Abstract
Sceptrin, a natural compound produced by various marine sponges, was tested for its effect on cell motility. We report for the first time that sceptrin inhibits cell motility in several cancer cell lines. The compound shows no toxicity at concentrations that are double the amount of sceptrin required for maximal inhibitory effect. Both random and factor-induced migration were impaired, suggesting that sceptrin targets a central process of cell motility machinery. Activity of de novo synthesized sceptrin was indistinguishable from sceptrin purified from Agelas nakamurai, and the inhibitory activity was found to be, at least partially, due to sceptrin’s capability to inhibit cell contractility. Additionally, sceptrin was found to bind to monomeric actin, further suggesting a mechanism involving the actin cytoskeleton. Close analogues of sceptrin were synthesized, tested for their effect on cell motility, and found to be either equimolar or less potent compared to the parental compound. Inadvertent cell motility is a key contributing factor in various human diseases, including cancer and chronic inflammation. Marine compounds isolated from sponges have been proven to be an excellent source of metabolites that show biological activities. Given the recently achieved total synthesis of sceptrin in multigram quantities, sceptrin could prove to be an attractive lead molecule for further preclinical testing and development for therapeutic purposes, as well as a useful research tool to elucidate the mechanisms involved in cell motility.
Natural compounds isolated from marine organisms have been found to be a very rich source of bioactive molecules. Reported biological effects of these compounds include anti-tumor, anti-inflammatory, and anti-viral activities, as well as immunomodulatory and analgesic properties (1,2). Among marine organisms, sponges have been proven to be excellent producers of secondary metabolites that exhibit biological activities. More than 5,300 compounds have been isolated from sponges with around 200 new molecules reported each year (3). Bromopyrrole alkaloids constitute a family of exclusively marine alkaloids and represent a fascinating example of the large variety of compounds formed by marine sponges (4). About 140 derivatives, with different structures and biological activities, have been isolated from more than 20 different sponges of various genera, most of them belonging to Agelasidae, Axinellidae, and Halichondridae families (5).
One of the members of this family is sceptrin. First isolated by Faulkner, Clardy, and co-workers (6) from Agelas sceptrum sponge in 1981, sceptrin is of considerable pharmaceutical interest because it exhibits a broad array of biological activities, including anti-bacterial/anti-fungal, anti-muscarinic and anti-histaminic (7−10). It has been reported that sceptrin is the most potent nonpeptidic inhibitor of somatostatin (11), implying its potential as a treatment for cystic fibrosis and Alzheimer’s disease. Encouragingly, sceptrin was also found to be noncytotoxic to monkey liver cells (12) and to mice at 50 mg kg−1(6). Interestingly, Rodriguez and co-workers (13) have recently demonstrated the interaction of sceptrin with the bacterial MreB protein. MreB protein is considered to be the actin homologue in bacteria, raising the possibility that sceptrin could have biological activities related to actin cytoskeleton functions in mammalian cells.
From a chemical standpoint, sceptrin provides a unique challenge. In particular, its architecture includes an asymmetrically oriented tetrasubstituted cyclobutane in the company of reactive and delicate heterocyclic species, such as the 2-aminoimidazoles and oxidation prone pyrroles. The first total synthesis of sceptrin was reported in 2004 (14), followed by an enantioselective variant in 2005 (15), and a refined route capable of delivering multigram quantities of the natural material was reported in 2007 (16). These advances in chemical synthesis overcome the common problem of product availability present for many natural compounds and have set the stage for an extensive evaluation of sceptrin’s potential in medicine.
In this work, we report the identification of novel biological activity of sceptrin as inhibitor of cell motility. Mechanistically, cell motility is effected through a combination of alternating phases of cell protrusion and contraction events. Polarized cells are able to induce lamellipodium protrusions at the front of the cell that attach to the underlying substrate. Once the cell front is attached to the underlying extracellular matrix, pulling forces are generated and cell motility ensues by contraction of the cell body and retraction of the cell tail in the rear (17,18). It is known that actin stress fibers are contractile (19,20) and play a central role in mediating cell contractility during cell migration. Tightly regulated signaling pathways control actin stress fiber polymerization and depolymerization during cell migration. Small GTPase Rho and its target Rho-associated kinase (ROCK) have been found to be key molecules in these regulatory pathways (21−24). Our studies here suggest that sceptrin affects cell motility primarily by regulating cell contractility. We further report that sceptrin binds mammalian monomeric actin, which could at least partially explain sceptrin’s effects on cell contractility. Importantly, cell migration governs numerous physiological and pathological cellular processes, including cancer metastasis. A nontoxic inhibitor of cell motility, such as sceptrin, could serve as an attractive lead molecule for further development of potent cell motility inhibitors for therapeutic use.
Results and Discussion
Sceptrin Inhibits Cell Motility in HeLa Cells
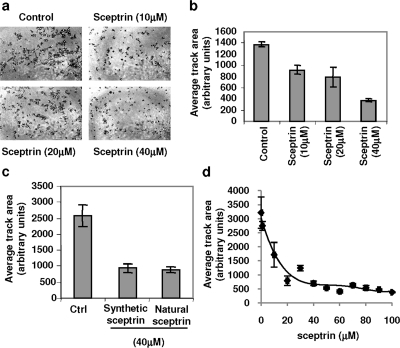
The putative effect of sceptrin on cell motility was studied in human cervical cancer HeLa cell line. Cellomics Cell Motility reagent kit (see Methods) was used to analyze and quantify cell motility changes. In this assay, exponentially growing HeLa cells were seeded on plates that had been covered with fluorescence beads in complete DMEM culture media supplemented with 10 ng mL−1 of hepatocyte growth factor (HGF) to promote cell motility. Cells were treated with the indicated concentrations of synthetic sceptrin (1, panel a) and allowed to migrate for 24 h at 37 °C. As shown in 1, panel a, sceptrin inhibits HeLa cell motility in a dose-dependent manner, as indicated by the shorter tracks produced by sceptrin-treated cells. Quantitative analysis of cell motility was carried out by ImageJ software, which segments the cell tracks and measures the track surface. 1, panel b shows average motility results of triplicates for each condition tested, expressed as a function of average track area (in pixels). It is important to note that track segmentation includes the surface area covered by cell bodies themselves. Thus, surface area of around 400 pixels consists of stationary cell bodies in this analysis, and accordingly, surface area value of around 400 pixels indicates lack of cell movement.
Figure 1.
Sceptrin inhibits HGF-induced cell motility in HeLa cells. a) HeLa cells were treated with sceptrin at indicated concentrations, and cell motility was induced by hepatocyte growth factor (HGF) (10 ng mL−1). Cells were allowed to migrate for 24 h before fixation. A representative picture (10x objective) of one well for each condition is shown. Three independent experiments done in triplicate for each condition were carried out. b) ImageJ software was used to segment and quantify cell tracks of the cell motility assay shown in panel a. Graph represents surface on arbitrary units of the cell tracks. c) Effect of synthetic and natural sceptrin (40 μM) in cell motility is compared. HeLa cells were induced to migrate as described for panel a. Each condition was tested in triplicate. Cell tracks were analyzed as described for panel b. d) Dose-dependent experiment was carried out in HeLa cells at indicated sceptrin concentrations. Each condition was tested in triplicate. Quantification of cell motility was done as described in panel b. Surface tracks were plotted, and a logarithmic trend line is shown.
Commercially available sceptrin that had been directly purified from the Agelas nakamurai sponge was tested in parallel with synthetic sceptrin. As shown in 1, panel c, activities of the purified and synthetic sceptrin were found to be indistinguishable. Synthetic sceptrin demonstrated maximum inhibitory effect of cell motility at a concentration of 50 μM, with an IC50 value of 15 μM.
Sceptrin Inhibits Cell Motility in a Variety of Cancer Cell Lines
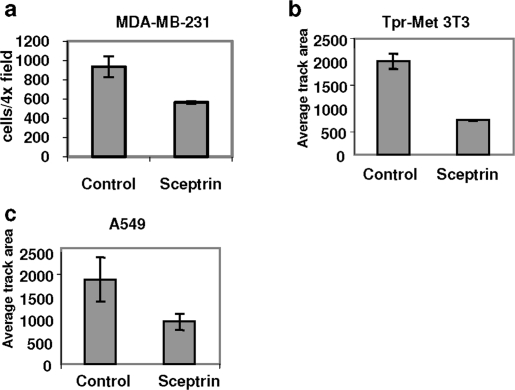
We next tested whether the effect of sceptrin on cell motility was cell-type or stimulus-dependent. The MDA-MB-231 metastatic breast cancer cell line, A549 lung cancer cell line and 3T3 mouse fibroblasts stably transfected with an active form of HGF receptor (Tpr-Met 3T3 cells) were treated with sceptrin (40 μM). Prior to the addition of sceptrin, A549 cells were treated with 10 ng mL−1 of HGF to induce chemotactic cell migration. No stimulus was added on the MDA-MB-231 cells, which exhibit high baseline cellular motility (and invasiveness). Tpr-Met cells are also highly motile due to activation of the HGF signaling pathway by the virtue of Tpr-Met oncogene expression in the cells. As shown in 2, sceptrin demonstrated broad inhibitory effect on cell motility in all the cell lines and conditions tested.
Figure 2.
Effect of sceptrin on cell motility in different cancer cell lines. a−c) Indicated cells were seeded on top of fluorescence beads and treated with sceptrin (40 μM). Basal random motility was measured for cells in panels a and b. Motility was stimulated by hepatocyte growth factor (HGF) (10 ng mL−1) in panel c. Cells were fixed after 24 h, a picture of each well (all the conditions were assayed in triplicate) was taken, and the surface of the cell tracks was segmented and quantified.
Sceptrin Does Not Prevent Cell Proliferation or Cell Survival
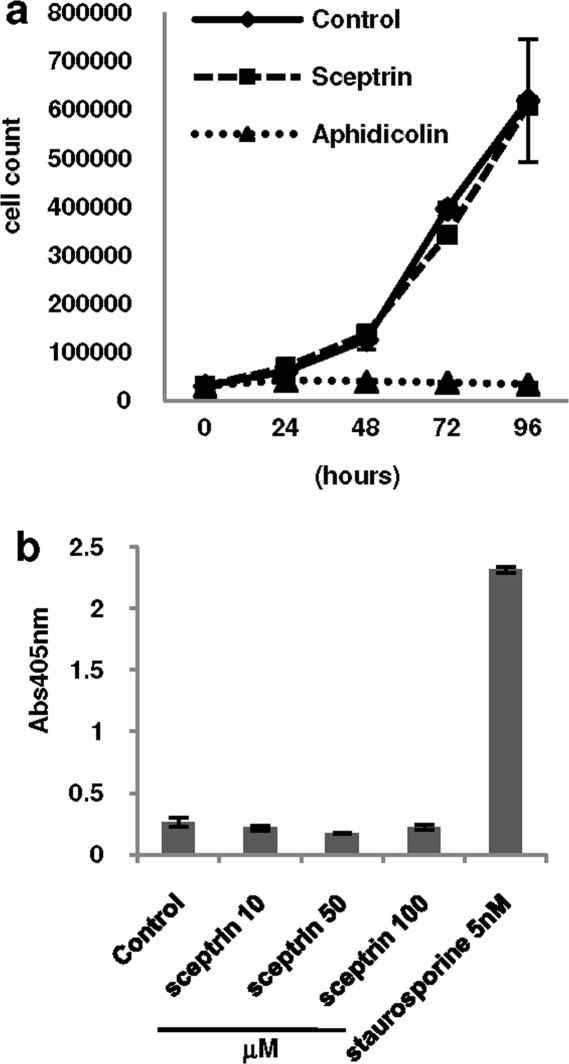
A reduction in cell motility by sceptrin could be secondary and caused by an induction of cell death or due to impaired cell proliferation. Apoptosis and cell proliferation assays were carried out next to study this possibility. HeLa cells were seeded on 6-well plates at low confluence and allowed to grow in the presence or absence of 40 μM sceptrin. Cell number was measured at indicated times while the cells were in an exponential growth phase. As shown in 3, panel a, sceptrin treatment had no effect on cell proliferation at any of the time points tested. As a positive control, 5 nM aphidicolin readily inhibited cell proliferation. To measure apoptosis, exponentially growing HeLa cells were treated by different concentrations of sceptrin, as indicated in 3, panel b. A Cell Death Detection ELISA kit (see Methods) was used to quantify apoptosis. As a positive control, cells were treated with 5 nM staurosporine. No apoptosis was observed at any tested concentrations of sceptrin, including twice (100 μM) the concentration of sceptrin that yields maximum inhibition of cell motility. These data confirm previous reports that show no toxicity by sceptrin in mice or monkey liver cells (6,12) and demonstrate that sceptrin did not exhibit any cytotoxic activity in the cell lines studied here. Since it was isolated by Walker and co-workers in 1981, sceptrin has been tested and found to exhibit a variety of biological activities including anti-microbial and anti-muscarinic activities, to inhibit depolarization-induced cellular calcium elevation, and to inhibit somatostatin and vasoactive intestinal polypeptide (VIP) activities (6,8,9,11). Here, we report a novel biological activity of sceptrin as inhibitor of cell migration. The inhibitory effect of sceptrin on cell motility is not restricted to a specific tissue or cell type, as we found sceptrin to be active in different cell lines, including human breast and lung cancer cell lines, as well as in transformed mouse fibroblast cells.
Figure 3.
Effect of sceptrin on apoptosis and cell proliferation. a) Effect of sceptrin on cell proliferation was tested in HeLa cells, which were seeded at low density and treated with vehicle, sceptrin (40 μM), or positive control aphidicolin (5 nM). Proliferation was quantified by cell counting at indicated times. b) Putative induction of apoptosis by sceptrin was assayed in HeLa cells, which were treated with sceptrin at indicated concentrations or with the positive control staurosporine (5 nM) for 24 h. Apoptosis was quantified by immunochemical determination of histone-complexed DNA fragments in a microplate well.
Effect of Various Sceptrin Analogues on Cell Motility
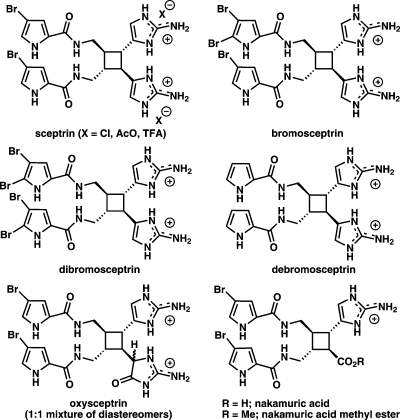
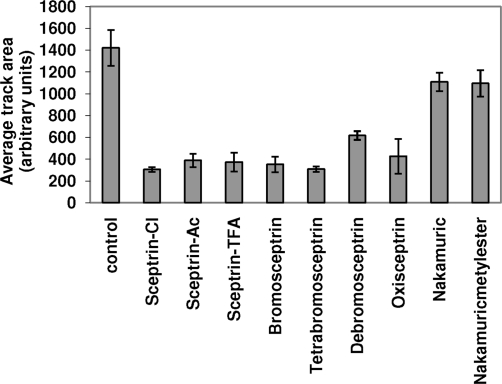
To identify which chemical group(s) of sceptrin are important for the observed inhibition of motility, various sceptrin derivatives were synthesized. 4 depicts the chemical structure of all tested sceptrin-derived compounds. On the basis of the fact that differences in the way sceptrin chemically reacts as a function of salt type have been observed (16), three different salts of sceptrin (chloride, acetate, and trifluoroacetate) were examined to assess the possible (although unlikely) unspecific effect of the anions in the effect of sceptrin on cell motility. As shown in 5, none of the salts showed significant differences in the motility inhibition when tested in HGF-treated HeLa cells. None of the analogues tested appeared to be superior compared to the parent sceptrin compound. In contrast, debromosceptrin and, more significantly, the two forms of the nakamuric acid compounds demonstrated reduced effect on cell motility compared to the parent compound. Thus, these studies suggest a critical role for the aminoimidazole group of sceptrin (missing in the nakamuric acid compounds) in the inhibition of cell motility.
Figure 4.
Structure of synthesized sceptrin-derived compounds. Five different sceptrin-derived compounds were synthesized to test their ability to block cell motility. In summary, extra bromide groups were added or original bromides were removed from the pyrrole group of sceptrin to generate the bromo, dibromo, or debromo compounds. Nakamuric compounds lack one of the aminoimidazole groups of sceptrin. On the oxysceptrin compound an oxygen is added to one of the aminoimidazole groups.
Figure 5.
Effect on cell motility of the various sceptrin-derived compounds. HeLa cells were allowed to migrate in the presence of hepatocyte growth factor (HGF) (10 ng mL−1). As indicated cells were treated with sceptrin or sceptrin-derivatives (40 μM) for 24 h as described above. Control cells were treated with vehicle (H2O). Cell motility was quantified as described in Methods.
Sceptrin Does Not Block Lamellipodia Formation
Motility is a complex process that involves several different mechanisms and steps. In broad terms, the various steps of cell motility include extension of the plasma membrane and formation of lamellipodia in the leading edge of the motile cell, generation of contractility forces that propels the cell forward, and finally, detachment of the rear tail to allow cell movement. Confocal video-microscopy analysis was performed to evaluate the effect of sceptrin on these various steps of cell motility. GFP-Actin expressing HeLa cells that had been treated with HGF (10 ng mL−1) or GFP-Actin expressing untreated MDA-MB-231 cells were allowed to exponentially grow for 24 h on 4-well LabTek chambers that had been coated with fibronectin, followed by automated temperature-controlled time-lapse confocal microscopy (see Methods for details). A still image of live cells treated or untreated with sceptrin was taken every 2 min up to 3 h. Images of MDA-MB-231 cells were taken using a 20x objective to capture a large number of cells for analysis. A 40x objective was used for HeLa cells to analyze individual cell dynamics in more detail. Movies were mounted using the NIH ImageJ software. 6 shows an RGB composite of the cell image, in which red color indicates time 0, green indicates the 1 h time-point, blue the 2 h time-point, and white the 3 h time-point. Actual composite videos can be found in the online Supporting Information. Time-lapse results show that sceptrin-treated cells demonstrate actin dynamics on the cell membrane, including lamellipodia formation, indistinguishable from those observed in nontreated cells. Thus, it appears that sceptrin has no effect on cortical actin polymerization required in the initial extension of the plasma membrane during cell motility. Confirming our previous data, sceptrin-treated cells demonstrated reduced cell motility compared to untreated cells in the time-lapse study. As shown in the composite movies (see online Supporting Information), initial protrusion of the cell body appears to occur normally in sceptrin-treated cells. However, directional movement of the cell fails to take place, resulting in subsequent retraction of the protrused lamellipodia. Thus, these results suggest that sceptrin might inhibit the generation of the contractile forces in the cell that are required for movement of the cell body, after the initial lamellipodia formation has taken place.
Figure 6.
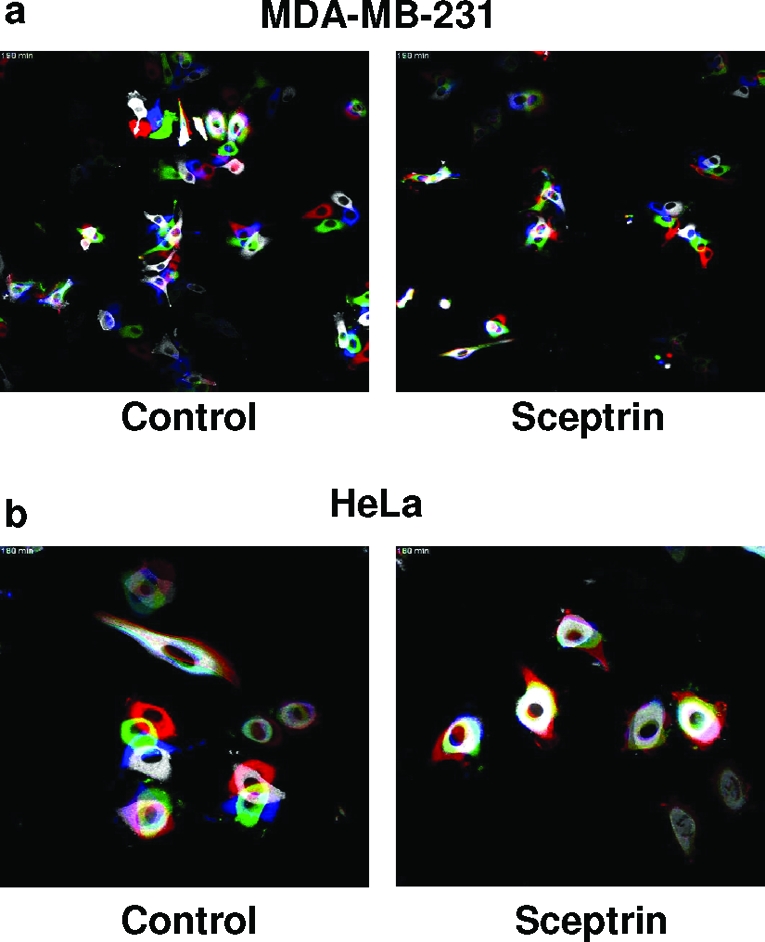
Time-lapse confocal video microscopy of cells treated with sceptrin. a) Effect of sceptrin on cell motility dynamics was tested on MDA-MB-231 cells transiently transfected with GFP-actin construct. Cells that had been seeded on fibronectin-coated LabTek chambers were pictured (20x objective) every 2 min up to 3 h. Panel a shows an RGB composite still image taken at time 0, 1, 2, and 3 h, where red represents 0 h, green 1 h, blue 2 h and white 3 h. Actual movies can be seen in online Supporting Information. b) Effect of sceptrin on HeLa cells was assayed as described for panel a. HeLa cells were induced to migrate by addition of hepatocyte growth factor (HGF) (10 ng mL−1). A 40x objective was used to take the pictures in this case.
Inhibition of Cell Contractility by Sceptrin
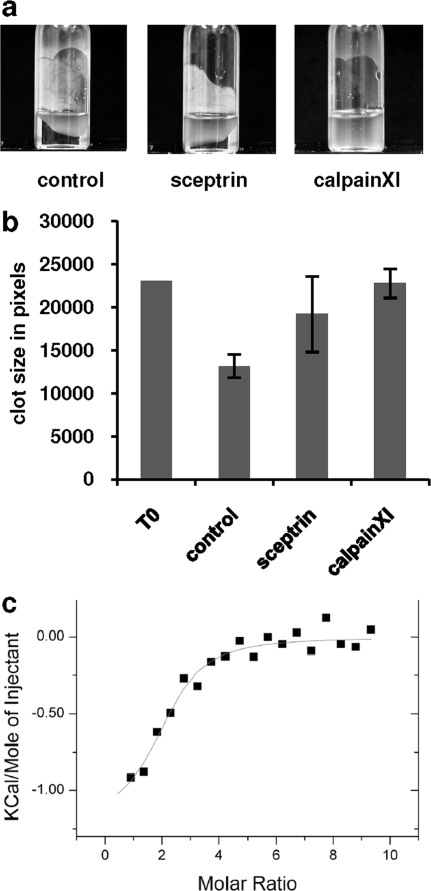
To study the possibility that sceptrin affects cell contractility to inhibit cell migration, a contractility assay known as clot retraction assay was performed (see Methods). Briefly, CHO cells stably transfected with human αIIbβ3 integrin (CHOαβ) were mixed with human plasma, thrombin, and CaCl2 in a Sigmacote-treated glass tube. Formed clot was allowed to retract for 2 h before pictures were taken. 2D images of the retracted clots were segmented and quantified using ImageJ software. As shown in 7, panel b, pretreatment of CHOαIIbβ3 cells with sceptrin significantly reduced clot retraction when compared to untreated cells. As a positive control, Calpain XI inhibitor was used, and as expected, calpain inhibitor completely blocked cell contractility. Thus, these results provide the first insights to the molecular mechanisms as to how sceptrin might inhibit cell motility, that is, by inhibiting cell contractility. To extend these studies further, we tested the possibility that sceptrin could directly bind actin. By using isothermal titration calorimetry, we show that sceptrin binds to monomeric actin with an equilibrium dissociation constant (Kd) of 19.2 ± 0.2 μM (7, panel c). Thus, these findings are consistent with the results obtained by Rodriguez and co-workers demonstrating that sceptrin interacts with the bacterial MreB protein, which is considered to be the actin homologue in bacteria (13).
Figure 7.
Inhibition of cell contractility and actin binding by sceptrin. a) Cell contractility was assayed using the clot retraction assay. CHOαIIbβ3 cells treated with vehicle, sceptrin (40 μM), or 10 μM Calpain inhibitor XI (positive control) were mixed with human serum, thrombin (2 U mL−1), and CaCl2 (5 mM). Clot was allowed to retract for 2 h, and still images were taken. All conditions were tested in triplicate and assayed in three independent experiments. Panel a shows a representative picture of each condition. b) 2D size of clots was segmented and quantified using the NIH ImageJ software. c) Isothermal titration calorimetry (ITC) binding curve after subtraction of the heat associated with sceptrin dilution. Kd = 19.4 ± 0.2 μM for sceptrin binding to G-actin is an average obtained from two independent measurements.
Other potential mechanisms how sceptrin affect cell contractility and cell motility beyond its binding to actin remain to be determined. It is known that the small GTPase Rho plays a central role in the regulation of cell contractility by controlling actin stress fiber formation (23−25); for a recent review see ref (26). In our unpublished data, sceptrin had no effect on the levels of active, GTP-bound Rho in cells. Whether sceptrin regulates downstream effectors of Rho, such as ROCK and mDIA1 (21,22,27), remains to be tested. Finally, sceptrin’s effect on cell motility could be mediated by its anti-muscarinic activity, as muscarine receptors have been implicated in migration in keratinocytes (28,29). In our unpublished studies, however, neither muscarine (a muscarinic agonist) or atropine (a muscarinic antagonist) demonstrated any effect on cell motility of the cell lines used in this study, nor did they influence the capability of sceptrin to inhibit cell migration.
Marine organisms have been found to be a very productive source of metabolites with clinically interesting properties (1). Two marine products, namely, Ziconotide (Prialt; Elan Pharmaceuticals) in 2004 and Trabectedin (Yondelis; PharmaMar) in 2007 have been approved for clinical use by the FDA and European Union, respectively (30). Development of marine products for pharmaceutical use has frequently been prevented by the scarceness of the compounds from the natural sources (1). Additionally, complete chemical synthesis of these molecules can be very complicated involving may reaction steps, making synthesis unappealing for industrial use. Importantly, the total synthesis of sceptrin was first resolved in 2004 (14), and in 2007 Dr. Baran’s group discovered a refined route capable of yielding multigram quantities of the product (16). Thus, the newly found biological activity of sceptrin in inhibiting cell motility, combined with the availability of synthetic sceptrin in multigram quantities, makes sceptrin an interesting candidate for further preclinical studies for treatment of diseases in which inadvertent cell motility is a key contributing factor, such as cancer and chronic inflammation.
Here, sceptrin was found to be capable of inhibiting both chemotactic HeLa cell migration induced by HGF and haptotactic cell motility of unstimulated MDA-MB-231 cells on fibronectin. Further, the inhibitory effect is not stimulus-dependent. These data suggest that sceptrin likely affects a signaling event common to all (or, a number of) motogenic pathways or exerts its effects through the cellular contractility machinery (e.g., actin stress fibers) directly. Given the lack of toxicity by sceptrin, and the recently achieved total synthesis of sceptrin in multigram quantities, sceptrin could prove to be an attractive lead molecule for further preclinical testing and development for therapeutic purposes, as well as a useful research tool to elucidate the mechanisms involved in cell motility.
Methods
Cell Culture and Transfections
HeLa, MDA-MB-231, and A549 cells were obtained from American Type Culture Collection (ATCC). NIH 3T3 cells stably transfected to express the Tpr-Met oncoprotein were generously provided by Dr. George Vande Woude. CHO cells expressing the αIIbβ3 integrin were generously provided by Dr. Sanford Shattil. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS), 2 mM glutamine, 100 U mL−1 streptomycin, and 100 μg mL−1 penicillin (Irvine Scientific). Cell transfections were performed using Lipofectamine and Plus Reagent (Invitrogen) following the manufacturer’s instructions.
Plasmids and Other Reagents
GFP-Actin construct was purchased from BD Biosciences. HGF was purchased from R&D Systems Inc. Natural sceptrin isolated from the sponge Agelas nakamurai was bought from A. G. Scientific, Inc. Calpain XI inhibitor was purchased from Calbiochem. Synthetic sceptrin, bromosceptrin, dibromosceptrin, debromosceptrin, oxysceptrin, and nakamuric acid and its methyl ester were synthesized as described in ref (16). Lyophilized human plasma, thrombin, and Sigmacote were purchased from Sigma. Aphidicolin was bought from Sigma. Staurosporine was purchased from Calbiochem.
Cell Migration Assays
Motility assays were performed by using the Cell Motility HitKit (Cellomics). In this assay, five hundred cells per well were plated on 96-well dishes coated with blue fluorescence beads and cultured in complete medium for 24 h in the absence or presence of HGF (10 ng mL−1) as indicated in figure legends. Cell tracks generated upon cell movement were analyzed by using a fluorescence microscopy at 10x magnification. Using the freeware NIH ImageJ software, pictures were converted to binary mode, cell tracks were segmented, and the track surface was quantified.
Cell Proliferation Assay
HeLa cells were seeded in 6-well dishes at low confluence (30,000 cells well−1), treated with vehicle (H2O) or 40 μM of sceptrin and allowed to grow in complete medium up to 96 h. Medium containing fresh sceptrin or vehicle were replaced every 24 h. Cells were manually counted in a Neubauer chamber at 24, 48, 72, and 96 h.
Cell Death Assay
HeLa cells seeded in 6-well dishes at 50% confluence were treated with 10, 50, or 100 μM sceptrin, vehicle (H2O), or 5 nM staurosporine as positive control for 24 h. After treatment, cell death was analyzed by quantitative detection of cytoplasmic histone-associated DNA fragments using the Cell Death Detection ELISA kit (Roche). Manufacturer’s provided instructions were followed without any modification.
Time-Lapse Confocal Microscopy
HeLa or MDA-MB-231 cells transiently transfected to express the GFP-actin fusion protein were seeded on 4-well LabTek chambers that had been coated with 10 μg mL−1 of fibronectin. When indicated, cells were treated with 40 μM of sceptrin for 3 h prior to placing the chambers under the microscope. Cell motility in HeLa cells was stimulated with HGF (10 ng mL−1), whereas MDA-MB-231 cells were allowed to freely migrate in the complete culture medium without addition of any chemotactic agent. Chambers with the living cells were placed under an automated, temperature-controlled Olympus confocal microscope at 37 °C. A still image was taken every 2 min up to 3 h. Time-lapse movies and RGB composite images were mounted using the freeware NIH Image software (movies can be seen online in Supporting Information).
Clot Retraction Assay
A 400 μL sample of CHOαIIbβ3 cells (4 × 106 mL−1), in complete DMEM in the presence or absence of sceptrin (40 μΜ) or calpain XI inhibitor (10 μM), was mixed with 100 μL of human plasma, 2 U mL−1 thrombin, and 5 mM CaCl2 in a partially Sigmacote-treated glass cuvette. The clots were allowed to retract for 2 h at 37 °C and were photographed. The two-dimensional areas of retracted clots in the photographs were quantified using the NIH ImageJ software and the results were expressed as clot size.
Isothermal Titration Calorimetry (ITC)
ITC was carried out on an ITC200 calorimeter (Microcal). Aliquots (2 μL) of 3.0 mM sceptrin in water were injected into the cell containing 65 mM monomeric (G) actin in G-actin buffer (5 mM Tris pH 8.3, 0.3 μM CaCl2, 0.1 mM NaN3, 0.1 mM EDTA, 0.5 mM DTT, 0.24 mM ATP, and 1 μg mL−1 Leupeptin). G-actin was a generous gift from Drs. Robert Jeng and Dorit Hanein and was purified as described in ref (31). Equilibrium association constant (Ka) was obtained by fitting experimental data to “One set of sites” binding model using the Origin 7 software package (Microcal). Equilibrium dissociation constant (Kd) was calculated as Kd = 1/Ka.
Acknowledgments
We thank A. Bobkov (Burnham Institute for Medical Research) for assistance with ITC and D. Hanein, R. Jeng, S. Shattil, and G. Vande Woude for various reagents and cell lines.
Supporting Information Available
This material is available free of charge via the Internet.
Supplementary Material
References
- Newman D. J.; Cragg G. M. (2004) Marine natural products and related compounds in clinical and advanced preclinical trials. J. Nat. Prod. 67, 1216–1238. [DOI] [PubMed] [Google Scholar]
- Butler M. S. (2008) Natural products to drugs: natural product-derived compounds in clinical trials. Nat. Prod. Rep. 25, 475–516. [DOI] [PubMed] [Google Scholar]
- Laport M. S.; Santos O. C.; Muricy G. (2009) Marine sponges: potential sources of new antimicrobial drugs. Curr. Pharm. Biotechnol. 10, 86–105. [DOI] [PubMed] [Google Scholar]
- Faulkner D. J. (2000) Marine natural products. Nat. Prod. Rep. 17, 7–55. [DOI] [PubMed] [Google Scholar]
- Aiello A.; Fattorusso E.; Giordano A.; Menna M.; Muller W. E.; Perovic-Ottstadt S.; Schroder H. C. (2007) Damipipecolin and damituricin, novel bioactive bromopyrrole alkaloids from the Mediterranean sponge Axinella damicornis. Bioorg. Med. Chem. 15, 5877–5887. [DOI] [PubMed] [Google Scholar]
- Walker RP F. D.; Van Engen D; Clardy J. (1981) Sceptrin, an antimicrobial agent from the sponge Agelas sceptrum. J. Am. Chem. Soc. 103, 6772–6773. [Google Scholar]
- Bernan V. S.; Roll D. M.; Ireland C. M.; Greenstein M.; Maiese W. M.; Steinberg D. A. (1993) A study on the mechanism of action of sceptrin, an antimicrobial agent isolated from the South Pacific sponge Agelas mauritiana. J. Antimicrob. Chemother. 32, 539–550. [DOI] [PubMed] [Google Scholar]
- Bickmeyer U.; Drechsler C.; Kock M.; Assmann M. (2004) Brominated pyrrole alkaloids from marine Agelas sponges reduce depolarization-induced cellular calcium elevation. Toxicon 44, 45–51. [DOI] [PubMed] [Google Scholar]
- Rosa R.; Silva W.; Escalona de Motta G.; Rodriguez A. D.; Morales J. J.; Ortiz M. (1992) Anti-muscarinic activity of a family of C11N5 compounds isolated from I sponges. Experientia 48, 885–887. [DOI] [PubMed] [Google Scholar]
- Mohammed R.; Peng J.; Kelly M.; Hamann M. T. (2006) Cyclic heptapeptides from the Jamaican sponge Stylissa caribica. J. Nat. Prod. 69, 1739–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassas A.; Bourdy G.; Paillard J. J.; Lavayre J.; Pais M.; Quirion J. C.; Debitus C. (1996) Naturally occurring somatostatin and vasoactive intestinal peptide inhibitors. Isolation of alkaloids from two marine sponges. Planta Med. 62, 28–30. [DOI] [PubMed] [Google Scholar]
- Keifer P. A. S., R. E.; Koker M. E. S.; Hughes R. G. Jr.; Rittschof D.; Rinehart K. L. (1991) Bioactive bromopyrrole metabolites from the Caribbean sponge Agelas conifera. J. Org. Chem. 56, 2965–2975. [Google Scholar]
- Rodriguez A. D.; Lear M. J.; La Clair J. J. (2008) Identification of the binding of sceptrin to MreB via a bidirectional affinity protocol. J. Am. Chem. Soc. 130, 7256–7258. [DOI] [PubMed] [Google Scholar]
- Baran P. S.; Zografos A. L.; O’Malley D. P. (2004) Short total synthesis of (±)-sceptrin. J. Am. Chem. Soc. 126, 3726–3727. [DOI] [PubMed] [Google Scholar]
- Baran P. S.; Li K.; O’Malley D. P.; Mitsos C. (2005) Short, enantioselective total synthesis of sceptrin and ageliferin by programmed oxaquadricyclane fragmentation. Angew. Chem., Int. Ed. 45, 249–252. [DOI] [PubMed] [Google Scholar]
- O’Malley D. P.; Li K.; Maue M.; Zografos A. L.; Baran P. S. (2007) Total synthesis of dimeric pyrrole-imidazole alkaloids: sceptrin, ageliferin, nagelamide e, oxysceptrin, nakamuric acid, and the axinellamine carbon skeleton. J. Am. Chem. Soc. 129, 4762–4775. [DOI] [PubMed] [Google Scholar]
- Ridley A. J.; Schwartz M. A.; Burridge K.; Firtel R. A.; Ginsberg M. H.; Borisy G.; Parsons J. T.; Horwitz A. R. (2003) Cell migration: integrating signals from front to back. Science 302, 1704–1709. [DOI] [PubMed] [Google Scholar]
- Small J. V.; Rottner K.; Kaverina I.; Anderson K. I. (1998) Assembling an actin cytoskeleton for cell attachment and movement. Biochim. Biophys. Acta 1404, 271–281. [DOI] [PubMed] [Google Scholar]
- Kreis T. E.; Birchmeier W. (1980) Stress fiber sarcomeres of fibroblasts are contractile. Cell 22, 555–561. [DOI] [PubMed] [Google Scholar]
- Isenberg G.; Wohlfarth-Bottermann K. E. (1976) Transformation of cytoplasmic actin. Importance for the organization of the contractile gel reticulum and the contraction-relasation cycle of cytoplasmic actomyosin. Cell Tissue Res. 173, 495–528. [DOI] [PubMed] [Google Scholar]
- Ishizaki T.; Maekawa M.; Fujisawa K.; Okawa K.; Iwamatsu A.; Fujita A.; Watanabe N.; Saito Y.; Kakizuka A.; Morii N.; Narumiya S. (1996) The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 15, 1885–1893. [PMC free article] [PubMed] [Google Scholar]
- Leung T.; Manser E.; Tan L.; Lim L. (1995) A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J. Biol. Chem. 270, 29051–29054. [DOI] [PubMed] [Google Scholar]
- Giry M.; Popoff M. R.; von Eichel-Streiber C.; Boquet P. (1995) Transient expression of RhoA, -B, and -C GTPases in HeLa cells potentiates resistance to Clostridium difficile toxins A and B but not to Clostridium sordellii lethal toxin. Infect. Immun. 63, 4063–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson H. F.; Self A. J.; Garrett M. D.; Just I.; Aktories K.; Hall A. (1990) Microinjection of recombinant p21rho induces rapid changes in cell morphology. J. Cell Biol. 111, 1001–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardin P.; Boquet P.; Madaule P.; Popoff M. R.; Rubin E. J.; Gill D. M. (1989) The mammalian G protein rhoC is ADP-ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilaments in Vero cells. EMBO J. 8, 1087–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumiya S.; Tanji M.; Ishizaki T. (2009) Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 12, 65–76. [DOI] [PubMed] [Google Scholar]
- Watanabe N.; Madaule P.; Reid T.; Ishizaki T.; Watanabe G.; Kakizuka A.; Saito Y.; Nakao K.; Jockusch B. M.; Narumiya S. (1997) p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 16, 3044–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyavsky A. I.; Arredondo J.; Wess J.; Karlsson E.; Grando S. A. (2004) Novel signaling pathways mediating reciprocal control of keratinocyte migration and wound epithelialization through M3 and M4 muscarinic receptors. J. Cell Biol. 166, 261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyavsky A. I.; Nguyen V. T.; Arredondo J.; Ndoye A.; Zia S.; Wess J.; Grando S. A. (2003) The M4 muscarinic receptor-selective effects on keratinocyte crawling locomotion. Life Sci. 72, 2069–73. [DOI] [PubMed] [Google Scholar]
- Molinski T. F.; Dalisay D. S.; Lievens S. L.; Saludes J. P. (2009) Drug development from marine natural products. Nat. Rev. Drug Discovery 8, 69–85. [DOI] [PubMed] [Google Scholar]
- Volkmann N.; Hanein D.; Ouyang G.; Trybus K. M.; DeRosier D. J.; Lowey S. (2000) Evidence for cleft closure in actomyosin upon ADP release. Nat. Struct. Biol. 7, 1147–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.