Summary
TRPM7 is a Ca2+ and Mg2+ permeant ion channel in possession of its own kinase domain. In a previous study we showed that overexpression of the channel-kinase in HEK-293 cells produced cell rounding and loss of adhesion which was dependent upon the Ca2+-dependent protease m-calpain. The TRPM7-elicited change in cell morphology was channel-dependent and occurred without any significant increase in cytosolic Ca2+. Here we demonstrate that overexpression of TRPM7 increased levels of cellular reactive oxygen species (ROS) and nitric oxide (NO), causing the activation of p38 MAP kinase (p38 MAPK) and c-Jun N-terminal kinase (JNK). Application of inhibitors of p38 MAPK and JNK blocked TRPM7-induced cell rounding and activation of m-calpain, without affecting the phosphorylation state of the protease. Overexpression of TRPM7 increased intracellular Mg2+; however, when the concentrations of either external Ca2+ or Mg2+ was increased to favor permeation of one divalent cation over the other, a similar increase in cell rounding and calpain activity was detected, indicating that TRPM7-mediated activation of m-calpain is not dependent on the nature of the divalent conducted by the channel. Application of inhibitors of nitric oxide synthase and mitochondrial-derived ROS reduced TRPM7-induced increases in nitric oxide and ROS production, blocked the change in cell morphology, and reduced cellular calpain activity. Collectively, our data reveal that excessive TRPM7 channel activity causes oxidative and nitrosative stress, producing cell rounding mediated by p38 MAPK/JNK dependent activation of m-calpain.
Keywords: TRPM7, calpain, magnesium, calcium, cell stress
Introduction
TRPM7 is a bifunctional protein with ion channel and kinase activities.1,2 The channel-kinase has been implicated in an array of physiological processes, including magnesium and rare metal homeostasis, melanopore maturation and kidney stone formation, cell volume regulation, sensing of osmolarity and sheer stress, synaptic vesicle fusion, thymopoiesis, and cell adhesion.3-12 TRPM7 channel activity has also been reported to be involved in cell death during ischemia by increasing intracellular Ca2+ overload and oxidative stress.13,14 However, the specific mechanisms by which TRPM7 contributes to these pathological processes have not been completely resolved. In an earlier study we revealed that TRPM7 overexpression in HEK-293 cells caused a loss of cell adhesion that was dependent upon m-calpain, a calcium-dependent protease that has also been implicated in cellular ischemic responses.12 Our studies further showed that TRPM7-mediated cell rounding was dependent upon the activity of the protein’s channel domain but not its kinase domain. However, overexpression of TRPM7 had little to no effect on cytoplasmic Ca2+ concentrations, obscuring insights into how exactly TRPM7 was able to stimulate m-calpain activity.
In our earlier work we also discovered that both TRPM7 and m-calpain localized to peripheral adhesion complexes in HEK-293 cells. We therefore considered, as an explanation for how TRPM7 activated m-calpain, the possibility that local Ca2+ influx mediated by the channel caused a local increase in Ca2+ that was sufficient to stimulate m-calpain without raising cytoplasmic Ca2+ concentrations. However, TRPM7’s extremely small inward conductance in conjunction with the high micromolar Ca2+ concentration required for activation of m-calpain led us to reexamine whether Ca2+ influx through the channel was sufficient to activate the protease in this manner.
In addition to Ca2+, m-calpain can also be activated through direct phosphorylation by ERK.15 We therefore sought to determine whether overexpression of TRPM7 activated ERK to stimulate m-calpain activity. To our surprise, overexpression of TRPM7 in HEK-293 cells suppressed ERK activation, but did stimulate activation of p38 MAP kinase (MAPK) and c-Jun N-terminal kinase (JNK), both of which are members of the MAP kinase family involved in cellular stress responses. Consistent with this result, our experiments also revealed that cell rounding elicited by TRPM7 could be prevented by a pharmacological blockade of p38 MAPK and JNK. The Stress Activated Protein Kinases (SAPK) p38 MAPK and JNK are activated by environmental insults to the cell which cause cell stress (free radicals, changes in osmolarity, sheer fluid flow) as well as by receptor signaling cascades.16,17 Our studies revealed that TRPM7 overexpression increased levels of reactive oxide species (ROS) and nitric oxide (NO) and that pharmacological inhibition of nitric oxide synthase (NOS) and mitochondrial-derived ROS attenuated TRPM7-induced cell rounding, indicating that TRPM7 overexpression caused oxidative and nitrosative stress to stimulate p38 MAPK and JNK. Surprisingly, application of inhibitors of p38 MAPK and JNK reduced m-calpain activity caused by channel overexpression without affecting the phosphorylation state of m-calpain. As the cell rounding caused by TRPM7 overexpression was not dependent on which divalent cation (Mg2+ or Ca2+) was permeated by the channel, these studies suggest that unrestrained influx of Mg2+ as well as Ca2+ through the channel elicits oxidative and nitrosative cell stress to cause p38 MAPK and JNK dependent activation of m-calpain without its direct phosphorylation.
Results
In a previous study we showed that TRPM7 caused cell rounding and loss of adhesion in a channel-dependent and kinase-independent manner.12 Cell rounding caused by TRPM7 could be prevented by pharmacological inhibition of m-calpain as well as by reduction of m-calpain protein levels by RNA interference. Ca2+ imaging revealed that TRPM7 overexpression did not induce a rise in cytoplasmic Ca2+ levels above basal levels. Immunocytochemical analysis of the distribution of TRPM7 and m-calpain in HEK-293 cells showed that both proteins localized to peripheral adhesion complexes, which supported the hypothesis that TRPM7 activated the Ca2+-dependent protease by raising local Ca2+ to a concentration sufficient to stimulate m-calpain. However, TRPM7’s extremely small inward conductance coupled with the elevated micromolar Ca2+ dependence of m-calpain motivated us to investigate whether TRPM7-mediated Ca2+ influx indirectly activated the protease.
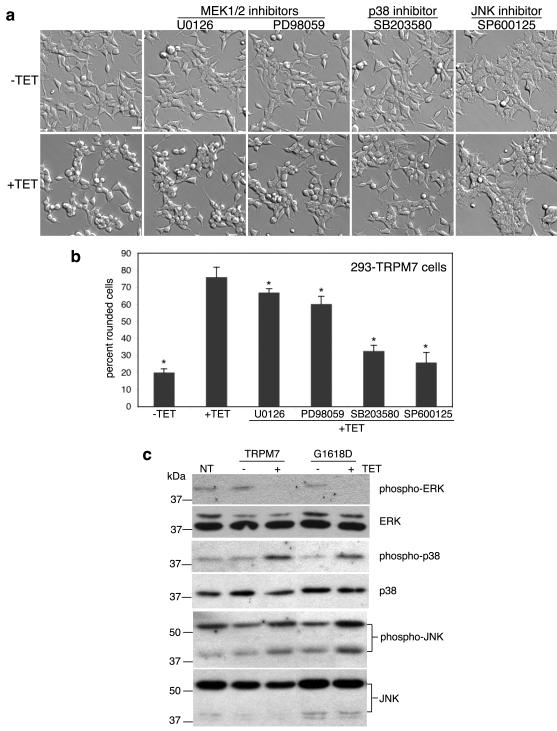
ERK, a member of the mitogen activated protein (MAP) kinase family, phosphorylates m-calpain on serine 50 resulting in its activation.15 ERK is itself activated by the MAP kinase kinase MEK1/2, whose activity can be stimulated downstream by growth factor receptors. To test whether ERK was involved in regulating TRPM7-mediated cell rounding, we utilized the 293-TRPM7 cell line that inducibly expresses TRPM7 when tetracycline (TET) is added to the growth media.12 Application of the MEK1/2 inhibitors U0126 (5 μM) and PD98059 (10 μM) to 293-TRPM7 cells partially blocked cell rounding (Fig 1a&b). Since the concentrations of the MEK1/2 inhibitors used were within the doses used to completely block MEK1/2 in vivo,18,19 we considered whether the blocking effect of these inhibitors was due to off-target effects on other MAP kinase family members. Related to ERK in structure and mechanism of activation are p38 MAPK and JNK. To test the involvement of p38 MAPK and JNK, we applied selective inhibitors of these kinases (SB203580 for p38 MAPK (1 μM) and SP600125 for JNK (20 μM)) to 293-TRPM7 expressing cells and found that both compounds effectively blocked TRPM7-induced cell rounding (Fig 1a&b). This data suggested that p38 MAPK and JNK quite possibly became activated in response to TRPM7 overexpression. Indeed, SDS-PAGE and western blot analysis of lysates derived from cells expressing TRPM7 or the kinase-inactive TRPM7-G1618D mutant revealed that p38 MAPK and JNK were stimulated by approximately 4- and 2-fold, respectively. In contrast, overexpression of TRPM7 and TRPM7-G1618D reduced ERK activation (Fig 1c). These results were in keeping with previous studies showing that an increase in p38 MAPK and JNK activity is often accompanied by a decrease in ERK activity during cell stress.20 Importantly, our findings suggested that TRPM7-mediated stimulation of p38 MAPK and JNK regulated m-calpain activation. Consistent with this conjecture, application of p38 MAPK and JNK inhibitors reduced in vivo calpain activity and suppressed proteolysis of the well-known m-calpain substrate talin in cells overexpressing TRPM7 (Fig 2a,b,c). In light of ERK’s ability to stimulate m-calpain through phosphorylation, we employed [32P]orthophosphate labeling experiments to determine whether the channel-kinase caused an increase in m-calpain phosphorylation; however, no increase in the incorporation of phosphate was detected upon TRPM7 overexpression (Fig 2d&e). We also ruled out that the SAPK inhibitors interfered with TRPM7-mediated activation of m-calpain by inhibiting TRPM7 channel activity or its expression. Electrophysiological analysis revealed that the p38 MAPK inhibitor SB203580 had no effect on current amplitude (Fig 3d) or on TRPM7 expression (Supplementary Fig 1a). However, the JNK inhibitor SP600125 blocked TRPM7 channel activity by approximately 63% at the concentration employed in our assay (Fig 3a,b,c) without affecting expression of the channel (Supplementary Fig 1a). This data indicated that the JNK inhibitor may be suppressing TRPM7-induced cell rounding by inhibiting TRPM7 channel conductance and by the compound’s blocking of JNK activity. Tellingly, this data also suggested that TRPM7-mediated m-calpain activation and cell rounding were a stress response caused by overexpression of the channel, because the SAPKs p38 MAPK and JNK are predominantly activated by cellular stress in the absence of receptor stimulation.
Figure 1.
Overexpression of TRPM7 in HEK-293 cells activates p38 MAPK and JNK, but suppresses ERK. (a) Application of the p38 MAPK inhibitor SB203580 (1 μM) and JNK inhibitor SP600125 (20 μM) to 293-TRPM7 cells treated with tetracycline (+TET) for 24 hours reduced TRPM7-induced cell rounding to levels similar to cells grown in the absence of tetracycline. In contrast, treatment of cells with the MEK1/2 inhibitors U0126 (5 μM) and PD98059 (10 μM) had only a partial effect. Scale bar is equal to 100 μm. (b) Quantification of the degree of cell rounding under the conditions depicted in (a). Values are mean ± standard deviation of at least three independent experiments. A χ2 test was employed to assess differences in cell rounding between 293-TRPM7 cells treated with the different inhibitors. An asterisk indicates treatments that produced a decrease in cell rounding that was significantly different from 293-TRPM7 cells grown in tetracycline. (c) Western blots showing expression of TRPM7 or kinase-inactive TRPM7-G1618D mutant (+TET) caused a modest increase in p38 MAPK and JNK activities and a reduction in ERK activation. “NT” is non-transfected HEK-293 cells.
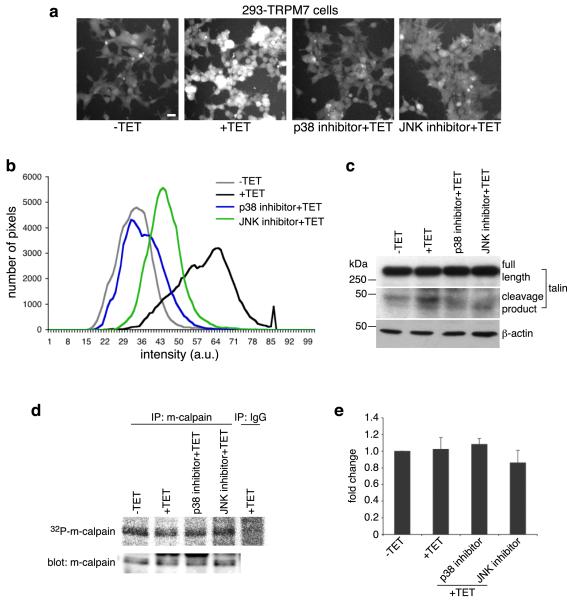
Figure 2.
p38 MAPK and JNK are required to stimulate m-calpain activity when TRPM7 is overexpressed. (a) Calpain activity assay using fluorogenic BOC-LM-CMAC substrates. Overexpression of TRPM7 (+TET) stimulated intracellular cleavage of the synthetic calpain substrate producing greater cellular fluorescence emission compared to control cells (−TET). Treatment of 293-TRPM7 expressing cells with p38 MAPK inhibitor SB203580 and JNK inhibitor SP600125 inhibited the TRPM7-dependent increase in fluorescence emission. Scale bar is equal to 100 μm. The experiment was repeated three times with similar results. (b) The intensity of fluorescence emission from cells under the conditions depicted in (a) was quantified and displayed as a histogram of the distribution of intensity. The x-axis represents fluorescence emission intensity and the y-axis indicates the number of pixels found for each respective intensity. (c) Western blots using a monoclonal antibody that recognizes the head domain of talin was used to probe cell lysates from 293-TRPM7 cells treated with tetracycline in the presence of various inhibitors. Expression of TRPM7 caused an increase in the cleavage of m-calpain substrate talin into its head and rod domains. The head domain cleavage product migrated at 47 kDa. Application of p38 MAPK inhibitor SB203580 and JNK inhibitor SP600125 reduced proteolysis of talin in response to TRPM7 expression. A western blot of β-actin is shown to demonstrate equal loading of samples. (d) In vivo [32P]orthophosphate labeling of 293-TRPM7 cells treated with tetracycline in the presence of various inhibitors. Shown is an autoradiograph of incorporation of [32P] into m-calpain immunoprecipitated from cell lysates. An anti-m-calpain blot is shown to demonstrate the quantity of immunoprecipitated protein. (e) Quantification of [32P]orthophosphate incorporation normalized to m-calpain protein. Results are presented as fold change compared to the negative control (−TET). Values are mean ± standard deviation of five independent experiments.
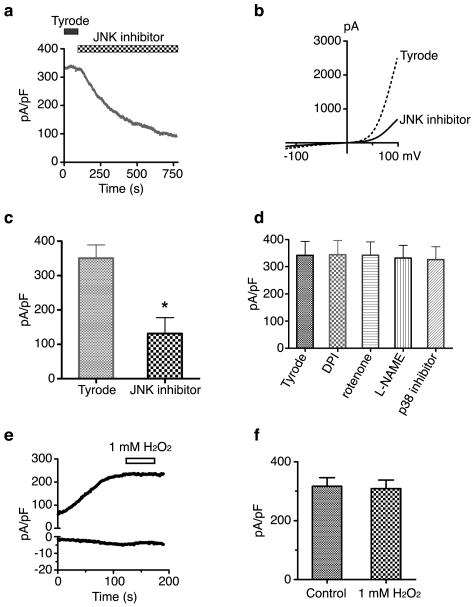
Figure 3.
The effects of SAPK, mitochondrial, and NOS inhibitors on TRPM7 channel activity. (a) Application of the JNK inhibitor SP600126 (20 μM) decreased TRPM7 current amplitude over time. (b) Representative trace showing TRPM7 current-voltage relationship before and after application of the JNK inhibitor. (c) TRPM7 outward current was blocked by the JNK inhibitor by approximately 63% at +100 mV (N=5). The asterisk indicates a significant difference in TRPM7 current compared to the negative control (Tyrode) using a Student t test (p<0.01). (d) TRPM7 current density at +100 mV was not changed by the application of DPI (1 μM), rotenone (100 nM), L-NAME (2 mM), and the p38 inhibitor SB203580 (40 μM) (N=4). (e) Representative trace showing that application of 1 mM H2O2 did not change TRPM7 inward or outward conductance over time. (f) TRPM7 current density at +100 mV was unchanged by the application of 1 mM H2O2 (N=8).
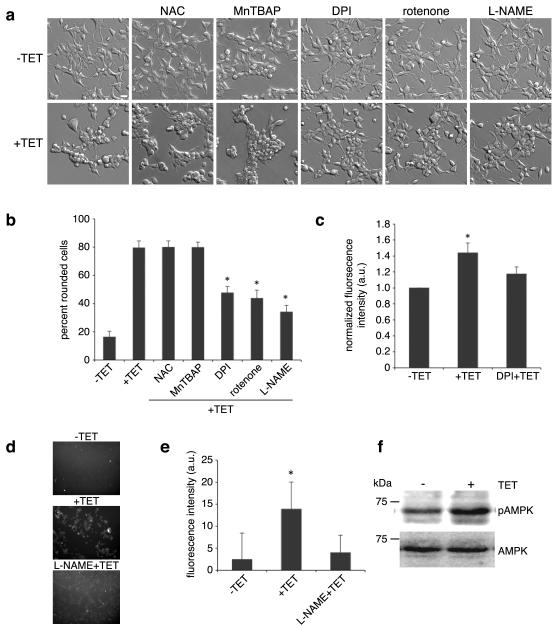
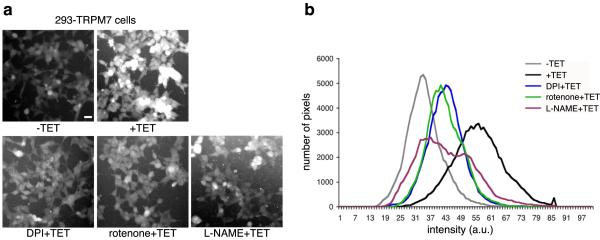
Environmental insults stimulate the production of reactive oxygen species (ROS) and nitrogen containing free radicals to cause the activation of p38 MAPK and JNK.16,17 A study by Aarts and colleagues demonstrated that TRPM7 contributes to Ca2+ overload leading to the production of ROS and nitrogen containing free radicals, which ultimately causes the demise of neurons subjected to oxygen glucose deprivation.13 Their study also suggested that TRPM7 participates in a positive feedback loop in which mitochondrially derived superoxide (O2−) combines with nitric oxide (NO) to form peroxynitrite (OONO−) radicals to further potentiate TRPM7 channel activity. This finding motivated us to examine whether ROS may be involved in TRPM7-mediated cell rounding. Application of the ROS scavenger N-acetyl cysteine (NAC) or the cell permeable superoxide dismutase mimetic MnTBAP failed to appreciably block TRPM7-induced cell rounding (Fig 4a&b). However, treatment of 293-TRPM7 cells with diphenyleneiodonium chloride (DPI) or rotenone, which interferes with the production of ROS through blockade of mitochondrial NADH-ubiquinone oxido-reductase in complex I of the mitochondrial respiratory chain, attenuated cell rounding (Fig 4a&b).21 DPI has been reported to inhibit nitric oxide synthase (NOS) as well as NADPH-ubiquinone oxido-reductase.22,23 To test the involvement of NOS-derived free radicals, such as peroxynitrite, in TRPM7-mediated cell rounding, we applied the NOS inhibitor L-NAME to 293-TRPM7 expressing cells and observed a large reduction in cell rounding, indicating that ROS and NOS-derived free radicals likely contribute to the observed change in cell morphology (Fig 4a&b). Consistent with this result, overexpression of TRPM7 stimulated an increase in ROS production that could be attenuated by the application of DPI (Fig 4c). In addition, the application of L-NAME reversed TRPM7-dependent NO production (Fig 4d&e). However, DPI, rotenone, and L-NAME did not alter TRPM7 channel activity (Fig 3d) or its expression (Supplementary Fig 1b). A similar finding for rotenone was obtained in an earlier study of the regulation of TRPM7 currents by mitochondria.24 To further clarify the role of ROS in regulating TRPM7 channel activity, we applied H2O2, from which hydroxyls are produced by the Fenton reaction at several concentrations during whole-cell clamp recordings of TRPM7. Application of H2O2 for approximately 1 to 2 minutes had no effect on TRPM7 inward or outward currents at concentrations up to 1 mM (Fig 3e&f). Together, these results indicated that L-NAME, DPI, and rotenone were not influencing TRPM7 channel activity, but were likely interfering with TRPM7-induced cell rounding by disrupting the production of free radicals. This conclusion is supported by the fact that AMP-dependent kinase (AMPK), which is stimulated by ROS, is activated 44% by TRPM7 overexpression (Fig 4f).25,26 In addition, we found that the application of DPI, rotenone, and L-NAME reduced calpain activity in TRPM7 expressing cells (Fig 5). Thus, the generation of ROS and nitrogen reactive compounds activated SAPKs, which in turn stimulated m-calpain activity to produce cell rounding. Whether Ca2+ entry by itself had a direct role in orchestrating these events remained unresolved.
Figure 4.
The effects of ROS scavengers or inhibitors of mitochondrial- and NOS-derived free radicals on TRPM7-mediated cell rounding. (a) Application of ROS scavenger NAC (5 μM) and MnTBAP (200 μM) had no effect on the TRPM7-induced cell rounding. In contrast, application of DPI (0.2 μM) and rotenone (10 nM) attenuated cell rounding. Treatment of cells with NOS inhibitor L-NAME (2 mM) also reduced cell rounding. Scale bar is equal to 100 μm. (b) Quantification of the degree of cell rounding under the conditions depicted in (a). An asterisk indicates treatments that produced a decrease in cell rounding that were significantly different from 293-TRPM7 cells grown in tetracycline using χ2 test. (c) Measurement of ROS generation in 293-TRPM7 cells. Overexpression of TRPM7 (+TET) resulted in a modest increase in ROS generation compared to control cells (−TET) that can be reversed with DPI. Average of fluorescence intensity was normalized to control cells (−TET). Values are mean ± standard deviation of three independent experiments. The asterisk indicates a signification difference in fluorescence intensity compare to control cells using a Student t test (p<0.05). (d) Fluorescence emission of 293-TRPM7 cells labeled with the nitric oxide (NO) indicator DAF-FM diacetate. Overexpression of TRPM7 (+TET) resulted in an increase in NO over non-expressing cells (−TET) that can be reversed with L-NAME (2 mM). (e) Quantification of the fluorescence intensity under the condition depicted in (d). Values are mean ± standard deviation of three independent experiments. The asterisk indicates a signification difference in fluorescence intensity compared to the negative control (−TET) using a Student t test (p<0.05). (f) Western blot with an antibody against AMP-dependent kinase (AMPK) and the Thr172-phosphorylated “activated-form” of AMPK indicates that overexpression of TRPM7 increases AMPK activation by 44%. The AMPK experiments were repeated three times with similar results.
Figure 5.
Inhibitors of ROS production and NOS attenuate TRPM7-dependent elevation of cellular calpain activity. (a) Calpain activity assay showing application of DPI, rotenone, and L-NAME caused a decrease in fluorescence emission intensity in 293-TRPM7 expressing cells (+TET). Scale bar is equal to 100 μm. (b) The intensity of fluorescence emission from cells under the conditions depicted in (a) was quantified and displayed as a histogram of the distribution of intensity as described in Figure 2b. The calpain activity assay was repeated three times with similar results.
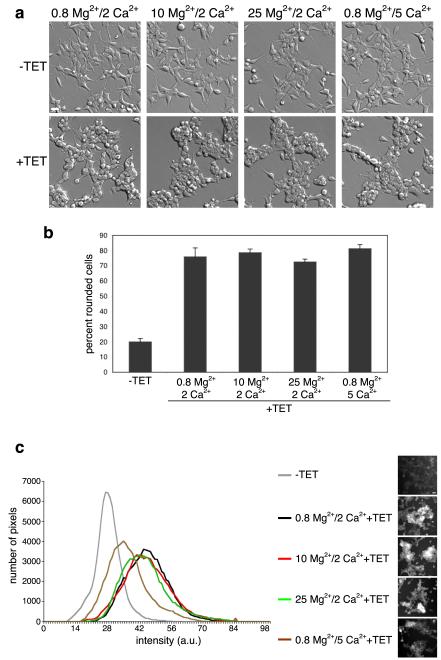
We had previously shown that overexpression of TRPM7 does not cause a significant increase in intracellular free Ca2+.12 However, an increase in local Ca2+ concentrations surrounding the channel may be sufficient to activate m-calpain. To test whether TRPM7-mediated Ca2+ influx was required for the cell rounding caused by overexpression of the channel, we tested whether increasing extracellular concentrations of Mg2+ would interfere with this effect. The rationale for this approach is based upon the observation that Ca2+ and Mg2+ bind with similar micromolar affinities to TRPM7’s pore: 3.6 μM for Mg2+ and 4.1 μM for Ca2+ at −120 mV.27 Thus, increasing the extracellular concentration of Mg2+ favors entry of this divalent cation over Ca2+ and should reduce TRPM7-induced cell rounding if this effect is solely dependent upon Ca2+ entry. To our surprise, raising the concentration of extracellular Mg2+ roughly 5 to 15 fold over Ca2+ had no effect on the change in cell morphology (Fig 6a&b). In addition, the cell rounding produced by TRPM7 overexpression persisted in the reverse experiment when the concentration of extracellular Ca2+ was 5 fold greater than Mg2+. For all the conditions tested, including when the concentration of Mg2+ was 15 fold higher than Ca2+, an increase in cellular calpain activity was detected (Fig 6c). Concentrations of free Ca2+ at 0 or above 5 mM were not tested, however, due to cell toxicity. Expression of a variant of TRPM7 containing the E1047K substitution, which renders the channel inactive, failed to produce cell rounding (Supplementary Fig 2).27 The above data indicates that TRPM7-induced cell rounding and activation of m-calpain were channel dependent, but were independent of the nature of the divalent cation conducted by the channel.
Figure 6.
TRPM7-mediated cell rounding is independent of the divalent cation permeated by the channel. (a) Cell rounding analysis of 293-TRPM7 expressing cells (+TET) after 24 hours of culture in regular DMEM media, which contained 0.8 mM Mg2+/ 2 mM Ca2+, or regular DMEM media with a total of 10 mM Mg2+, 25 mM Mg2+ or 5 mM Ca2+. (b) Quantification of the degree of cell rounding under the conditions depicted in (a). (c) Calpain activity assay of 293-TRPM7 expressing cells (+TET) under the conditions described in (a). Scale bar is equal to 100 μm. The experiments were repeated three times with similar results.
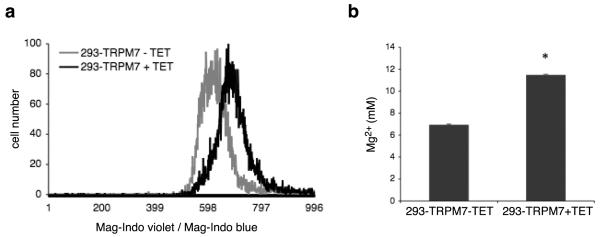
Since Mg2+ influx through the channel appeared sufficient to cause cell rounding, we asked whether overexpression of TRPM7 caused an increase in cellular Mg2+. Interestingly, we detected an elevation in free and total Mg2+ concentrations in HEK-293 cells overexpressing TRPM7 by flow cytometry using Mag-Indo-1 and by ICP-MS, respectively (Fig 7a&b). The detected increase in free Mg2+ did not appear to be due to binding of Ca2+ in the endoplasmic reticulum (ER) to the Mag-Indo-1 probe used in these experiments, as treatment of cells with thapsigargin, which depletes the ER of Ca2+, failed to prevent the TRPM7-induced increase in cellular free Mg2+ (Supplementary Fig 3). As a positive control, we showed that application of CCCP, which causes release of Mg2+ from mitochondria, increased free Mg2+ (Supplementary Fig 3).28 Interestingly, knockdown of TRPM7 in HEK-293 cells did not lower free Mg2+ concentrations or affect the levels of activated p38 MAPK and JNK (Supplementary Fig 4). Since the JNK inhibitor SP600125 blocks TRPM7 channel activity (Fig 3a,b,c), we next asked whether application of the inhibitor to 293-TRPM7 cells would prevent the increase in cellular free Mg2+ caused by TRPM7 overexpression. Application of the JNK inhibitor did not block the TRPM7-induced increase in free Mg2+ or the increase in ROS levels, indicating that TRPM7 is not the primary target of SP600125 within the context of our cell rounding assays (Supplementary Fig 5). So while cytoplasmic Ca2+ levels are not increased upon TRPM7 overexpression, the concentration of Mg2+ does become elevated (Fig 7).12 However, Mg2+ itself does not activate m-calpain, but likely contributes to cellular stress.29 Together the data support a model in which TRPM7-induced cell rounding is caused by p38 MAPK/JNK-dependent activation of m-calpain, which is initiated by increased production of ROS and nitrogen-containing free radicals as cells respond to heightened conductance of divalent cations through the channel.
Figure 7.
Overexpression of TRPM7 elevates cellular Mg2+ levels. (a) Measurement of intracellular free Mg2+ in 293-TRPM7 cells. Cells were loaded with the fluorescent ratiometric indicator Mag-Indo-1. Baseline Mag-Indo violet to Mag-Indo blue ratios were measured using a Beckman Coulter MoFlo XDP cell sorter. Fluorescence emission ratios were displayed using a histogram. Overexpression of TRPM7 (+TET) caused an increase in intracellular free Mg2+ compared to control cells (293-TRPM7-TET). Data are representative of five independent experiments. (b) ICP-MS quantitation of total Mg2+ in HNO3 extracts of 293-TRPM7 cells with and without tetracycline treatment, where average concentration of total cellular Mg2+ was calculated by normalizing to a [K+] of 120 mM. Values are mean ± standard deviation of at least two independent experiments. The asterisk indicates a significant difference in total Mg2+ compared to the negative control (293-TRPM7-TET) using a Student t test (p<0.05).
Discussion
Excessive TRPM7 channel activity has been implicated in neuronal cell death during anoxia, in which the demise of the cells has been largely attributed to the detrimental effects of Ca2+ overload.13 In this study, however, we reveal that overexpression of TRPM7 produces a cascade of events as a result of cell stress caused by increased constitutive permeation of Ca2+ as well as Mg2+ into the cell. The cell rounding resulting from increased TRPM7 channel activity could be successfully blocked by inhibitors of the SAPKs p38 MAPK and JNK as well as by DPI, rotenone, and L-NAME, compounds that interfere with the concomitant production of ROS and other nitrogen-containing free radicals which are key to the activation of these stress-dependent MAP kinases.
The results of our study also indicate that m-calpain can be activated independent of a large increase in cytoplasmic Ca2+ concentrations. Indeed, supplementation of the growth media with up to an approximately 15 fold excess of Mg2+ over Ca2+, which overwhelming favors Mg2+ permeation by TRPM7, still produced cell rounding and activation of m-calpain. We can therefore confidently conclude that Ca2+ overload is not responsible for m-calpain activation under these circumstances. Rather, our data demonstrate the involvement of SAPKs in the stimulation of m-calpain activity, a result supported by an earlier study of the glycoprotein VI-dependent procoagulant response in platelets, in which application of the p38 MAPK inhibitor SB203580 (employed in our experiments) downregulated m-calpain activity.30 ERK has been shown to stimulate m-calpain through direct phosphorylation on Ser50, however, our in vivo labeling experiments did not reveal an increase in 32P incorporation into m-calpain, indicating that the regulation of m-calpain by p38 MAPK and JNK is likely not direct. Numerous proteins are known to bind to m-calpain and modulate its activity, including calpastatin. Thus, there could be multiple ways by which p38 MAPK and JNK promote m-calpain proteolysis of its target substrates. Future work will be devoted to investigating SAPKs’ regulation of m-calpain activity and the role of TRPM7 in this process. Indeed, our results strongly indicate that SAPKs should not be overlooked as potential regulators of this important Ca2+-dependent protease, especially during stress-related disease states and conditions such as ischemia during which TRPM7 channel activity is reported to be elevated.13 However, as p38 MAPK and JNK can presumably be activated independent of TRPM7, it will also be important to investigate the role of these kinases in regulating m-calpain in processes in which the channel is not necessarily involved.
Nevertheless, given TRPM7’s association with anoxic neuronal cell death, the results from our study support the hypothesis that unrestrained entry of divalent cations other than Ca2+ may also contribute to the demise of cells. Indeed, permeation of Mg2+ through TRPM7 was as effective as Ca2+ in stimulating cell rounding. Aside from the established role of Ca2+ overload in several pathological conditions, the accumulation of the trace metal ion Zn2+ has been implicated in ischemic brain damage and the pathogenesis of chronic neurodegenerative conditions such as Alzheimer’s disease.31,32 During pathological states unrestrained entry of multiple types of divalent cations, such as Ca2+, Mg2+ as well as the trace metal ion Zn2+, all of which are capable of permeating this unique bifunctional channel, may be more overwhelming to a cell’s defenses than previously imagined.8
Finally, our study shows that TRPM7 by itself is not solely required for cellular Mg2+ homeostasis. As the concentration of free Mg2+ in TRPM7 knockdown cells was not lower than controls, our data indicate that other cellular processes are at work in the maintenance of Mg2+ homeostasis. For example, Zhou and colleagues recently showed that the Mg2+ transporter MagT1 is up-regulated in HEK-293 cells when the concentration of extracellular Mg2+ in the growth media is reduced.33 It will be important to test whether other Mg2+ transport mechanisms can compensate for depletion or loss of TRPM7. Our future work will be directed at testing this hypothesis and uncovering the feedback signaling pathways involved in these compensatory responses.
Materials and Methods
Chemical Reagents
All chemicals, unless otherwise stated, were from Sigma (St. Louis, MI). The inhibitors U0126 (MEK1/2), PD98059 (MEK1/2), SP600125 (JNK), SB203580 (p38 MAPK), diphenyleneiodonium chloride or DPI (mitochondrial NADH-ubiquinone oxido-reductase), rotenone (mitochondrial NADH-ubiquinone oxido-reductase), Mn(III)tetrakis(4-benzoic acid)porphyrin chloride or MnTBAP (cell-permeable superoxide dismutase (SOD) mimetic and peroxynitrite scavernger), and NG-Nitro-L-arginine Methyle Ester or L-NAME (NOS) were from Calbiochem/EMD Biosciences (San Diego, CA). Mag-Indo-1, Fluo-4-AM, CM-H2DCFDA, t-butoxycarbonyl-Leu-Met-chloromethylaminocoumarin (BOC-LM-CMAC), and 4-amino-5-methylamino-2′,7′-difluorescein diacetate (DAF-FM diacetate) were from Molecular Probes (Eugene, OR).
Cell Lines
A description and characterization of the 293-TRPM7 cell line expressing Hemagglutinin (HA) tagged murine TRPM7 (GenBank Accession # AF376052), of the 293-TRPM7-G1618D expressing the kinase-dead G1618D mutant of the channel, of the 293-M7shRNA2 cells expressing a shRNA targeting human TRPM7 under tetracycline control, and of the 293-shRNA-C cells expressing a non-silencing control shRNA was previously described.12 The HEK-293 cell line expressing TRPM7 containing the amino acid substitution E1047K (293-TRPM7-E1047K), which renders the channel inactive, was constructed using the Flp-In system (Invitrogen) as previously described.12,27
Cell Rounding Assay
Phase-contrast images of 293-TRPM7 cells were obtained with a phase-contrast 10X UPlanFI objective using an Olympus IX70 microscope equipped with an environmental chamber at a temperature of 37°C. Changes in cell morphology were scored manually employing the following criteria: Cells which had a fully-rounded cell body with no membrane extension processes were given one point. Partially-rounded cells with one or two membrane extension processes were assigned half-a-point. Non-rounded cells having three or four membrane extension processes and with a cell morphology similar to wildtype HEK-293 cells, were given zero points. A χ2 test was used to test differences in cell rounding.
Detection of TRPM7 Protein Expression
Detection of recombinant HA-tagged TRPM7 and the channel-inactive mutant in HEK-293 cells was previously described.12 Briefly, cells were lysed in ice-cold RIPA buffer (50 mM Tris (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% IGEPAL CA-630, 0.5% (w/v) deoxycholate, 0.1% (w/v) SDS, and 10 mM iodoacetamide). Proteins were immunoprecipitated overnight using anti-HA affinity matrix and resolved by SDS-PAGE and western blotting using the anti-TRPM7 antibody α-CTERM. The SuperSignal West Dura Maximum Sensitive Substrate (Pierce, Rockford, IL) was used for immunochemiluminescence detection.
Detection of MAP Kinases, AMP Kinase and Talin Cleavage
293-TRPM7, 293-shRNA-C, and 293-M7shRNA2 cells were lysed following a 24-hour treatment with tetracycline with 2 ml of ice-cold RIPA buffer. Samples of the lysates were resolved by SDS-PAGE and analyzed by western blotting using standard procedures. ERK was detected with the rabbit polyclonal (K-23) antibody from Santa Cruz Biotechnology (Santa Cruz, CA). p38 MAPK and JNK were detected with rabbit polyclonal antibodies from Cell Signaling Technology (Danvers, MA). Levels of activated ERK, p38 MAPK and JNK were detected with Phospho-p44/42 MAPK (Thr202/Tyr204), Phospho-p38 MAPK (Thr180/Tyr182) and Phospho-JNK (Thr183/Tyr185) rabbit polyclonal antibodies from Cell Signaling Technology. Talin was detected using a monoclonal antibody (clone TA205) from Upstate Biotechnology (Temecula, CA), which recognizes an epitope within the head domain of human talin (a.a. 139-433). Levels of AMP-dependent kinase (AMPK) and its activated form were detected with a mouse monoclonal antibody against AMPKα1/2 (clone D6) and a rabbit polyclonal against p-AMPKα(Thr 172) from Santa Cruz Biotechnology (Santa Cruz, CA). The SuperSignal West Dura Maximum Sensitive Substrate (Pierce, Rockford, IL) was used for immunochemiluminescence detection.
Metabolic Labeling
293-TRPM7 cells were incubated with 1 mCi/ml [32P]orthphosphate (900-1100 mCi/mmol; PerkinElmer, Waltham, MA) in phosphate-free DMEM for 24 hours. Cells were lysed in RIPA buffer, and m-calpain protein was immunoprecipitated using an anti-m-calapin antibody from Abcam (Cambridge, MA). The samples were separated by 8% SDS-PAGE and subsequently transferred to a PVDF membrane. The membrane was used for autoradiography and subsequently blotted with an anti-m-calpain antibody.
BOC Calpain Assay
We employed the BOC calpain assay to detect calpain activity in live cells.34 Briefly, 8×105 293-TRPM7 cells were plated onto a 35 mm culture dish and then treated with tetracycline for 24 hours. The cells were then incubated for 10 minutes in the presence of the 10 μM BOC-LM-CMAC, a fluorogenic calpain substrate. 293-TRPM7 cells without tetracycline treatment were used as the negative control. The unhydrolyzed BOC-LM-CMAC was retained within the cells by its conjugation to intracellular thiol groups. Cleavage of the synthetic calpain substrate increased the fluorescence yield of the chloromethylaminocoumarin moiety, which is also retained in cells.35 Fluorescence emission from the labeled cells was collected using an Olympus IX70 microscope equipped with 10X UPlanFI objective and a standard DAPI bandpass filter set. Changes in cell fluorescence were analyzed using ImageJ software (http://rsbweb.nih.gov/ij/).
Measurement of ROS and NO Production
293-TRPM7 cells were trypsinized, washed with PBS, and resuspended in phenol red-free culture medium. Cells were then labeled with 5 μM CM-H2DCFDA for 20 min at 37°C under 5% CO2. After washing twice with PBS, cells were resuspended in phenol red-free DMEM, and the samples were immediately analyzed using a Beckman Coulter Cytomics FC500 Flow cytometry equipped with a 488 nm argon-ion laser and a 525 nm bandpass emission filter. The nitric oxide (NO) indicator DAF-FM diacetate was employed to detect increases in NO production caused by TRPM7 overexpression. Cells were labeled with 5 μM DAF-FM diacetate in phenol red-free DMEM for 20 min at 37°C under 5% CO2, and fluorescence emission from the labeled cells was collected using an Olympus IX70 microscope equipped with 10X UPlanFI objective and a standard fluorescein bandpass filter set.
Ca2+ Imaging
293-TRPM7 cells were plated onto 35 mm dishes and then loaded the following day with 5 μM of the Ca2+-sensitive fluorophore Fluo-4-AM for 30 minutes. Non-incorporated dye was washed from cells using a HEPES-Buffered Saline Solution (HBSS) containing (in mM): 20 HEPES, 10 D-glucose, 1.2 MgCl2, 1.2 KH2PO4, 4.7 KCl, 140 NaCl, 1.3 CaCl2 (pH 7.4). Images of cells bathed in HBSS following treatment with 300 nM thapsigargin, a specific inhibitor of the endoplasmic reticulum Ca2+-ATPase, were obtained with a 20X UPlanFI objective using an Olympus IX70 microscope equipped with an environmental chamber at a temperature of 37°C.
Measurement of Intracellular Mg2+
293-TRPM7, 293-shRNA-C and 293-M7shRNA2 cells were trypsinzed, washed twice with PBS, and resuspended in phenol red-free DMEM. Cells were then labeled with 5 μM Mag-Indo-1 and incubated for 35 min at 37°C under 5% CO2. After washing twice with PBS, cells were resuspended in phenol red-free DMEM, and blue/violet fluorescence emission were collected using a Beckman Coulter MoFlo XDP cell sorter equipped with a 355 nm laser at room temperature. Total Mg2+ content of cells was quantified using ICP-MS (Inductively Coupled Plasma Mass Spectrometry) as described with the following modification6- freshly isolated cells were washed twice in Mg2+-free buffer containing 20 mM Hepes, pH 7.4 and 280 mM Sucrose.
Electrophysiological Recordings
The voltage-clamp technique was used to evaluate the whole-cell currents of TRPM7 expressed in HEK-293 cells as described.12 Briefly, whole-cell current recordings of TRPM7 expressing cells were elicited by voltage stimuli lasting 250 ms delivered every 1 second using voltage ramps from −120 to +100 mV. Data was digitized at 2 or 5 kHz and digitally filtered off-line at 1 kHz. The internal pipette solution for macroscopic current recordings contained (in mM) 145 Cs-methanesulfonate, 8 NaCl, 10 EGTA, and 10 HEPES, pH adjusted to 7.2 with CsOH. The extracellular solution for whole-cell recordings contained (in mM) 140 NaCl, 5 KCl, 2 CaCl2, 20 HEPES, and 10 glucose, pH adjusted to 7.4 with NaOH.
Supplementary Material
Supplementary Figure 1. The effect of inhibitors on TRPM7 protein expression. (a) Application of the MEK1/2 inhibitors (U0126 and PD98059), the p38 MAPK inhibitor SB203580 and the JNK inhibitor SP600125 had no effect on TRPM7 protein expression in 293-TRPM7 expressing cells (+TET). A western blot of β-actin is shown to demonstrate equal loading of the samples. (b) Application of MnTBAP, DPI, rotenone and L-NAME to 293-TRPM7 expressing cells (+TET) had no effect on TRPM7 protein expression. A western blot of β-actin is shown to demonstrate equal loading of the samples.
Supplementary Figure 2. Expression of the channel-inactive TRPM7 mutant E1047K in 293-TRPM7-E1047K cells does not cause cell rounding. (a) Western blot showing similar expression of wildtype TRPM7 and TRPM7-E1047K in 293-TRPM7 and 293-TRPM7-E1047K cells grown in the presence of tetracycline. A western blot of β-actin is shown to demonstrate equal loading of samples. (b) Overexpression of TRPM7-E1047K does not cause rounding of 293-TRPM7-E1047K cells. (c) Quantification of the degree of cell rounding under the conditions depicted in (b). Values are mean ± standard deviation of at least three independent experiments.
Supplementary Figure 3. The effect of thapsigargin on intracellular free Mg2+ measurement. (a) Fluo-4 Ca2+ imaging revealed that thapsigargin (TG, 300 nM) elicited a rapid increase in cytosolic Ca2+ in HEK-293 cells. (b) Overexpression of TRPM7 in 293-TRPM7 cells (+TET) caused an increase in intracellular free Mg2+ compared to control cells (293-TRPM7-TET). Application of thapsigargin (300 nM) to 293-TRPM7 cells for 10 minutes had no effect on the TRPM7-induced increase in intracellular free Mg2+. (c) Application of carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (CCCP), analogs of which have been shown to release Mg2+ from mitochondria, produced a robust increase in Mag-Indo-1 fluorescence intensity.
Supplementary Figure 4. The effect of knockdown of TRPM7 on SAPKs activation and intracellular free Mg2. (a) Western blots showing that knockdown of TRPM7 in HEK-293 cells (293-M7shRNA2) had no effect on the activation of p38 MAPK and JNK. (b) The cellular free Mg2+ concentration of TRPM7-knockdown cells (293-M7shRNA2) was similar to controls cells expressing a non-silencing shRNA (293-shRNA-C).
Supplementary Figure 5. The effect of the JNK inhibitor on TRPM7-induced increase in intracellular free Mg2+ and ROS production. Application of the JNK inhibitor SP600125 (20 μM) to 293-TRPM7 cells (JNK inhibitor +TET) had no effect on the TRPM7-induced increase in intracellular free Mg2+ (a) and TRPM7-mediated ROS production (b).
Acknowledgements
We are grateful to Dr. Raymond Habas and Dr. Alexey Ryazanov (UMDNJ-Robert Wood Johnson Medical School), and to Elizabeth Puccini for their constructive suggestions and comments. We thank Dr. Peter Lobel (UMDNJ-Robert Wood Johnson Medical School) for his technical assistance. This work was supported by the generous support of the National Institutes of Health, NIGMS (1R01GM080753).
Abbreviations used
- ERK
extracellular signal-regulated kinase
- MAP kinase
mitogen-activated protein kinase
- JNK
c-Jun N-terminal kinase
- SAPK
stress activated protein kinase
- ROS
reactive oxygen species
- NOS
nitric oxide synthase
- RNA
ribonucleic acid
- TET
tetracycline
- MEK1/2
mitogen-activated protein kinase kinase
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- NAC
N-acetyl cysteine
- DPI
diphenyleneiodonium chloride
- NADH
nicotinamide adenine dinucleotide
- L-NAME
NG-nitro-L-arginine methyle ester
- MnTBAP
Mn(III)tetrakis(4-benzoic acid)porphyrin chloride
- SOD
superoxide dismutase
- BOC-LM-CMAC
t-butoxycarbonyl-Leu-Met-chloromethylaminocoumarin
- HA
hemagglutinin
- shRNA
short hairpin ribonucleic acid
- RIPA
radioimmunoprecipitation assay
- EDTA
ethylenediaminetetraacetic acid
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- EGTA
ethylene glycol tetraacetic acid
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- TG
thapsigargin
- ER
endoplasmic reticulum
- CCCP
carbonyl cyanide m-chlorophenylhydrazone
- AMPK
AMP-dependent kinase
- PVDF
polyvinylidene difluoride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nadler MJS, Hermosura MC, Inabe K, Perraud A-L, Zhu Q, Stokes AJ, Kurosaki T, Kine J-P, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a Mg·ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 2.Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–7. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- 3.Bessac BF, Fleig A. TRPM7 channel is sensitive to osmotic gradients in human kidney cells. J Physiol. 2007;582:1073–86. doi: 10.1113/jphysiol.2007.130534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark K, Langeslag M, van Leeuwen B, Ran L, Ryazanov AG, Figdor CG, Moolenaar WH, Jalink K, van Leeuwen FN. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. Embo J. 2006;25:290–301. doi: 10.1038/sj.emboj.7600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elizondo MR, Arduini BL, Paulsen J, MacDonald EL, Sabel JL, Henion PD, Cornell RA, Parichy DM. Defective skeletogenesis with kidney stone formation in dwarf zebrafish mutant for trpm7. Curr Biol. 2005;15:667–71. doi: 10.1016/j.cub.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 6.Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, Clapham DE. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 2008;322:756–60. doi: 10.1126/science.1163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krapivinsky G, Mochida S, Krapivinsky L, Cibulsky SM, Clapham DE. The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron. 2006;52:485–96. doi: 10.1016/j.neuron.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 8.Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol. 2003;121:49–60. doi: 10.1085/jgp.20028740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Numata T, Shimizu T, Okada Y. TRPM7 is a stretch- and swelling-activated cation channel involved in volume regulation in human epithelial cells. Am J Physiol Cell Physiol. 2007;292:C460–7. doi: 10.1152/ajpcell.00367.2006. [DOI] [PubMed] [Google Scholar]
- 10.Oancea E, Wolfe JT, Clapham DE. Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circ Res. 2006;98:245–53. doi: 10.1161/01.RES.0000200179.29375.cc. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 12.Su LT, Agapito MA, Li M, Simonson WT, Huttenlocher A, Habas R, Yue L, Runnels LW. TRPM7 regulates cell adhesion by controlling the calcium-dependent protease calpain. J Biol Chem. 2006;281:11260–70. doi: 10.1074/jbc.M512885200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–77. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- 14.Wei WL, Sun HS, Olah ME, Sun X, Czerwinska E, Czerwinski W, Mori Y, Orser BA, Xiong ZG, Jackson MF, Tymianski M, MacDonald JF. TRPM7 channels in hippocampal neurons detect levels of extracellular divalent cations. Proc Natl Acad Sci U S A. 2007;104:16323–8. doi: 10.1073/pnas.0701149104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glading A, Bodnar RJ, Reynolds IJ, Shiraha H, Satish L, Potter DA, Blair HC, Wells A. Epidermal growth factor activates m-calpain (calpain II), at least in part, by extracellular signal-regulated kinase-mediated phosphorylation. Mol Cell Biol. 2004;24:2499–512. doi: 10.1128/MCB.24.6.2499-2512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717–26. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 17.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–69. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 18.Hotokezaka H, Sakai E, Kanaoka K, Saito K, Matsuo K, Kitaura H, Yoshida N, Nakayama K. U0126 and PD98059, specific inhibitors of MEK, accelerate differentiation of RAW264.7 cells into osteoclast-like cells. J Biol Chem. 2002;277:47366–72. doi: 10.1074/jbc.M208284200. [DOI] [PubMed] [Google Scholar]
- 19.Mills J, Laurent Charest D, Lam F, Beyreuther K, Ida N, Pelech SL, Reiner PB. Regulation of amyloid precursor protein catabolism involves the mitogen-activated protein kinase signal transduction pathway. J Neurosci. 1997;17:9415–22. doi: 10.1523/JNEUROSCI.17-24-09415.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–31. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 21.Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci. 2000;25:502–8. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- 22.Majander A, Finel M, Wikstrom M. Diphenyleneiodonium inhibits reduction of iron-sulfur clusters in the mitochondrial NADH-ubiquinone oxidoreductase (Complex I) J Biol Chem. 1994;269:21037–42. [PubMed] [Google Scholar]
- 23.Wang YX, Poon CI, Poon KS, Pang CC. Inhibitory actions of diphenyleneiodonium on endothelium-dependent vasodilatations in vitro and in vivo. Br J Pharmacol. 1993;110:1232–8. doi: 10.1111/j.1476-5381.1993.tb13947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim BJ, Jeon JH, Kim SJ, So I, Kim KW. Regulation of transient receptor potential melastatin 7 (TRPM7) currents by mitochondria. Mol Cells. 2007;23:363–9. [PubMed] [Google Scholar]
- 25.Choi SL, Kim SJ, Lee KT, Kim J, Mu J, Birnbaum MJ, Soo Kim S, Ha J. The regulation of AMP-activated protein kinase by H(2)O(2) Biochem Biophys Res Commun. 2001;287:92–7. doi: 10.1006/bbrc.2001.5544. [DOI] [PubMed] [Google Scholar]
- 26.Hardie DG. The AMP-activated protein kinase pathway--new players upstream and downstream. J Cell Sci. 2004;117:5479–87. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 27.Li M, Du J, Jiang J, Ratzan W, Su LT, Runnels LW, Yue L. Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7. J Biol Chem. 2007;282:25817–30. doi: 10.1074/jbc.M608972200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kubota T, Shindo Y, Tokuno K, Komatsu H, Ogawa H, Kudo S, Kitamura Y, Suzuki K, Oka K. Mitochondria are intracellular magnesium stores: investigation by simultaneous fluorescent imagings in PC12 cells. Biochim Biophys Acta. 2005;1744:19–28. doi: 10.1016/j.bbamcr.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 29.David LL, Shearer TR. Purification of calpain II from rat lens and determination of endogenous substrates. Exp Eye Res. 1986;42:227–38. doi: 10.1016/0014-4835(86)90057-6. [DOI] [PubMed] [Google Scholar]
- 30.Siljander P, Farndale RW, Feijge MA, Comfurius P, Kos S, Bevers EM, Heemskerk JW. Platelet adhesion enhances the glycoprotein VI-dependent procoagulant response: Involvement of p38 MAP kinase and calpain. Arterioscler Thromb Vasc Biol. 2001;21:618–27. doi: 10.1161/01.atv.21.4.618. [DOI] [PubMed] [Google Scholar]
- 31.Choi DW, Koh JY. Zinc and brain injury. Annu Rev Neurosci. 1998;21:347–75. doi: 10.1146/annurev.neuro.21.1.347. [DOI] [PubMed] [Google Scholar]
- 32.Lee JY, Cole TB, Palmiter RD, Suh SW, Koh JY. Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proc Natl Acad Sci U S A. 2002;99:7705–10. doi: 10.1073/pnas.092034699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou H, Clapham DE. Mammalian MagT1 and TUSC3 are required for cellular magnesium uptake and vertebrate embryonic development. Proc Natl Acad Sci U S A. 2009;106:15750–5. doi: 10.1073/pnas.0908332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosser BG, Powers SP, Gores GJ. Calpain activity increases in hepatocytes following addition of ATP. Demonstration by a novel fluorescent approach. J Biol Chem. 1993;268:23593–600. [PubMed] [Google Scholar]
- 35.Glading A, Uberall F, Keyse SM, Lauffenburger DA, Wells A. Membrane proximal ERK signaling is required for M-calpain activation downstream of epidermal growth factor receptor signaling. J Biol Chem. 2001;276:23341–8. doi: 10.1074/jbc.M008847200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. The effect of inhibitors on TRPM7 protein expression. (a) Application of the MEK1/2 inhibitors (U0126 and PD98059), the p38 MAPK inhibitor SB203580 and the JNK inhibitor SP600125 had no effect on TRPM7 protein expression in 293-TRPM7 expressing cells (+TET). A western blot of β-actin is shown to demonstrate equal loading of the samples. (b) Application of MnTBAP, DPI, rotenone and L-NAME to 293-TRPM7 expressing cells (+TET) had no effect on TRPM7 protein expression. A western blot of β-actin is shown to demonstrate equal loading of the samples.
Supplementary Figure 2. Expression of the channel-inactive TRPM7 mutant E1047K in 293-TRPM7-E1047K cells does not cause cell rounding. (a) Western blot showing similar expression of wildtype TRPM7 and TRPM7-E1047K in 293-TRPM7 and 293-TRPM7-E1047K cells grown in the presence of tetracycline. A western blot of β-actin is shown to demonstrate equal loading of samples. (b) Overexpression of TRPM7-E1047K does not cause rounding of 293-TRPM7-E1047K cells. (c) Quantification of the degree of cell rounding under the conditions depicted in (b). Values are mean ± standard deviation of at least three independent experiments.
Supplementary Figure 3. The effect of thapsigargin on intracellular free Mg2+ measurement. (a) Fluo-4 Ca2+ imaging revealed that thapsigargin (TG, 300 nM) elicited a rapid increase in cytosolic Ca2+ in HEK-293 cells. (b) Overexpression of TRPM7 in 293-TRPM7 cells (+TET) caused an increase in intracellular free Mg2+ compared to control cells (293-TRPM7-TET). Application of thapsigargin (300 nM) to 293-TRPM7 cells for 10 minutes had no effect on the TRPM7-induced increase in intracellular free Mg2+. (c) Application of carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (CCCP), analogs of which have been shown to release Mg2+ from mitochondria, produced a robust increase in Mag-Indo-1 fluorescence intensity.
Supplementary Figure 4. The effect of knockdown of TRPM7 on SAPKs activation and intracellular free Mg2. (a) Western blots showing that knockdown of TRPM7 in HEK-293 cells (293-M7shRNA2) had no effect on the activation of p38 MAPK and JNK. (b) The cellular free Mg2+ concentration of TRPM7-knockdown cells (293-M7shRNA2) was similar to controls cells expressing a non-silencing shRNA (293-shRNA-C).
Supplementary Figure 5. The effect of the JNK inhibitor on TRPM7-induced increase in intracellular free Mg2+ and ROS production. Application of the JNK inhibitor SP600125 (20 μM) to 293-TRPM7 cells (JNK inhibitor +TET) had no effect on the TRPM7-induced increase in intracellular free Mg2+ (a) and TRPM7-mediated ROS production (b).