Abstract
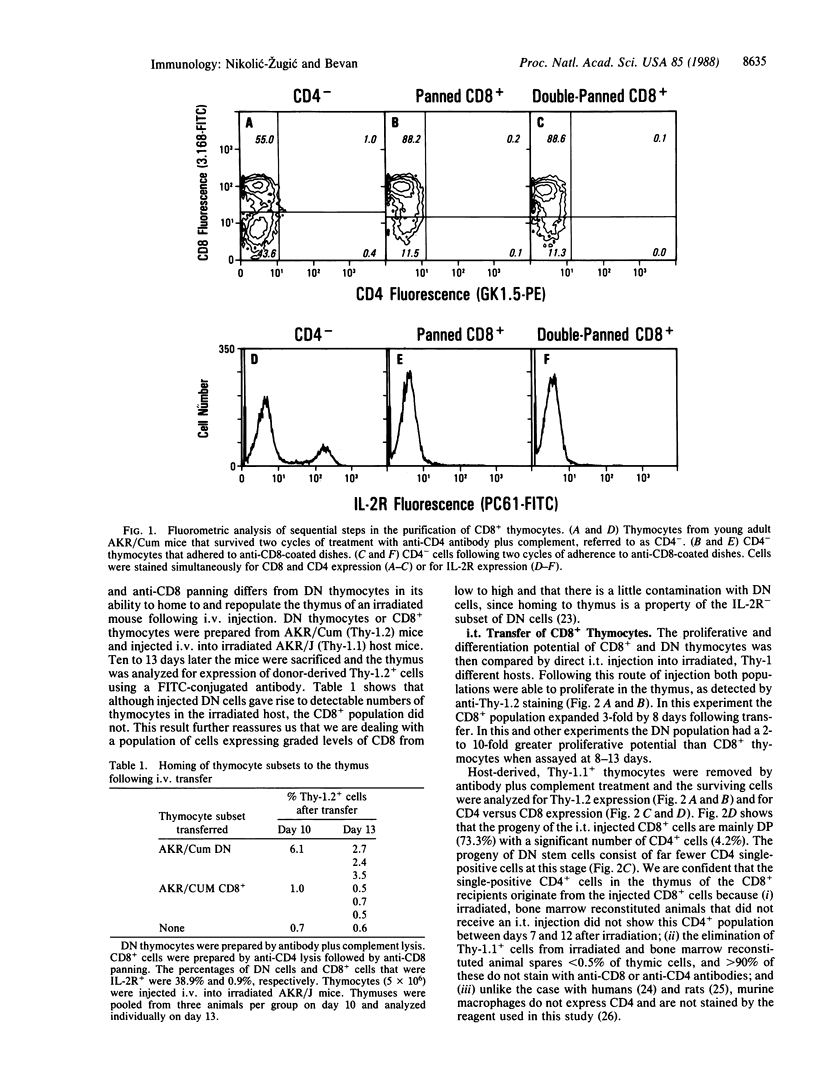
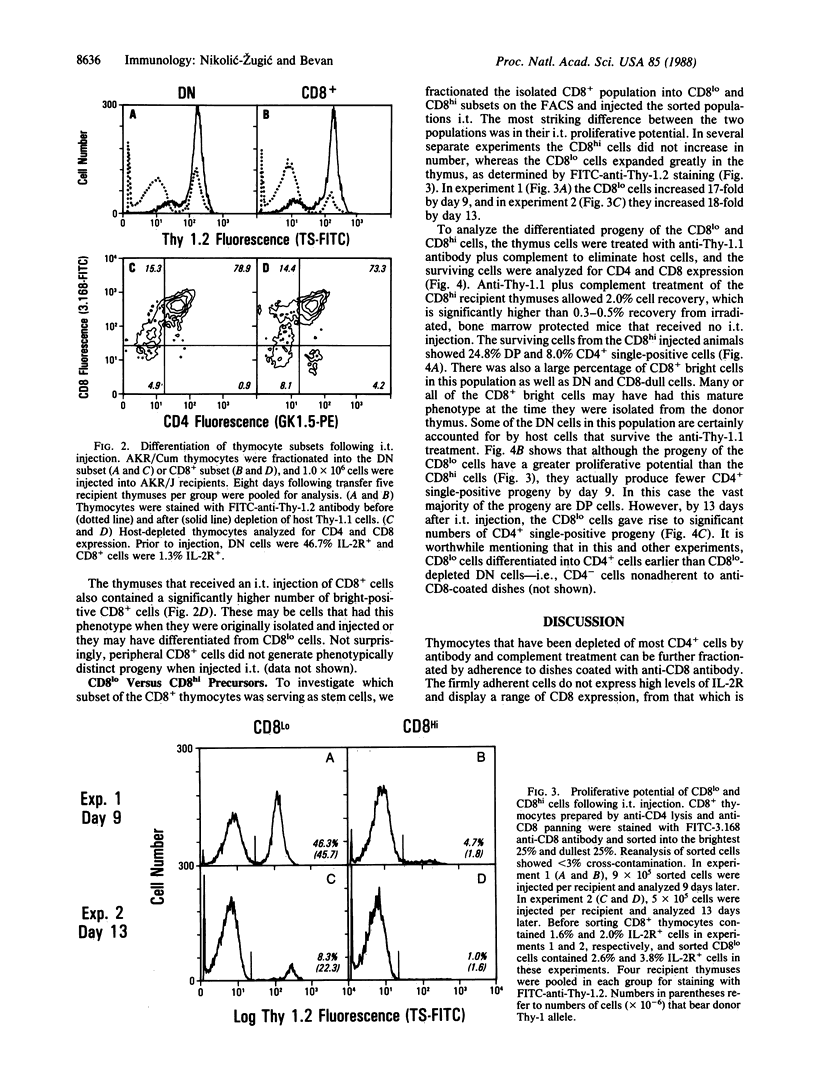
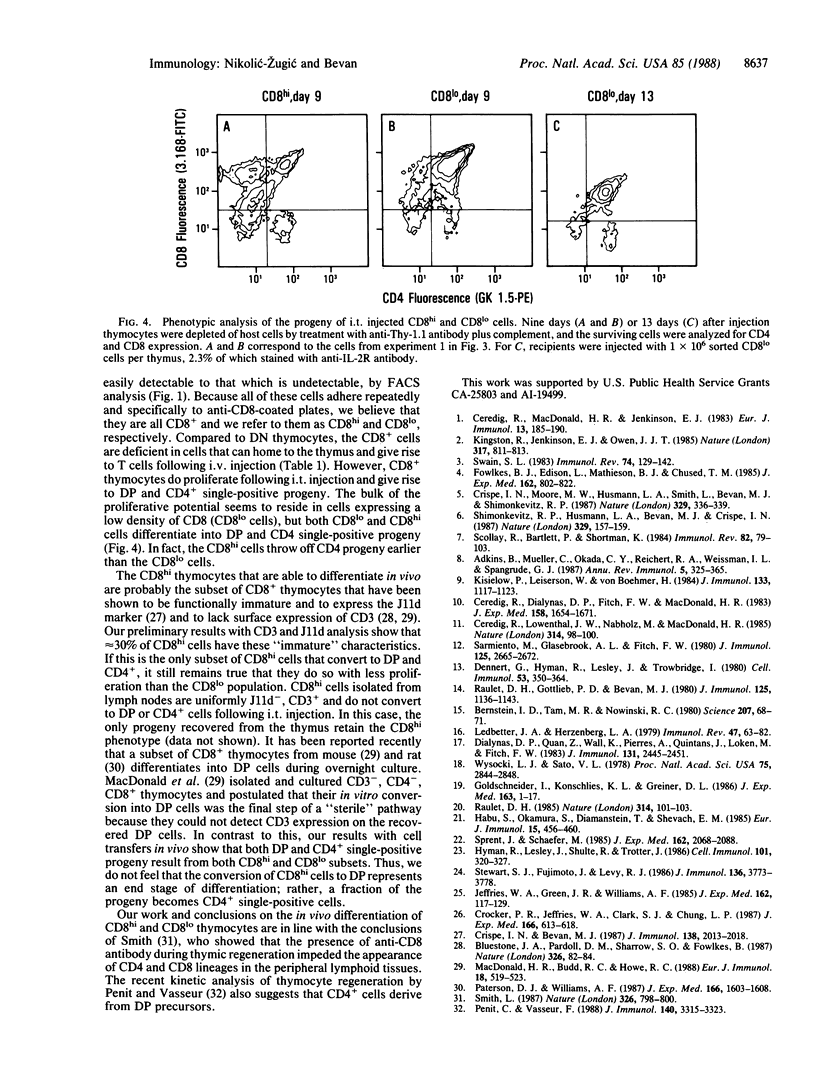
Based on the cell surface expression of CD4 and CD8 molecules, murine thymocytes can be divided into four populations: these include CD4-, CD8- double-negative and CD4+, CD8+ double-positive subpopulations, both of which consist largely of immature cells, and the single positive, CD4+ or CD8+, subsets that contain functional helper or killer cells, respectively. The double-negative subset contains precursors of the other three populations and can reconstitute the thymus following intravenous or intrathymic transfer into irradiated hosts. In an attempt to establish the sequence of CD4 and CD8 expression during intrathymic development, we investigated the differentiation potential of highly purified CD8+ thymocytes by using intravenous or intrathymic adoptive transfer. Unlike the double-negative thymocyte subset, CD8+ cells did not have the ability to home to the thymus following intravenous transfer. However, when CD8+ thymocytes were injected directly into the thymus, they increased in number and gave rise to CD4+, CD8+ double-positive and CD4+ single-positive progeny. Furthermore, the rate of appearance of CD4+ cells from injected CD8+ precursors was faster than from the double-negative subset. Cells expressing a high surface density of CD8 convert to double-positive and CD4+ progeny without increasing in number, whereas CD8+ cells expressing a low surface density of the marker expand greatly and give rise to differentiated progeny. The results suggest that CD8 expression is an intermediate step on the differentiation pathway of mature CD4+ T cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins B., Mueller C., Okada C. Y., Reichert R. A., Weissman I. L., Spangrude G. J. Early events in T-cell maturation. Annu Rev Immunol. 1987;5:325–365. doi: 10.1146/annurev.iy.05.040187.001545. [DOI] [PubMed] [Google Scholar]
- Bernstein I. D., Tam M. R., Nowinski R. C. Mouse leukemia: therapy with monoclonal antibodies against a thymus differentiation antigen. Science. 1980 Jan 4;207(4426):68–71. doi: 10.1126/science.6965328. [DOI] [PubMed] [Google Scholar]
- Bluestone J. A., Pardoll D., Sharrow S. O., Fowlkes B. J. Characterization of murine thymocytes with CD3-associated T-cell receptor structures. Nature. 1987 Mar 5;326(6108):82–84. doi: 10.1038/326082a0. [DOI] [PubMed] [Google Scholar]
- Ceredig R., Dialynas D. P., Fitch F. W., MacDonald H. R. Precursors of T cell growth factor producing cells in the thymus: ontogeny, frequency, and quantitative recovery in a subpopulation of phenotypically mature thymocytes defined by monoclonal antibody GK-1.5. J Exp Med. 1983 Nov 1;158(5):1654–1671. doi: 10.1084/jem.158.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Ceredig R., MacDonald H. R., Jenkinson E. J. Flow microfluorometric analysis of mouse thymus development in vivo and in vitro. Eur J Immunol. 1983 Mar;13(3):185–190. doi: 10.1002/eji.1830130302. [DOI] [PubMed] [Google Scholar]
- Crispe I. N., Bevan M. J. Expression and functional significance of the J11d marker on mouse thymocytes. J Immunol. 1987 Apr 1;138(7):2013–2018. [PubMed] [Google Scholar]
- Crispe I. N., Moore M. W., Husmann L. A., Smith L., Bevan M. J., Shimonkevitz R. P. Differentiation potential of subsets of CD4-8- thymocytes. Nature. 1987 Sep 24;329(6137):336–339. doi: 10.1038/329336a0. [DOI] [PubMed] [Google Scholar]
- Crocker P. R., Jefferies W. A., Clark S. J., Chung L. P., Gordon S. Species heterogeneity in macrophage expression of the CD4 antigen. J Exp Med. 1987 Aug 1;166(2):613–618. doi: 10.1084/jem.166.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennert G., Hyman R., Lesley J., Trowbridge I. S. Effects of cytotoxic monoclonal antibody specific for T200 glycoprotein on functional lymphoid cell populations. Cell Immunol. 1980 Aug 1;53(2):350–364. doi: 10.1016/0008-8749(80)90335-4. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Fowlkes B. J., Edison L., Mathieson B. J., Chused T. M. Early T lymphocytes. Differentiation in vivo of adult intrathymic precursor cells. J Exp Med. 1985 Sep 1;162(3):802–822. doi: 10.1084/jem.162.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Komschlies K. L., Greiner D. L. Studies of thymocytopoiesis in rats and mice. I. Kinetics of appearance of thymocytes using a direct intrathymic adoptive transfer assay for thymocyte precursors. J Exp Med. 1986 Jan 1;163(1):1–17. doi: 10.1084/jem.163.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habu S., Okumura K., Diamantstein T., Shevach E. M. Expression of interleukin 2 receptor on murine fetal thymocytes. Eur J Immunol. 1985 May;15(5):456–460. doi: 10.1002/eji.1830150508. [DOI] [PubMed] [Google Scholar]
- Hyman R., Lesley J., Schulte R., Trotter J. Progenitor cells in the thymus: most thymus-homing progenitor cells in the adult mouse thymus bear Pgp-1 glycoprotein but not interleukin-2 receptor on their cell surface. Cell Immunol. 1986 Sep;101(2):320–327. doi: 10.1016/0008-8749(86)90145-0. [DOI] [PubMed] [Google Scholar]
- Jefferies W. A., Green J. R., Williams A. F. Authentic T helper CD4 (W3/25) antigen on rat peritoneal macrophages. J Exp Med. 1985 Jul 1;162(1):117–127. doi: 10.1084/jem.162.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston R., Jenkinson E. J., Owen J. J. A single stem cell can recolonize an embryonic thymus, producing phenotypically distinct T-cell populations. 1985 Oct 31-Nov 6Nature. 317(6040):811–813. doi: 10.1038/317811a0. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Leiserson W., Von Boehmer H. Differentiation of thymocytes in fetal organ culture: analysis of phenotypic changes accompanying the appearance of cytolytic and interleukin 2-producing cells. J Immunol. 1984 Sep;133(3):1117–1123. [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Budd R. C., Howe R. C. A CD3- subset of CD4-8+ thymocytes: a rapidly cycling intermediate in the generation of CD4+8+ cells. Eur J Immunol. 1988 Apr;18(4):519–523. doi: 10.1002/eji.1830180405. [DOI] [PubMed] [Google Scholar]
- Paterson D. J., Williams A. F. An intermediate cell in thymocyte differentiation that expresses CD8 but not CD4 antigen. J Exp Med. 1987 Nov 1;166(5):1603–1608. doi: 10.1084/jem.166.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penit C., Vasseur F. Sequential events in thymocyte differentiation and thymus regeneration revealed by a combination of bromodeoxyuridine DNA labeling and antimitotic drug treatment. J Immunol. 1988 May 15;140(10):3315–3323. [PubMed] [Google Scholar]
- Raulet D. H. Expression and function of interleukin-2 receptors on immature thymocytes. Nature. 1985 Mar 7;314(6006):101–103. doi: 10.1038/314101a0. [DOI] [PubMed] [Google Scholar]
- Raulet D. H., Gottlieb P. D., Bevan M. J. Fractionation of lymphocyte populations with monoclonal antibodies specific for LYT-2.2 and LYT-3.1. J Immunol. 1980 Sep;125(3):1136–1143. [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A. L., Fitch F. W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980 Dec;125(6):2665–2672. [PubMed] [Google Scholar]
- Scollay R., Bartlett P., Shortman K. T cell development in the adult murine thymus: changes in the expression of the surface antigens Ly2, L3T4 and B2A2 during development from early precursor cells to emigrants. Immunol Rev. 1984 Dec;82:79–103. doi: 10.1111/j.1600-065x.1984.tb01118.x. [DOI] [PubMed] [Google Scholar]
- Shimonkevitz R. P., Husmann L. A., Bevan M. J., Crispe I. N. Transient expression of IL-2 receptor precedes the differentiation of immature thymocytes. Nature. 1987 Sep 10;329(6135):157–159. doi: 10.1038/329157a0. [DOI] [PubMed] [Google Scholar]
- Smith L. CD4+ murine T cells develop from CD8+ precursors in vivo. Nature. 1987 Apr 23;326(6115):798–800. doi: 10.1038/326798a0. [DOI] [PubMed] [Google Scholar]
- Sprent J., Schaefer M. Properties of purified T cell subsets. I. In vitro responses to class I vs. class II H-2 alloantigens. J Exp Med. 1985 Dec 1;162(6):2068–2088. doi: 10.1084/jem.162.6.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S. J., Fujimoto J., Levy R. Human T lymphocytes and monocytes bear the same Leu-3(T4) antigen. J Immunol. 1986 May 15;136(10):3773–3778. [PubMed] [Google Scholar]
- Swain S. L. T cell subsets and the recognition of MHC class. Immunol Rev. 1983;74:129–142. doi: 10.1111/j.1600-065x.1983.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]


