survivors compared to the age and gender matched comparison group (p<0.05). There was no significant difference between the ADL motor ability of sarcoma survivors compared to the comparison group that was 10 years older, but sarcoma survivors had significantly better ADL motor ability (p<0.05) compared to the oldest comparison group (20 years older, Figure 1).
Table II.
Comparison of mean ADL motor and ADL process ability measures (±95% confidence intervals) for sarcoma survivors and comparison groups (p<0.05)
| Mean ± 95% confidence interval | |||
|---|---|---|---|
| Groups | Sample size |
ADL Motor Ability Measures (logits) |
ADL Process Ability Measures (logits) |
| Sarcoma survivors | 32 | 2.76 (2.49, 3.03) | 1.69 (1.58, 1.80) |
| Well adults matched for age and gender |
32 | 3.08 (2.92, 3.25)* | 2.28 (2.11, 2.44)* |
| Well adults matched for gender and 10 years older |
32 | 2.55 (2.41, 2.68) | 1.87 (1.71, 2.02) |
| Well adults matched for gender and 20 years older |
32 | 2.35 (2.18, 2.52)* | 1.57 (1.40, 1.74) |
Significantly different from sarcoma survivors
Figure 1. ADL Motor Ability Measures (logits) of Sarcoma Survivors and Comparison Groups. Mean (± 95% CI).
▲ Significantly different from sarcoma survivors
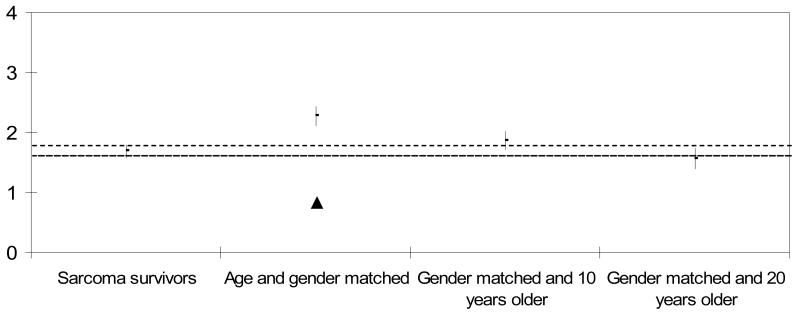
Figure 2. ADL Process Ability Measures of Sarcoma Survivors and Comparison Groups. Mean (± 95% CI).
▲ Significantly different from sarcoma survivors
There was a significant difference in ADL process ability between the sarcoma survivors and the age matched comparison group (p<0.05), such that the process ability of the sarcoma survivors was lower. There was no difference in ADL process ability between the sarcoma survivors and the comparison groups that were 10 and 20 years older (Figure 2).
Subgroup analyses of sarcoma survivors
There were no significant relationships between ADL motor or ADL process ability measures and 1) age at evaluation, 2) age at diagnosis, or 3) time since treatment among the sarcoma survivors. There were no gender differences in ADL motor and ADL process ability measures among the sarcoma survivors. Sarcoma survivors with extremity lesions requiring amputation had significantly lower ADL motor ability measures (1.60 +/− 0.23) than those with extremity lesions that did not require amputation (2.86 +/− 0.53) or those with axial lesions (2.98 +/− 0.73). However, there was no difference in ADL process ability measures for these three groups.
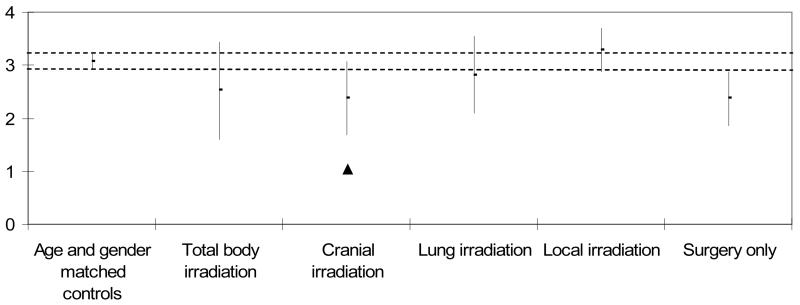
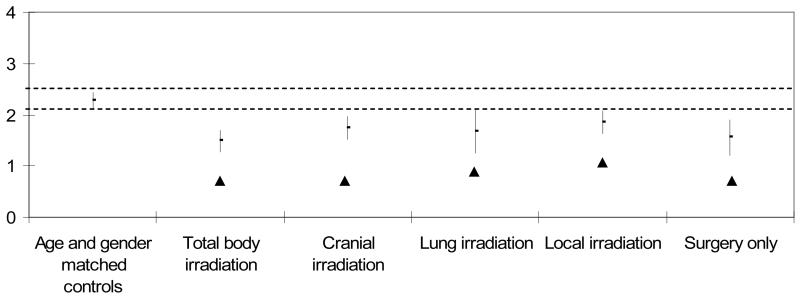
Table III and Figures 3 and 4 compare means and 95% confidence intervals for ADL motor and process ability measures for the five sarcoma survivor treatment subgroups to the age matched comparison group. Only the subgroup receiving cranial irradiation had significantly lower ADL motor ability measures compared to the age matched comparison group (p<0.05, Figure 3). ADL motor ability measures were equivalent between all other treatment subgroups compared to the age matched comparison group. All five treatment subgroups had significantly lower ADL process ability compared to the age matched comparison group (p<0.05, Figure 4).
Table III.
Comparison of mean ADL motor and ADL process ability measures (+95% confidence intervals) for treatment subgroups (among sarcoma survivors) and age matched comparisons. (p<0.05)
| Mean ± 95% confidence interval | |||
|---|---|---|---|
| Groups | Sample size |
ADL Motor Ability Measures (logits) |
ADL Process Ability Measures (logits) |
| Well adults matched for age and gender |
32 | 3.08 (2.92, 3.25) | 2.28 (2.11, 2.44) |
| Sarcoma survivors: (N=32) | |||
| Total body radiation | 7 | 2.52 (1.61, 3.43) | 1.49 (1.28, 1.70)† |
| Cranial radiation | 6 | 2.38 (1.69, 3.06)† | 1.74 (1.52, 1.96)† |
| Whole lung radiation | 5 | 2.82 (2.09, 3.55) | 1.68 (1.25, 2.11)† |
| Local irradiation | 10 | 3.29 (2.88, 3.69) | 1.86 (1.64, 2.08)† |
| No irradiation (surgery only) | 4 | 2.37 (1.86, 2.88) | 1.56 (1.21, 1.90)† |
Significantly lower than age and gender matched comparisons
Figure 3. ADL Motor Ability Measures (logits) by Cancer Treatment Group. Mean (± 95% CI).
▲ Significantly different from age and gender matched controls
Figure 4. ADL Process Ability Measures (logits) by Cancer Treatment Group. Mean (± 95% CI).
▲ Significantly different from age and gender matched controls
DISCUSSION
The purpose of this study was to examine the ADL functioning of a group of sarcoma survivors using an objective, well standardized performance-based assessment. The study showed that the ADL functioning of sarcoma survivors was worse than that of an age-matched comparison group.
Once this finding was established, the degree of deficit was characterized by comparison to standard performance of well subjects who were older than survivors. Selection of this approach was informed by the metabolic syndrome literature which shows “early senescence” of some organs as a late effect of sarcoma treatment, and the approach acknowledges results suggesting a higher incidence of metabolic syndrome traits in our cohort [49]. Although not suggesting a causative relationship between the metabolic syndrome traits of subjects and their ADL performance deficits, the results are analogous to findings for those with metabolic syndrome, who appeared to have early senescence of certain organs and systems secondary to sarcoma therapy. In this study, survivors performed worse than age matched well comparisons, but more like those 10 years older, and not as much reduced as those 20 years older on ADL motor ability. They performed worse than age matched comparisons, and more like those comparison groups both 10 and 20 years older on ADL process ability. It is possible that some of the factors hypothesized to contribute to the pathogenesis of the metabolic syndrome, such as increased stress, sedentary behavior, or decreased activity levels [49], also played a role in the lower functional performance measures on the AMPS.
Further data analysis was prompted by reports of specific effects of radiation therapy widely noted in the cancer literature [50,51]. Since there were differences between study subjects and well, age-matched comparison groups, ADL motor and process ability measures were analyzed to detect differences related to radiation therapy. The sarcoma survivor group was, therefore, divided into sub-groups by type of radiation therapy. The results are consistent with the findings of other authors [46] regarding adverse events and functional effects of radiation therapy: sarcoma survivors who received cranial radiation had significantly lower ADL motor ability measures than well comparison groups; all sub-groups of radiation therapy, as well as those who received surgery only, had significantly lower ADL process ability measures than well comparisons.
As explanation for these lower ADL process ability measures, several subjects demonstrated clear short-term memory problems, for example having trouble locating items previously shown to them in the kitchen. Fisher says, however, that even more than the direct demonstration of ability to organize a task and adapt when problems are encountered, the AMPS process scale provides further information regarding the extent to which the person has overcome residual neuromuscular, biomechanical, cognitive, and psychosocial impairments and capacity limitations by using alternative or compensatory strategies during task performance [1]. As a group, the study subjects demonstrated measurable limitations in their ability to overcome residual deficits that affect functional performance in daily life. For some sarcoma survivors, generalized motor difficulty affected overall ability to organize and adapt actions to complete a task, which in turn contributed to lower ADL process ability measures.
Over the past two decades, the cancer literature has reported variable results on functional abilities of long-term sarcoma survivors [52]. The selection of functional assessment tools for cancer patients has been particularly problematic, especially since many questionnaires completed by cancer patients were not designed specifically to elicit information about function [8].
Measurements of functional ability or deficit are only as valid as the instruments used to measure the ability; generic instruments, self-reports, and other commonly used tools lack accuracy in measuring actual ADL ability. Information from contradictory reports about ADL status in literature is not useful to clinicians who are following cancer survivors for late effects. Depending on the approach to data collection on functional status, there is a risk of “inflation” of the functional level of survivors, who may not be aware of their limitations or may not report them accurately due to short-term memory problems or other mild cognitive impairments. Functional impairments may be more widespread than currently suspected, because reported study outcomes are directly related to the type of instrument used to detect impairments. Continuing the practice of using a large gauge screen/sieve to evaluate survivors' functional impairments will mean that many subtle but life affecting problems/issues can be missed. Using a narrower, standardized, and witnessed screen is likely to give more accurate, real-life information on the functional status of survivors, particularly when ability to accurately self-report functional status is questionable.
Other authors [52] have confirmed our findings that cancer survivors are at increased risk for functional limitations in physical performance and in participation in activities needed for daily living, based on data abstracted from medical records and from completed questionnaires. The current study actually measured functional ability by a standardized observed performance assessment. This study was designed to see what could be measured by using an observed evaluation of functional performance with documented validity and reliability, expansive literature, but no accounts in this patient population.
This study is different from others cited, because it employed a direct observation using a well standardized, objective assessment to evaluate functional performance. Significant differences were found between our subjects' performance and that of matched comparisons on both ADL motor and ADL process skills. Patients treated for sarcoma as children may continue to have subtle effects on their daily life, even absent more obvious limiting conditions such as amputation or central nervous system involvement.
This study had several limitations, including a small cohort representing a broad diversity of age at diagnosis and variable time since completion of sarcoma therapy. It is not possible to attribute causative treatment factors to the diminished functional outcomes observed on testing; it is, however, worthy of note that the group had significantly worse motor and process skills than well, age-matched peers. Subtle functional deficits can affect independence in self-care, community skills, and vocational/academic success, and some of the impairments that yielded lower AMPS ability measures may also play a role in outcomes regarding employment, relationships, etc.
The results suggest that daily problems may be more routine than has been suggested previously, and consequently survivors will have effects that influence their success, effort, and satisfaction in personal and vocational pursuits [4]. The point in bringing this finding to the attention of practitioners whose caseload includes adult survivors of childhood sarcoma is that clinicians need to be aware of these potential vulnerabilities and ask more pointed questions concerning functional ability; ideally, clinicians and caregivers need to directly observe the performance of survivors.
It is clear that survivors need long-term follow up by informed caregivers who are able to give suitable advice that will help them to maintain their health and maximum social/functional level of performance [9,53-57]. During the course of long-term follow up, survivors are best helped by understanding their own potential vulnerabilities and risk factors so as to track them with appropriate regularity. In order to improve the health status of survivors, health care professionals are obligated to comprehensively educate their patients about anxiety-provoking cancer-related risks using methods to promote ongoing health monitoring and adherence to lifestyle practices that support risk reduction [25].
Finally, it is worth mentioning that many functional limitations from late effects may be remediable by appropriate referrals for rehabilitation interventions along the course of long-term survivorship [29], if survivors' health care providers are aware of the potential for these limitations and refer their patients timely to qualified rehabilitation professionals for appropriate evaluation and treatment [52,58-60].
CONCLUSIONS
Results from this study which employed a standardized, objective observational assessment suggest that the influences of treatment late effects on performance of daily life activities among pediatric sarcoma survivors are more widespread than reported, even among many survivors with no noticeable physical impairment. Knowledge of potential areas of functional deficit is important for clinicians of all types, and the findings reinforce the fact that these deficits may be underreported [61]. Such potential vulnerabilities should be added to the established list of possible late effects and this information incorporated into the educational offerings of clinics and practitioners following sarcoma survivors, so as to properly inform them of susceptibility profiles [62], risk factors, and predisposing factors that may have an influence on their effectiveness and success in daily life.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute. The opinions presented in this article reflect the views of the authors and not necessarily those of the National Institutes of Health or the U.S. Public Health Service. As we are U.S. federal employees, this article fits the description of a ‘U.S. Government Work’ and cannot be copyrighted (Copyright Revision Act, 1976). It is available for publication and there are no restrictions on its use, now or subsequently.
REFERENCES
- 1.Fisher AG. Assessment of Motor and Process Skills, vol. 1: Development, standardization, and administration manual. Three Star Press; Ft. Collins, CO: 2003. [Google Scholar]
- 2.Ries LG, Percy CL, Bunin GR. Introduction--SEER pediatric monograph. In: Ries LG, Smith MA, Gurney JG, et al., editors. Cancer incidence and survival among children and adolescents: United States SEER program 1975-1995. National Cancer Institute; Bethesda, MD: 1999. pp. 1–15. SEER program. NIH (Pub. No. 99-4649) [Google Scholar]
- 3.Rowland J, Mariotto A, Aziz N, et al. Cancer survivorship--United States, 1971-2001. MMWR. 2004;53(24):526–529. [PubMed] [Google Scholar]
- 4.Stiller CA. Population-Based Survival Rates for Childhood-Cancer in Britain, 1980-91. British Medical Journal. 1994;309(6969):1612–1616. doi: 10.1136/bmj.309.6969.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulhern RK, Palmer SL. Neurocognitive late effects in pediatric cancer. Curr Probl Cancer. 2003;27(4):177–197. doi: 10.1016/s0147-0272(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 6.Anderson DM, Rennie KM, Ziegler RS, et al. Medical and neurocognitive late effects among survivors of childhood central nervous system tumors. Cancer. 2001;92(10):2709–2719. doi: 10.1002/1097-0142(20011115)92:10<2709::aid-cncr1625>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Bhatia S. Late effects among survivors of leukemia during childhood and adolescence. Blood Cells Mol Dis. 2003;31(1):84–92. doi: 10.1016/s1079-9796(03)00072-x. [DOI] [PubMed] [Google Scholar]
- 8.Friedman DL, Meadows AT. Late effects of childhood cancer therapy. Pediatr Clin North Am. 2002;49(5):1083–1106, x. doi: 10.1016/s0031-3955(02)00032-9. [DOI] [PubMed] [Google Scholar]
- 9.Monaco GP, Fiduccia D, Smith G. Legal and societal issues facing survivors of childhood cancer. Pediatr Clin North Am. 1997;44(4):1043–1058. doi: 10.1016/s0031-3955(05)70544-7. [DOI] [PubMed] [Google Scholar]
- 10.Neglia JP. Late effects of treatment in children with cancer. Semin Pediatr Surg. 1993;2(1):29–36. [PubMed] [Google Scholar]
- 11.Sklar CA. Overview of the effects of cancer therapies: the nature, scale and breadth of the problem. Acta Paediatr Suppl. 1999;88(433):1–4. doi: 10.1111/j.1651-2227.1999.tb14395.x. [DOI] [PubMed] [Google Scholar]
- 12.Murray RD, Brennan BM, Rahim A, et al. Survivors of childhood cancer: long-term endocrine and metabolic problems dwarf the growth disturbance. Acta Paediatr Suppl. 1999;88(433):5–12. doi: 10.1111/j.1651-2227.1999.tb14396.x. [DOI] [PubMed] [Google Scholar]
- 13.Marina N. Long-term survivors of childhood cancer. The medical consequences of cure. Pediatr Clin North Am. 1997;44(4):1021–1042. doi: 10.1016/s0031-3955(05)70543-5. [DOI] [PubMed] [Google Scholar]
- 14.Boulad F, Sands S, Sklar C. Late complications after bone marrow transplantation in children and adolescents. Curr Probl Pediatr. 1998;28(9):273–297. doi: 10.1016/s0045-9380(98)80030-3. [DOI] [PubMed] [Google Scholar]
- 15.Gillis TA, Donovan ES. Rehabilitation following bone marrow transplantation. Cancer. 2001;92(s 4):998–1007. doi: 10.1002/1097-0142(20010815)92:4+<998::aid-cncr1412>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 16.Escalante CP, Weiser MA, Manzullo E, et al. Outcomes of treatment pathways in outpatient treatment of low risk febrile neutropenic cancer patients. Supportive Care in Cancer. 2004;12(9):657–662. doi: 10.1007/s00520-004-0613-6. [DOI] [PubMed] [Google Scholar]
- 17.Robinson DL, Carr BA. Delayed Vomiting in Children With Cancer After Receiving Moderately High or Highly Emetogenic Chemotherapy. Journal of Pediatric Oncology Nursing. 2007;24(2):70. doi: 10.1177/1043454206298840. [DOI] [PubMed] [Google Scholar]
- 18.Apajasalo M, Sintonen H, Siimes MA, et al. Health-related quality of life of adults surviving malignancies in childhood. European Journal of Cancer. 1996;32(8):1354–1358. doi: 10.1016/0959-8049(96)00024-x. [DOI] [PubMed] [Google Scholar]
- 19.Novakovic B, Fears TR, Horowitz ME, et al. Late effects of therapy in survivors of Ewing's sarcoma family tumors. J Pediatr Hematol Oncol. 1997;19:220–225. doi: 10.1097/00043426-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Karnofsky D. The clinical evaluation of chemotherapeutic agents in cancer. In: Macleod C, editor. Evaluation of chemotherapeutic agents. Columbia University; New York: 1949. pp. 199–205. [Google Scholar]
- 21.Langeveld NE, Stam H, Grootenhuis MA, et al. Quality of life in young adult survivors of childhood cancer. Support Care Cancer. 2002;10(8):579–600. doi: 10.1007/s00520-002-0388-6. [DOI] [PubMed] [Google Scholar]
- 22.Hutchinson TA, Boyd NF, Feinstein AR, et al. Scientific problems in clinical scales, as demonstrated in the Karnofsky index of performance status. J Chronic Dis. 1979;32(9-10):661–666. doi: 10.1016/0021-9681(79)90096-1. [DOI] [PubMed] [Google Scholar]
- 23.Yates JW, Chalmer B, McKegney FP. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer. 1980;45(8):2220–2224. doi: 10.1002/1097-0142(19800415)45:8<2220::aid-cncr2820450835>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 24.Franklin DJ. Cancer Rehabilitation: Challenges, Approaches, and New Directions. Physical Medicine & Rehabilitation Clinics of North America. 2007;18(4):899–924. doi: 10.1016/j.pmr.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Hudson M. Health Status of Adult Long-term Survivors of Childhood Cancer. JAMA. 2003;290(12):1583–1592. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- 26.Nagarajan R, Neglia JP, Clohisy DR, et al. Education, employment, insurance, and marital status among 694 survivors of pediatric lower extremity bone tumors - A report from the Childhood Cancer Survivor Study. Cancer. 2003;97(10):2554–2564. doi: 10.1002/cncr.11363. [DOI] [PubMed] [Google Scholar]
- 27.Mansky P, Arai A, Stratton P, et al. Treatment late effects in long-term survivors of pediatric sarcoma. Pediatr Blood Cancer. 2007;48(2):192–199. doi: 10.1002/pbc.20871. [DOI] [PubMed] [Google Scholar]
- 28.Wiener L, Battles H, Bernstein D, et al. Persistent psychological distress in long-term survivors of pediatric sarcoma: the experience at a single institution. Psychooncology. 2006;15(10):898–910. doi: 10.1002/pon.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerber LH, Hoffman K, Chaudhry U, et al. Functional outcomes and life satisfaction in long-term survivors of pediatric sarcomas. Arch Phys Med Rehabil. 2006;87(12):1611–1617. doi: 10.1016/j.apmr.2006.08.341. [DOI] [PubMed] [Google Scholar]
- 30.The Assessment of Motor and Process Skills (AMPS) 2009 Apr 2; < http://www.ampsintl.com/>. Accessed 2/4/2009. [PubMed]
- 31.Hayase D, Mosenteen D, Thimmaiah D, et al. Age-related changes in activities of daily living ability. Australian Occupational Therapy Journal. 2004 Dec;51(4):192–8. (42 ref) [Google Scholar]
- 32.Merritt BK, Fisher AG. Gender differences in the performance of activities of daily living. Arch Phys Med Rehabil. 2003;84(12):1872–1877. doi: 10.1016/s0003-9993(03)00483-0. [DOI] [PubMed] [Google Scholar]
- 33.Cooke KZ, Fisher AG, Mayberry W, et al. Differences in Activities of Daily Living Process Skills of Persons With and Without Alzheimer's Disease. OCCUPATIONAL THERAPY JOURNAL OF RESEARCH. 2000;20(2):87–104. [Google Scholar]
- 34.Graff MJL, Vernooij-Dassen MJM, Thijssen M, et al. Community based occupational therapy for patients with dementia and their care givers: randomised controlled trial. British Medical Journal. 2006;333(7580):1196–1201. doi: 10.1136/bmj.39001.688843.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kottorp A, Hällgren M, Bernspång B, et al. Client-centred occupational therapy for persons with mental retardation: implementation of an intervention programme in activities of daily living tasks. Scandinavian Journal of Occupational Therapy. 2003;10(2):51–60. [Google Scholar]
- 36.Linden A, Boschian K, Eker C, et al. Assessment of Motor and Process Skills reflects brain-injured patients' ability to resume independent living better than neuropsychological tests. Acta Neurologica Scandinavica. 2005;(111):48–53. doi: 10.1111/j.1600-0404.2004.00356.x. [DOI] [PubMed] [Google Scholar]
- 37.Oakley F, Duran L, Fisher AG, et al. Differences in motor skills in persons with and without Alzheimer's disease. Australian Occupational Therapy Journal. 2003;(50):72–78. [Google Scholar]
- 38.Oakley F, Khin N, Parks R, et al. Improvement in activitis of daily living in elderly following treatment for post-bereavement depression. Acta Psychiatrica Scandinavica. 2002;(105):231–234. doi: 10.1034/j.1600-0447.2002.1sc021.x. [DOI] [PubMed] [Google Scholar]
- 39.Oakley F, Sunderland T. The Assessment of Motor and Process Skills as a measure of IADL functionining in pharmacologic studies of people with Alzheimer's disease: a pilot study. International Psychogeriatrics. 1997;(9):197–206. doi: 10.1017/s1041610297004341. [DOI] [PubMed] [Google Scholar]
- 40.Wittenberg GF, Chen R, Ishii K, et al. Constraint-induced therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabilitation and Neural Repair. 2003;17(1):48–57. doi: 10.1177/0888439002250456. [DOI] [PubMed] [Google Scholar]
- 41.Fisher AG, Atler K, Potts A. Effectiveness of occupational therapy with frail community living older adults. Scandinavian Journal of Occupational Therapy. 2007;14(1):240–249. doi: 10.1080/11038120601182958. [DOI] [PubMed] [Google Scholar]
- 42.Fisher AG. AMPS computer-scoring software: Version 2005 [computer program] Three Star Press; Fort Collins, CO: 2005. 2005. [Google Scholar]
- 43.Gershon R. Computer Software. 3rd ed. Computer Adaptive Technologies; Evanston: 1999. Assessment of Motor and Process Skills Computer-Scoring Program. [Google Scholar]
- 44.Linacre J. Many-facet Rasch measurement. 2nd Edition MESA Press; Chicago: 1993. [Google Scholar]
- 45.Vannatta K, Gerhardt CA, Wells RJ, et al. Intensity of CNS treatment for pediatric cancer: Prediction of social outcomes in survivors. Pediatric Blood & Cancer. 2007;49(5):716–722. doi: 10.1002/pbc.21062. [DOI] [PubMed] [Google Scholar]
- 46.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. Jama. 2007;297(24):2705–2715. doi: 10.1001/jama.297.24.2705. [DOI] [PubMed] [Google Scholar]
- 47.Dickerson AE, Fisher AG. Age differences in functional performance. Am J Occup Ther. 1993;47(8):686–692. doi: 10.5014/ajot.47.8.686. [DOI] [PubMed] [Google Scholar]
- 48.Hayase D, Mosenteen D, Thimmaiah D, et al. Age-related changes in activities of daily living ability. Australian Occupational Therapy Journal. 2004;51(4):192–198. [Google Scholar]
- 49.Hoffman KE, Derdak J, Bernstein D, et al. Metabolic syndrome traits in long-term survivors of pediatric sarcoma. Pediatr Blood Cancer. 2008;50(2):341–346. doi: 10.1002/pbc.21363. [DOI] [PubMed] [Google Scholar]
- 50.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic Health Conditions in Adult Survivors of Childhood Cancer. New England Journal of Medicine. 2006;355(15):1572. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 51.Raney RB, Asmar L, Vassilopoulou-Sellin R, et al. Late complications of therapy in 213 children with localized, nonorbital soft-tissue sarcoma of the head and neck: A descriptive report from the Intergroup Rhabdomyosarcoma Studies (IRS)-II and - III. IRS Group of the Children's Cancer Group and the Pediatric Oncology Group. Med Pediatr Oncol. 1999;33(4):362–371. doi: 10.1002/(sici)1096-911x(199910)33:4<362::aid-mpo4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 52.Ness KK, Mertens AC, Hudson MM, et al. Limitations on physical performance and daily activities among long-term survivors of childhood cancer. Ann Intern Med. 2005;143(9):639–647. doi: 10.7326/0003-4819-143-9-200511010-00007. [DOI] [PubMed] [Google Scholar]
- 53.Rowland JH. Foreword: looking beyond cure: pediatric cancer as a model. J Pediatr Psychol. 2005;30(1):1–3. doi: 10.1093/jpepsy/jsi010. [DOI] [PubMed] [Google Scholar]
- 54.Neglia JP. Care and treatment of long-term survivors of childhood cancer. Cancer Supplement. 1993;71(10):3386–3391. doi: 10.1002/1097-0142(19930515)71:10+<3386::aid-cncr2820711742>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 55.Friedman DL, Freyer DR, Levitt GA. Models of care for survivors of childhood cancer. Pediatr Blood Cancer. 2006;46(2):159–168. doi: 10.1002/pbc.20611. [DOI] [PubMed] [Google Scholar]
- 56.Landier W, Wallace WH, Hudson MM. Long-term follow-up of pediatric cancer survivors: education, surveillance, and screening. Pediatr Blood Cancer. 2006;46(2):149–158. doi: 10.1002/pbc.20612. [DOI] [PubMed] [Google Scholar]
- 57.Ginsberg JP, Hobbie WL, Carlson CA, et al. Delivering long-term follow-up care to pediatric cancer survivors: transitional care issues. Pediatr Blood Cancer. 2006;46(2):169–173. doi: 10.1002/pbc.20610. [DOI] [PubMed] [Google Scholar]
- 58.Mellette SJ. Cancer rehabilitation. Vol. 85. © Oxford University Press; 1993. pp. 781–784. [DOI] [PubMed] [Google Scholar]
- 59.de Boer AG, Verbeek JH, van Dijk FJ. Adult survivors of childhood cancer and unemployment: A metaanalysis. Cancer. 2006;107(1):1–11. doi: 10.1002/cncr.21974. [DOI] [PubMed] [Google Scholar]
- 60.Marchese VG, Chiarello LA, Lange BJ. Effects of physical therapy intervention for children with acute lymphoblastic leukemia The study was performed at The Children's Hospital of Philadelphia. Pediatric Blood & Cancer. 2004;42(2) doi: 10.1002/pbc.10481. [DOI] [PubMed] [Google Scholar]
- 61.Eiser C, Levitt G, Leiper A, et al. Clinic audit for long-term survivors of childhood cancer. Arch Dis Child. 1996;75(5):405–409. doi: 10.1136/adc.75.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Snyderman R, Yoediono Z. Prospective care: a personalized, preventative approach to medicine. Pharmacogenomics. 2006;7(1):5–9. doi: 10.2217/14622416.7.1.5. [DOI] [PubMed] [Google Scholar]