Abstract
Aim
To identify alcohol biomarker and psychometric measures that relate to drivers’ blood alcohol concentration (BAC) patterns from ignition interlock devices (IIDs).
Design, Setting, Participants, Measurements
In Alberta, Canada, 534 drivers, convicted of driving under the influence of alcohol (DUI), installed IIDs and agreed to participate in a research study. IID BAC tests are an established proxy for predicting future DUI convictions. Three risk groups were defined by rates of failed BAC tests. Program entry and followup blood samples (n=302, 171) were used to measure phosphatidyl ethanol (PETH), carbohydrate deficient transferrin (%CDT), gamma glutamyltransferase (GGT) and other biomarkers. Program entry urine (n=130) was analyzed for ethyl glucuronide (ETG) and ethyl sulfate (ETS). Entry hair samples were tested for fatty acid ethyl esters (FAEE) (n=92) and ETG (n=146). Psychometric measures included the DSM-4 Diagnostic Interview Schedule Alcohol Module, Alcohol Use Disorders Identification Test (AUDIT), the Timeline Followback (TLFB), the Drinker Inventory of Consequences (DRINC), and the Temptation and Restraint Inventory (TRI).
Findings
Except for FAEE, all alcohol biomarkers were significantly related to the interlock BAC test profiles; higher marker levels predicted higher rates of interlock BAC test failures. PETH, the strongest with an overall ANOVA F ratio of 35.5, had significant correlations with all nine of the other alcohol biomarkers and with 16 of 19 psychometric variables. Urine ETG and ETS were strongly correlated with the IID BAC tests.
Conclusions
The findings suggest several alcohol biomarkers and assessments could play an important role in the prediction and control of driver alcohol risk when relicensing.
Keywords: alcohol, driver, ignition interlock, biomarkers, assessment, DUI, phosphatidyl ethanol (PETH)
INTRODUCTION
Alcohol-related Risk on the Roadways
In the United States, alcohol use is associated with the road death of approximately 17,000 people annually. Annually, over the past 10 years, 21–22% of all fatal crashes have had a driver with a BAC (blood alcohol concentration) of more than .08 g/dL (the USA legal limit) [1]. Alcohol ignition interlocks (IIDs), which require a breath test before an engine start, were devised to prevent alcohol-impaired driving by requiring a driver convicted of DUI (driving under the influence of alcohol) to blow a low BAC air sample (usually set to lock the ignition at .02 – .04 g/dL) before a car can be started.
Interlocks have been studied in North American for 20 years. Ten studies with different subgroups and durations show a 40 to 90% reduction in recidivism while the IID is installed on the vehicle [2; 3; 4]. A meta-analysis by Willis, Lybrand, and Bellamy [5] estimated that IIDs reduced the relative risk of DUI recidivism by 64%. Once the IID is removed from the vehicle, recidivism increases to control rates.
Interlock BAC Test Patterns
In addition to blocking vehicle starts by a driver with an elevated BAC, the IID also logs a cumulative record of BAC tests. Studies of the IID BAC test records have been reported on 2000+ DUI offenders in Alberta, Canada (1995–1999 [6, 7, 8]), 7500+ offenders in Quebec, Canada (2000–2003 [9]), and 7000+ offenders in New Mexico (2003–2008). This data from over 16,000 DUI offenders show a consistent pattern of behavior in which the rate of positive (and generally locked out/failed) BAC tests in the IID record reliably predicts post-interlock repeat DUI. That is, attempts to start a car with positive BAC predict subsequent recidivism (after IID removal). The positive BAC tests occur most frequently during the first start up of the day (7 to 9 AM on working weekdays, 10 AM to noon on weekends) and likely reflect drinking the night before resulting in a BAC that has still not fallen below .02 g/dL by morning. The morning pattern was confirmed in Texas with another 11,000 offenders providing 20 million additional tests [10]. In all three sites, the most vehicle starts Monday-Friday occur around 5 PM.
Alcohol Biomarkers for Triangulating Driver Risk
Additional predictors of driver alcohol-related risk are still warranted for forensic purposes. The IID is a vehicle sanction; others are permitted to drive the car and, possibly, contribute breath-test results to the log file. More importantly, some high-risk drivers defy the IID requirement and drive cars without interlocks. Alcohol biomarkers can provide driver-specific information. While biomarkers seem to have not had systematic use in North America as an aid in road safety, they are increasingly used in Europe for driver re-licensing decisions.
In North America, biomarkers are most commonly used to supplement diagnostic assessments among alcohol-dependent patients participating in treatment or in clinical trials [11]. Because clinical diagnosis is part art, part science, using it as a criterion for validating markers is unsatisfying. There is no gold standard in the clinic, but interlock data can provide a useful high-sensitivity criterion. Together, alcohol biomarkers and interlock BAC test profiles may prove to be useful independent methods for quantifying the risk posed by convicted DUI offenders.
Indirect Markers: CDT, GGT, MCV, ALT, AST
The indirect alcohol markers primarily reflect organ responses to repeated ethanol exposure. The most widely used are carbohydrate deficient transferrin (CDT) and gamma glutamyltransferase (GGT), both of which can be measured in serum for several days to several weeks after high levels of regular drinking. GGT is dependable, widely used, and inexpensive but less specific than CDT; GGT can be elevated for reasons having to do with hepatic or biliary disease unrelated to alcohol [12]. GGT was reported to be elevated in one-fourth to one-half of those arrested for alcohol-related driving infractions [13].
Regarding CDT, transferrin is a glycoprotein made in the liver that transports iron throughout the body. Each molecule has several carbohydrate chains with sialic acid end groups, some of which are lost after heavy drinking. As heavy drinking progresses, more end groups are deficient in their carbohydrate residue. The percent deficiency (%CDT) renders it a very specific alcohol exposure marker. Consumption of five or more drinks per day (60 g ethanol), for a week or more, is widely cited as sufficient to elevate CDT. Age-related or gender-related factors may affect CDT measurement [14]. Among the widely used indirect markers, macrocytic (red cell) volume (MCV), although moderately specific to ethanol, is far less sensitive than other markers.
Several European nations include indirect alcohol biomarkers, especially CDT, GGT, and MCV as part of their re-licensing decisions. In Luxembourg, Appenzeller, Schneider, Maul, and Wennig [15] found that drivers with arrest BAC levels in the ranges of .25–.30, .30–.35 and .35+ g/dL had CDT levels higher than 3% at rates of 22%, 47% and 67% of the sample, respectively. Gilg, Bucholtz, and Huth [16] in Germany found 24% of drivers arrested for DUI had elevated CDT, 26% had elevated GGT, but only 7% had both. The method of combining and the types of markers can improve sensitivity or specificity. Korzec et al. [13] found 74 and 82% of his driver sample were heavy drinkers based on the biomarker evidence, but a dependence diagnostic instrument identified just 4% as alcoholic. A Norwegian study [17] found that GGT was more likely to be elevated in arrested males older than age 29. GGT levels were at least twice as high in those with arrest BACs ≥ .25 g/dL than in those between .05 and .15 g/dL. There is no standard protocol for selecting biomarkers or cutoff levels. Sweden, for example, has a medical review program for DUI offenders that links reductions of alcohol markers to successful completion of their interlock program [18; 19]. Relicensing decisions are made using GGT, CDT, and MCV but also the nonspecific liver enzymes AST (aspartate aminotransferase) and ALT (alanine aminotransferase) in a Boolean “OR” combination to maximize sensitivity. Specificity of driver fitness decisions are improved by looking for markers at or above the 97.5 percentile of national mean population levels. Bjerre and colleagues have reported that this monitoring program reduces sick leave and hospital utilization [20].
Direct Markers: PETH, FAEE, ETG, ETS
Markers that directly reflect acute alcohol ingestion have had little study with DUI drivers. Unlike many indirect markers that reflect end-organ response to alcohol, the direct markers are both very specific and very sensitive.
This class of markers includes phosphatidyl ethanol (PETH), a membrane phospholipid found in the erythrocyte fraction of whole blood and formed only in the presence of ethanol [21]. PETH’s half-life is about 4 days, and no substances besides alcohol cause positives on PETH [22; 23; 24]. Hartmann et al. [25] reported that PETH is detectable in blood for 2 weeks after sustained ethanol intake.
Minor pathways of ethanol metabolism synthesize ethyl glucuronide (ETG) (via conjugation with glucuronic acid) or ethyl sulfate (ETS) (via sulfation). ETG and ETS can be found in serum, urine, or hair, and ETG concentrations are 1/1000 that of ethanol. Both ETG and ETS can be measured up to 2 days in the urine [26] after dosing to .08 g/dL, or longer in clinical samples of heavy drinkers [27]. Wurst and others [28; 29] suggest that ETS may be a more stable indicator of ethanol exposure than ETG, as ETS is less likely to be altered by bacteria if there is a urinary tract infection at the time of sampling [30]. Kip et al. [31] reported that in the emergency room, ETG and PETH are more reliable indicators of drinking than the Alcohol Use Disorders Identification Test (AUDIT).
Fatty acid ethyl esters (FAEE), another direct alcohol marker found in both serum and hair, has shown promise as an indicator of alcohol use proclivity [32]. Pragst and Yegles [32] reported that fatal alcohol crash victims had an average combined FAEE level 10 times higher than social drinkers. A cutoff of 0.5 ng/mg is thought to distinguish moderate to heavy drinking. To detect high-risk drinkers for diagnostic purposes, the investigators suggest using the combined FAEE cutoff value of 1.0 ng/mg and/or a positive hair ETG result of 25 pg/mg [33]. Kintz et al. [34] recommended 50 pg/mg cutoff for judging hair ETG as high.
Significance
The convergence of information in interlock BAC test records, alcohol psychometric assessments, and alcohol biomarkers holds promise for objectifying the often-difficult decisions related to driver licensing. This research study was designed to examine how well these indicators converge, whether they can be usefully combined, and to determine levels of the marker types most associated with high-risk BAC profiles in the interlock record. This initial paper provides simple descriptive summaries and cross-relationships between the markers, the interlock BAC tests, and the psychometric measures. Alcohol dependence is not a precondition for DUI; better tools can improve the evaluation of public risk exposure to drinking drivers.
METHODS
This research was conducted by the Pacific Institute for Research and Evaluation (PIRE) in the Washington DC area; subjects were recruited in Alberta, Canada (province population ~ 3.3 million), at the Guardian Interlock Systems (GIS) service center in Edmonton (city population ~ 730,000). This project—a three-partner collaboration of the Alberta Transportation Safety Board (ATSB) of the provincial Transport Ministry, GIS, and PIRE—is the second interlock research collaboration since 1994 for these organizations.
DUI offenders from central Alberta who want an IID installed do so at the GIS service center in Edmonton. The interlock business and this interlock research were completely separate activities managed by different people. For DUI offenders participation was voluntary, confidential, and had no bearing on the offender’s relationship with the government. All subjects signed informed-consent documents, and all research staff completed the National Institutes of Health training protocols for human subjects’ research. This research was reviewed and approved annually by the PIRE East IRB under FWA00003078. Intake proceeded continuously as prospective subjects became available between the dates of December 2003 and May 2007.
Procedures: Subjects and Measures
At the time of IID installation, 942 DUI offenders were asked to participate in the research: 58% of first offenders (n=346) and 53% of multiple offenders (n=183) agreed. Among multiple offenders, 125 were second offenders, and 58 had three or more prior DUI convictions; 6 could not be matched. The “look-back” period for defining prior offenses in Alberta driver records is 10 years. The 534 participants were 87% male, mean age 38.7 ±11.5 ranging from ages 19 to 74. Participants reported Caucasian ancestry (91%) with the remaining 9% split among nine ethnic categories, none more than 2.5%. The most prevalent income group was the highest (24%), earning more than $90,000 Canadian Dollars (CDN) per year (likely reflecting Alberta’s booming oil industry); Canada’s 2004 median income was $70,000/year. Just 44% of participants lived in or near Edmonton; the others were from rural towns or farms (39%), and 16% were from small urban areas beyond Edmonton.
Participants reported on general demographics, the Timeline Followback (past 30 days of drinking) [35], DSM-IV (Diagnostic and Statistical Manual IV) Module R (alcohol abuse and dependence) via the Computerized Diagnostic Interview Schedule (C-DIS) interview [36], the (AUDIT) [37], the Drinkers Inventory of Consequences (DRINC) [38], and the Temptation and Restraint Inventory (TRI) [39]. The research coordinator, who administered the C-DIS, had trained on C-DIS during a week in St. Louis with the Washington University staff. Thirty-nine percent of the study subjects met DSM-IV criteria for alcohol dependence, 12% met criteria for alcohol abuse. Nearly half (49%) were either negative or could not be definitively diagnosed by the software; 13% (69 of 536) were fully negative for both diagnoses (e.g., without inconclusive diagnoses).
Most subjects were asked to provide blood samples twice, both at the start and near the end of their IID requirement. The lag between the initial and subsequent blood samples was about 8 months (mean=8.4 and median=7.8). About 55% of those asked provided a second blood sample; the drop off may have reflected the inadequacy of the participant fees relative to the time burden for reporting to the blood lab.
After the research began, informed consent was adjusted and approved so hair samples and urine samples could be collected. Table 1 shows the count of different markers that were measured by source collected and whether they were baseline or followup samples. The smaller number of urine (prefix “u”) and hair (prefix “h”) samples reflects their late addition to the protocol. Samples for PETH, CDT, uETG, and uETS were held at −80C for long-term storage, and then were batched and packed in dry ice for shipment. Hair was sampled from the scalp near the inion, packed in aluminum foil and shipped for measurement of hFAEE and/or hETG.
Table 1.
Alcohol biomarkers measured
| Marker | Source/unit | Initial sample n= |
Followup sample n= |
|---|---|---|---|
| Macrocytic volume (MCV) | Red cell fL | 300 | 170 |
| Alanine aminotransferase (ALT) | Serum U/L | 302 | 171 |
| Aspartate Aminotransferase, (AST) | Serum U/L | 302 | 171 |
| Gamma glutamyltransferase (GGT) | Serum U/L | 302 | 171 |
| Carbohydrate deficient transferrin (CDT) | Serum % isoforms to total | 298 | 165 |
| Phosphatidyl ethanol (PETH) | Whole Blood µmol/L | 298 | 167 |
| Fatty acid ethyl esters (hFAEE) | Hair ng/mg | 92 | 0 |
| Ethyl glucuronide (hETG) | Hair pg/mg | 146 | 0 |
| Ethyl glucuronide (uETG) | Urine ng/ml | 130 | 0 |
| Ethyl sulfate (uETS) | Urine ng/ml | 130 | 0 |
Analytic Methods—Alcohol Markers
Heparinized whole blood was collected, stored at −80C, and shipped in three batches over 4 years of sample collection. In Lund, Sweden, in the laboratory of Drs. Christer Alling, Steina Aradottir, and Therese Hansson, phosphatidyl ethanol was extracted and measured by high performance liquid chromatography (HPLC) via published protocols [23; 24; 40; 41]. The limit of quantitation (LOQ) was 0.22 µmol PETH/L; 31.5% of baseline samples and 40.7% of the followup samples were below the LOQ. Samples were analyzed with control samples on two levels (low control 0.63 µmol/L, and high control 0.82 µmol/L) as a quality check above and below typical clinical cutoff values of 0.70 µmol/L. High variability is found below 0.70 µmol/L.
CDT was measured in the laboratory of Dr. Martin Javors at the University of Texas in San Antonio with the use of Axis-Shield (Oslo, Norway) immunoassay “%CDT” kits, which measure the percentage of total transferrin in serum that consists of asialo-, monosialo-, and disialo-transferrin isoforms. Javors determined that the Axis-Shield kit has high-test/retest reliability through analysis of 197 human samples randomly selected from approximately 20% of all samples to verify reliability. Results showed r2 = .78, with x and y intercepts not statistically different from zero [42].
ETG and FAEE in hair [32] were determined, respectively, at the laboratories of Dr. Michel Yegles at the University of Luxembourg and Dr. Fritz Pragst at Humbodlt University in Berlin, Germany. When there was sufficient material, a sample of hair was used for both measurements. Methods for extraction and analysis of ETG in hair are found in Yegles et al. [33]. The analytic procedures for FAEE extraction and measurement in hair were documented in Pragst et al. [43].
Urine samples, collected in years 3 and 4, were frozen and shipped for measurement of ethyl glucuronide and ethyl sulfate at the United States Drug Testing Laboratory (USDTL), a commercial facility near Chicago, Illinois. The specimens were analyzed for creatinine and ethanol; uETG and uETS determinations were performed using standard protocols on an API 2000 high-flow liquid chromatographic-tandem mass spectrometry (LC-MS/MS) (Applied Biosystems, Foster City, CA, USA). The limit of detection for uETG and uETS was 38.7 ng/mL and 7.2 ng/mL, respectively. High values were clipped at 10,001 ng/mL.
The measurement of GGT, AST, ALT, and MCV was conducted with standard clinical chemistry protocols at the Dynacare Kasper Medical Laboratory in Edmonton promptly following blood collection.
Interlock Data
The IID installation duration and vehicle usage vary across subjects. To account for differences, interlock usage (tests taken) is set as a denominator and failed interlock BAC tests are expressed as a rate relative to all tests taken by each subject. Study subjects used the interlock car an average of 277 days; subjects performed a mean 2800 BAC tests during the study. This reflects a median of 9.9 (mean 10.7) BAC tests per day that the car was used, reflecting a mix of startup tests and the required running retests. The overall mean number of BAC tests during the full time the IID was installed was 9.8 tests per day of which 5.8 were startup tests; accordingly, 63% of all tests were startup tests. The target for analysis is startup tests because startup tests have a higher density of positive BAC than retests (e.g., all retests must be preceded by a passed startup test). In Alberta, the ignition locks out at .04 g/dL
Statistical Methods
All analyses were done with SPSS ver. 15. Procedures include order and interval correlations, one-way analyses of variance (ANOVAs), and when appropriate square root and log normalizing transformations. All tables are based on raw values to allow readers to compare levels across studies.
RESULTS
Results are organized to show (1) alcohol biomarker interrelationships, (2) the relationship between alcohol biomarker and interlock BAC test results, (3) the relationship between psychometric or diagnostic assessments and the interlock BAC test results, and (4) the relationship between biomarkers and the psychometric measures.
Marker Interrelationships
Table 2 shows biomarker cross correlations calculated for both Pearson (interval correlations on the right above the diagonal) and Spearman (ordinal correlations shaded on the left below the diagonal tests). PETH levels were significantly correlated with all other blood markers in both Pearson and Spearman at P<.0005. The exception to the generally significant correlations between PETH and other markers was its Pearson correlation with hair ETG. FAEE showed weak positive correlations using Pearson and strongly significantly correlations using Spearman. Spearman correlations are not influenced by outliers or other departures from distributional normality, as is Pearson's test.
Table 2.
Initial alcohol biomarker correlations: Pearson (top right, above diagonal) and Spearman (shaded bottom left, below diagonal)
| MCV | ALT | AST | GGT | %CDT | PETH | Hair_FAEE | Hair_ETG | Urine_ETG | Urine_ETS | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MCV | r | 1 | .05 | .15(**) | .16(**) | .20(**) | .26(***) | .13 | .13 | .18(*) | .10 |
| n | 300 | 300 | 300 | 300 | 297 | 297 | 62 | 101 | 129 | 129 | |
| ALT | r | −.02 | 1 | .85(***) | .57(***) | −.03 | .34(***) | .22 | −.04 | .29(**) | .24(**) |
| n | 300 | 302 | 302 | 302 | 298 | 298 | 63 | 102 | 130 | 130 | |
| AST | r | .08 | .74(***) | 1 | .59(***) | .08 | .41(***) | .26(*) | .02 | .34(***) | .30(***) |
| n | 300 | 302 | 302 | 302 | 298 | 298 | 63 | 102 | 130 | 130 | |
| GGT | r | .03 | .66(***) | .47(***) | 1 | −.02 | .44(***) | .09 | .18 | .35(***) | .34(***) |
| n | 300 | 302 | 302 | 302 | 298 | 298 | 63 | 102 | 130 | 130 | |
| %CDT | r | .17(**) | −.10 | .04 | −.09 | 1 | .39(***) | .10 | .01 | .17 | .17 |
| n | 297 | 298 | 298 | 298 | 298 | 297 | 62 | 101 | 130 | 130 | |
| PETH | r | .30(***) | .22(***) | .27(***) | .42(***) | .36(***) | 1 | .26(*) | .18 | .41(***) | .38(***) |
| n | 297 | 298 | 298 | 298 | 297 | 298 | 62 | 101 | 129 | 129 | |
| Hair_FAEE | r | .19 | .13 | .19 | .35(**) | .08 | .52(***) | 1 | .01 | .40 | .03 |
| n | 62 | 63 | 63 | 63 | 62 | 62 | 92 | 92 | 4 | 4 | |
| Hair_ETG | r | .12 | .08 | .19 | .18 | .07 | .20(*) | .28(**) | 1 | .25 | .43(**) |
| n | 101 | 102 | 102 | 102 | 101 | 101 | 92 | 146 | 42 | 42 | |
| Urine_ETG | r | .27(**) | .18(*) | .29(**) | .12 | .29(**) | .47(***) | .40 | .13 | 1 | .90(***) |
| n | 129 | 130 | 130 | 130 | 130 | 129 | 4 | 42 | 130 | 130 | |
| Urine_ETS | r | .24(**) | .21(*) | .30(***) | .19(*) | .25(**) | .47(***) | .20 | .14 | .93(**) | 1 |
| n | 129 | 130 | 130 | 130 | 130 | 129 | 4 | 42 | 130 | 130 |
Correlation is significant at P< 0.05 level (2-tailed).
Correlation is significant at P < 0.01 level (2-tailed).
Correlation is significant at P < .0005 level (2-tailed).
Statistical significance of correlations can be a misleading criterion, especially when there are several hundred xy pairs. With large samples, even small correlations can be statistically significant. The correlation itself, the r value, is an effect size indicator for judging the strength of the relationship between two variables. Although all the markers have positively skewed distributions, strong correlations in both Pearson and Spearman minimizes the possibility that a few outliers account for spurious relationships or that the effect sizes are biased. The strongest relationships for PETH are with GGT, %CDT, uETG, and uETS, with r values in the range of 0.4 for both types of correlations. The sensitivity of PETH is underscored by its strong relationship with urine ETG (r>.4) and urine ETS, both of which, like PETH, are direct markers that reflect recent drinking. We found, as others often report, there was no correlation between GGT and %CDT.
Interlock BAC Measures
Interlock results were aggregated at 2-month intervals to examine change over time. At the group level, the mean vehicle use over time during each of three 2-month intervals varied very little for either first-time DUI offenders or multiple offenders. The (mean, median) vehicle startup tests were stable, in each 2-month period: 0–2 months (355, 308), 2–4 months (345, 288) and 4–6 months (346, 288). While driving per se did not change, there were reductions in the rates of positive BAC tests across those intervals. For example, relative to BAC test rates during months 0 to 2, rates of positive BAC tests in months 4 to 6 declined to about half (49% lower for first offenders, 45% lower for multiple offenders). Because total driving holds steady (as indicated by start tests), the decline in positive BAC tests suggests that over time interlock offenders learn to separate their drinking and driving. A similar finding reported in an earlier study [6] also showed a reduction over time in the proportion of all tests that were elevated. Is the decline in failed interlock BAC tests over time related to a decline in drinking, or just drinking and driving? A partial answer to this question comes from biomarker evidence.
Baseline and Followup Markers
Table 3 shows the initial and average 8 months later sample of the six markers that could be paired. In Table 3, the follow-up marker results end with a “2.” On the right of Table 3 are paired t statistics showing that only MCV (168 pairs) was significantly lower in the second sample (P=.014), but the mean levels of MCV of less than 91.25 are within the normal range. No marker, other than MCV, showed change from the first to the second samples. This failure to find much evidence of change in the drinking level may partly explain why, even though IID programs reduce impaired driving by an average of 64% during the installed period [5], control levels of recidivism resume once the IID is removed from the car. The alcohol biomarkers’ levels at the start and at follow up show little evidence that overall drinking declined. Program participants may be learning to alter drinking and driving without reducing overall drinking. There was no treatment intervention concurrent with the IID.
Table 3.
Initial and follow-up alcohol marker pairs
| Mean | N | Std. Dev. | r | t | df | Sig (2- tailed) |
||
|---|---|---|---|---|---|---|---|---|
| Pair 1 | MCV | 91.22 | 168 | 4.02 | ||||
| MCV2 | 90.85 | 168 | 3.78 | |||||
| MCV pairs | .88 | 2.49 | 167 | .01 | ||||
| Pair 2 | ALT | 33.75 | 169 | 20.22 | ||||
| ALT2 | 34.59 | 169 | 26.40 | |||||
| ALT pairs | .78 | −.66 | 168 | .51 | ||||
| Pair 3 | AST | 28.36 | 169 | 13.46 | ||||
| AST2 | 28.02 | 169 | 16.42 | |||||
| AST pairs | .77 | .42 | 168 | .67 | ||||
| Pair 4 | GGT | 53.20 | 169 | 91.58 | ||||
| GGT2 | 51.38 | 169 | 76.41 | |||||
| GGT pairs | .88 | .55 | 168 | .58 | ||||
| Pair 5 | %CDT | 2.58 | 161 | .91 | ||||
| %CDT2 | 2.56 | 161 | 1.04 | |||||
| %CDT pairs | .60 | .38 | 160 | .70 | ||||
| Pair 6 | PETH | .70 | 163 | .72 | ||||
| PETH2 | .69 | 163 | .78 | |||||
| PETH pairs | .65 | .22 | 162 | .82 | ||||
Biomarkers and Interlock BAC Fail Groups
Categorization of interlock BAC test rates into a small number of bins facilitates presentation of the results. Because ignition lockout rate (in Alberta when BAC ≥ .04 g/dL) is a discrete indicator of drinking control, the 136 participants who had zero lockouts are defined as one subset of the overall group and represent 26.8% of the sample. At the other extreme, the high-rate group was set by inspection of the data series. The rate interval of failed tests began to widen at about the 80th percentile, so the definition of the high rate group was set at a fail rate above .0145 (fail starts to all starts). There were 20.5% of the subjects above this cut. The remaining cases between the zero group and the high group define the middle group representing 52.8% of the total. In summary, the zero group included n=136, a low group had n=268, and a high group had n=104. The total sample with useable interlock data was N=508.
The data in Table 4 show the descriptive summaries of initial biomarkers broken out by the interlock BAC categories. One-way ANOVA of these results, with Scheffe post hoc tests, shows that, with the exception of hair markers (hFAEE and hETG), there were differences (all at least P<.001) in which the high-BAC group was always unique. The overall F value is shown for each analysis and the subgroups are flagged with an asterisk.
Table 4.
Initial alcohol biomarkers by interlock BAC fail-rate bins
| Fail rate bins |
Mean | N | Minimum | Maximum | Std. deviation |
|
|---|---|---|---|---|---|---|
| MCV | None | 90.21 | 75 | 84 | 97 | 3.33 |
| Low | 90.90 | 152 | 79 | 101 | 3.86 | |
| High* | 93.16 | 58 | 84 | 105 | 4.20 | |
| F=10.7, df=2/282, P=.000 | Total | 91.18 | 285 | 79 | 105 | 3.93 |
| ALT | None | 28.87 | 75 | 10 | 103 | 16.31 |
| Low | 30.43 | 154 | 11 | 107 | 17.62 | |
| High* | 42.31 | 58 | 11 | 241 | 36.99 | |
| F=7.1, df=2/284, P=.001 | Total | 32.42 | 287 | 10 | 241 | 23.09 |
| AST | None | 24.83 | 75 | 14 | 87 | 10.61 |
| Low | 25.74 | 154 | 13 | 55 | 7.56 | |
| High* | 33.86 | 58 | 15 | 142 | 23.56 | |
| F=9.7, df=2/284, P=.000 | Total | 27.14 | 287 | 13 | 142 | 13.49 |
| GGT | None | 28.28 | 75 | 7 | 116 | 20.98 |
| Low | 41.14 | 154 | 6 | 374 | 43.17 | |
| High* | 91.60 | 58 | 10 | 955 | 159.15 | |
| F=11.8, df=2/284, P=.000 | Total | 47.98 | 287 | 6 | 955 | 81.68 |
| %CDT | None | 2.50 | 73 | 1.3 | 5.49 | 0.84 |
| Low | 2.65 | 152 | 1.06 | 10.27 | 1.08 | |
| High* | 3.28 | 58 | 0.81 | 10.92 | 1.56 | |
| F=8.6, df=2/280, P=.000 | Total | 2.74 | 283 | 0.81 | 10.92 | 1.17 |
| PETH | None | 0.43 | 74 | 0.22 | 2.67 | 0.51 |
| Low | 0.61 | 151 | 0.22 | 3.28 | 0.61 | |
| High* | 1.45 | 58 | 0.22 | 5.49 | 1.17 | |
| F=35.5, df=2/280. P=.000 | Total | 0.74 | 283 | 0.22 | 5.49 | 0.83 |
| FAEE hair | None | 0.46 | 13 | 0.05 | 2.23 | 0.69 |
| Low | 0.56 | 50 | 0.03 | 3.05 | 0.74 | |
| High | 1.11 | 22 | 0.07 | 5.79 | 1.57 | |
| F=2.7, df=2/82, P=.075 | Total | 0.69 | 85 | 0.03 | 5.79 | 1.03 |
| ETG hair | None | 43.02 | 29 | 0.7 | 357 | 76.56 |
| Low | 49.95 | 78 | 0.7 | 487 | 76.64 | |
| High | 68.68 | 30 | 5.8 | 411 | 94.79 | |
| F=0.8, df=2/134, P=.435 | Total | 52.59 | 137 | 0.7 | 487 | 80.80 |
| ETG urine | None | 846.15 | 34 | 38.7 | 10001 | 2250.89 |
| Low | 1095.43 | 65 | 38.7 | 10001 | 2489.12 | |
| High* | 5232.45 | 22 | 38.7 | 10001 | 4471.87 | |
| F=19.3, df=2/118, P=.000 | Total | 1777.57 | 121 | 38.7 | 10001 | 3299.00 |
| ETS urine | None | 465.44 | 34 | 7.2 | 10001 | 1744.10 |
| Low | 625.35 | 65 | 7.2 | 10001 | 1940.78 | |
| High* | 3496.09 | 22 | 7.2 | 10001 | 3998.85 | |
| F=13.5, df=2/118, P=.000 | Total | 1102.37 | 121 | 7.2 | 10001 | 2632.96 |
Subgroup significantly different from the others.
To ensure that the strong significant relationships in Table 4 for all the blood and urine biomarkers and the interlock BAC groups were not a secondary consequence of skewed outliers in the biomarker data series, all markers were subjected to both square root and natural log transformations to force them into more normally distributed data series. The ANOVA and post hoc analyses of these transformed data determined that the statistical significance and the interlock subgroup separation were at least as strong as found with the raw data. Moreover, after log transformation of the strongly skewed hair ETG data, it also yielded a statistically significant split between the high to low BAC groups.
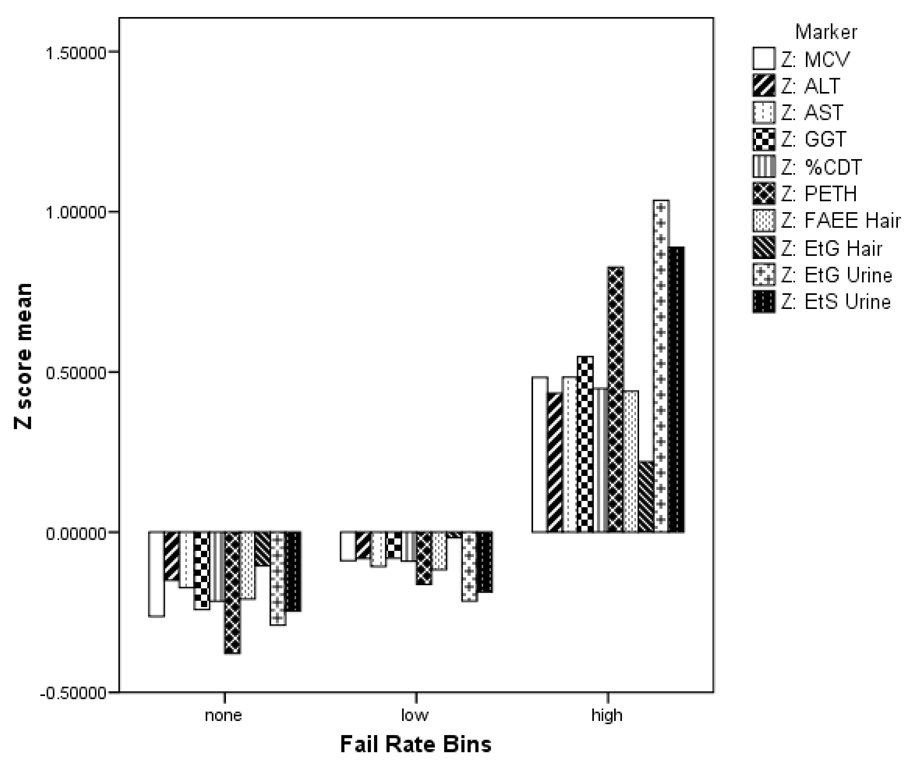
Most alcohol biomarker studies categorize responses as above or below some cutoff level. This is not the tactic adopted here because the subject population of DUI offenders is heterogeneous and overlaps a more socially normative drinking population. Accordingly, cutoff levels derived from clinical samples do not serve our purpose. The alcohol biomarkers are used here as a tool to characterize DUI drivers. The mean levels of markers for the high risk group shown in Table 4 could be used as cutoff estimates for other DUI risk studies. The biomarker means for the 3 interlock BAC subgroups matched our assumption that more BAC lockouts on the interlock would be associated with higher marker levels. To make this point graphically, a Z score (standard deviation [SD] unit) chart allows for direct comparison of markers by interlock group in a way that is free of underlying measurement units; figure 1 shows the breakout of the biomarker Z scores (mean=0, SD=1). All the biomarkers of the high BAC interlock group are much higher than the two lower risk groups.
Figure 1.
Z score representation of 10 baseline alcohol biomarkers by fail-rate bins of elevated interlock BAC tests
Alcohol Assessments and Interlock BAC Fail Groups
The three interlock BAC test bins were evaluated against baseline psychometrics measures via ANOVA with Scheffe post hoc Scheffe tests to determine subgroup differences. Psychometric assessments shown in Table 5 include the full AUDIT, the AUDIT-C (the first three consumption items), four questions from the 30-day TLFB (maximum drinks in one day, total drinks in the month, longest abstinence period, longest drinking period); DRINC total score plus the five subscales (physical, interpersonal, intrapersonal, social responsibility, impulse control), the five basic subscales from the TRI are included (govern, restrict, emotion, preoccupation, concern); and DIS-C DSM-IV abuse and dependence are scored categorically as positive or negative (those that could not be definitively diagnosed as positive for abuse or dependence are scored as negative). The results show that, with the exception of the DSM abuse and dependence variables and two TRI variables, the rest of the assessments were significantly related in the predicted direction to the interlock BAC positive subgroups. In most cases, the Scheffe post hoc tests found the high-BAC interlock subgroup was uniquely different from the other two, and is shown in Table 5 with asterisks.
Table 5.
Initial alcohol assessments by interlock BAC fail-rate bins
| Fail rate bins | Mean | N | Std. deviation | |
|---|---|---|---|---|
| AUDIT total | None | 7.76 | 135 | 4.86 |
| Low | 9.00 | 268 | 5.09 | |
| Total F=15.3, df=2/504, P=.000 | High* | 11.34 | 104 | 4.90 |
| AUDIT_C | None | 4.27 | 135 | 1.79 |
| Low | 4.59 | 268 | 1.69 | |
| Total F=11.1, df=2/504, P=.000 | High* | 5.29 | 104 | 1.54 |
| TLFB max drinks one day | None* | 6.87 | 120 | 5.69 |
| Low | 9.04 | 243 | 6.90 | |
| Total F=8.5, df= 2/459, P=.000 | High | 10.37 | 99 | 6.12 |
| TLFB total drinks | None | 34.34 | 120 | 34.45 |
| Low | 41.47 | 243 | 51.04 | |
| Total F=7.9, df=2/459, P=.000 | High* | 59.10 | 99 | 50.44 |
| TLFB longest abstinence | None | 9.86 | 120 | 7.38 |
| Low | 10.28 | 243 | 7.08 | |
| Total F=5.7, df=2/459, P=.003 | High* | 7.49 | 99 | 6.04 |
| TLFB longest drinking | None* | 4.72 | 120 | 7.03 |
| Low | 3.90 | 243 | 6.15 | |
| Total F=3.9, df=2/459, P=.000 | High* | 6.21 | 99 | 8.58 |
| DRINC total score | None* | 4.75 | 126 | 6.22 |
| Low* | 7.28 | 248 | 8.70 | |
| Total F=18.7, df=2/473, P=.000 | High* | 12.44 | 102 | 14.01 |
| DRINC physical | None | 1.00 | 129 | 1.44 |
| Low | 1.49 | 261 | 2.03 | |
| Total F=16.5, df=2/490, P=.000 | High* | 2.54 | 103 | 2.72 |
| DRINC interpersonal | None | 0.91 | 129 | 1.74 |
| Low | 1.59 | 259 | 2.51 | |
| Total F=16.3, df=2/487, P=.000 | High* | 2.96 | 102 | 4.05 |
| DRINC intrapersonal | None | 0.85 | 133 | 1.77 |
| Low | 1.58 | 263 | 2.76 | |
| Total F=13.3, df=2/496, P=.000 | High* | 2.80 | 103 | 4.12 |
| DRINC social responsibility | None | 0.61 | 132 | 1.33 |
| Low | 0.81 | 257 | 1.50 | |
| Total F=10.9, df=2/489, P=.000 | High* | 1.61 | 103 | 2.52 |
| DRINC impulse control | None | 1.47 | 132 | 1.60 |
| Low | 1.91 | 263 | 2.10 | |
| Total F=8.4, df=2/495, P=.000 | High* | 2.61 | 103 | 2.71 |
| TRI govern | None* | 6.52 | 133 | 5.01 |
| Low* | 8.85 | 266 | 6.46 | |
| Total F=12.3, df=2/498, P=.000 | High* | 10.44 | 102 | 6.76 |
| TRI restrict | None | 11.26 | 133 | 7.32 |
| Low | 11.86 | 265 | 7.43 | |
| Total F=2.4, df=2/497, P=.093 | High | 13.33 | 102 | 7.24 |
| TRI emotion | None | 6.19 | 134 | 4.11 |
| Low | 6.97 | 267 | 4.83 | |
| Total F=12.5, df=2/500, P=.000 | High* | 9.31 | 102 | 6.01 |
| TRI cognitive preocc | None | 4.10 | 133 | 2.96 |
| Low | 4.66 | 266 | 3.80 | |
| Total F=5.6, df=2/498, P=.004 | High* | 5.76 | 102 | 4.55 |
| TRI concern drink | None | 7.63 | 133 | 5.85 |
| Low | 7.41 | 267 | 5.37 | |
| Total F=1.6, df=2/498, P=.192 | High | 8.59 | 101 | 5.79 |
| DSM abuse | None | 0.28 | 136 | 0.45 |
| Low | 0.40 | 268 | 0.49 | |
| Total F=3.1, df=2/505, P=.047 | High | 0.38 | 104 | 0.49 |
| DSM dependence | None | 0.32 | 136 | 0.47 |
| Low | 0.39 | 268 | 0.49 | |
| Total F=2.1, df=2/505, P=.126 | High | 0.45 | 104 | 0.50 |
Subgroup significantly different from others
Alcohol Biomarkers and Alcohol Assessments
Although the primary focus of this paper is on the interlock BAC relationships, review of the bivariate correlations of the markers and the psychometric assessments is warranted as these are commonly reported by others. The same assessment variables found in Table 5 were correlated with the markers shown in Table 4, and the results are found in Table 6.
Table 6.
Spearman correlation of initial alcohol assessments by initial biomarker level
| MCV | ALT | AST | GGT | %CDT | PETH | Hair_FAEE | Hair_ETG | Urine_ETG | Urine_ETS | |
|---|---|---|---|---|---|---|---|---|---|---|
| Range ( n ) = | 264–300 | 266–302 | 266–302 | 266–302 | 263–298 | 263–298 | 89–92 | 140–144 | 125–130 | 125–130 |
| AUDIT total | 0.09 | 0.05 | 0.10 | .14(*) | .16(**) | .35(***) | 0.11 | .23(**) | .34(***) | .39(***) |
| AUDIT_C | .11(*) | 0.08 | .12(*) | .15(*) | .20(***) | .38(***) | 0.14 | .27(**) | .47(***) | .50(***) |
| TLFB max drinks 1 day | 0.04 | .13(*) | 0.11 | .19(**) | .13(*) | .30(***) | 0.12 | .23(**) | .31(***) | .34(***) |
| TLFB total drinks | .17(**) | .12(*) | .16(**) | .21(***) | .23(***) | .49(***) | .31(**) | .30(***) | .52(***) | .56(***) |
| TLFB long. abstinence | −.23(***) | −0.11 | −.18(**) | −.17(**) | −.28(***) | −.44(***) | −.29(**) | −.22(**) | −.47(***) | −.52(***) |
| TLFB longest drinking | .25(***) | 0.09 | .13(*) | .13(*) | .23(***) | .41(***) | .33(**) | .29(**) | .46(***) | .48(***) |
| Drinc total score | 0.06 | 0.01 | 0.09 | .12(*) | 0.10 | .30(***) | 0.16 | .24(**) | .27(**) | .30(**) |
| Drinc physical | 0.03 | 0.01 | 0.08 | .12(*) | .16(**) | .32(***) | 0.20 | .30(***) | .21(*) | .23(**) |
| Drinc interpersonal | 0.04 | 0.07 | .15(*) | .13(*) | 0.05 | .25(***) | 0.05 | .20(*) | .19(*) | .21(*) |
| Drinc intrapersonal | 0.07 | 0.02 | 0.09 | .13(*) | 0.08 | .27(***) | 0.14 | 0.15 | .20(*) | .21(*) |
| Drinc social responsible | 0.09 | −0.02 | 0.07 | 0.05 | 0.09 | .20(***) | 0.09 | 0.07 | 0.17 | .21(*) |
| Drinc impulse control | 0.06 | 0.07 | 0.06 | 0.05 | −0.00 | .15(**) | 0.06 | 0.10 | .22(*) | .25(**) |
| TRI govern | 0.08 | −0.07 | −0.05 | 0.07 | .14(*) | .25(***) | 0.12 | .21(*) | 0.11 | 0.13 |
| TRI restrict | −0.01 | 0.01 | −0.04 | 0.11 | −0.01 | 0.09 | 0.03 | 0.15 | −0.01 | −0.00 |
| TRI emotion | 0.05 | −0.03 | −0.03 | 0.09 | 0.01 | .24(***) | 0.02 | 0.15 | 0.10 | 0.09 |
| TRI cognitive preocc | .12(*) | −0.00 | 0.01 | 0.02 | 0.06 | .22(***) | 0.14 | 0.04 | 0.13 | 0.12 |
| TRI concern drink | 0.03 | 0.01 | −0.03 | 0.01 | 0.02 | 0.08 | 0.07 | 0.04 | −0.04 | −0.07 |
| DSM abuse | 0.07 | −0.01 | −0.04 | 0.06 | −0.00 | 0.04 | 0.06 | .24(**) | 0.11 | 0.11 |
| DSM dependence | −0.01 | 0.04 | 0.07 | 0.09 | .13(*) | .17(**) | 0.01 | .29(***) | .27(**) | .29(**) |
Correlation is significant at P< 0.05 level (2-tailed).
Correlation is significant at P< 0.01 level (2-tailed).
Correlation is significant at P< .0005 level (2-tailed)
The traditional markers—MCV, ALT, and AST—relative to the other biomarkers, correlate poorly with the self-report psychometric variables. Of all the psychometrics, the four TLFB variables were the most consistently correlated with biomarkers. The TLFB and AUDIT variables had the strongest correlations with the direct markers (PETH, FAEE, ETG, and ETS), whether measured in blood, hair, or urine. GGT and CDT were correlated less strongly, but nonetheless positively, with the TLFB and AUDIT measures.
PETH was strongly correlated with all but 3 of the 19 assessment variables. Hair ETG was well correlated with AUDIT and TLFB and with the DSM-IV dependence variable. In all cases, the TLFB variable of “longest abstinence” period was negatively correlated with the biomarkers, as it should be. The TLFB 30-day longest drinking and total drinks variables were, among all self-report assessments, the ones most strongly related to the markers with Spearman correlations as high as r=.56 and several with r>.40. The TRI subscales were not related to any biomarkers other than PETH. Other than ETG or ETS the DSM variables were unassociated with other biomarkers, much as they were unassociated with the interlock BAC risk bins.
Finally, because not all of the participants would give blood samples, we evaluated whether those who provided blood for analysis were different from those who did not. Subsets splits based on whether blood was provided were evaluated on the most consistent psychometric measures. Results from independent group t tests for AUDIT, TLFB, and DRINC scales showed that the blood donor and nondonor subgroups were either not different on those measures or, when different, the blood group had the higher risk. Based on these results, the blood donors are not a lower-risk subset of the total and therefore the results probably do not underestimate the biomarker levels of interlock-using DUI drivers.
DISCUSSION
The public risk exposure to alcohol-impaired drivers is significant. For example, nearly all U.S. states have drivers with 10–20 or more DUI convictions on their records. California alone has more than 300,000 drivers with three or more prior DUI convictions [44]. In the United States, there is a daily average of about 22 fatal crashes in which a driver’s BACs was ≥.08 g/dL [1]. Our ability to deter drinking drivers is very limited, as is our ability to intervene effectively. Better, more objective, tools are needed to make licensing decisions.
This study tested the relationships of three categories of alcohol-risk measures. These included: nine alcohol biomarkers in three matrices (blood, urine, and hair), an average of approximately 2800 alcohol interlock BAC tests per vehicle reflecting about 8 months of driving, and self-report data captured on five standard psychometric assessments.
Interlock BAC test profiles reflect actual attempts to drive with an elevated BAC. Published biomarker cutoffs that have been used as criteria for making severity judgments in alcohol treatment populations may not be the appropriate ones for DUI samples. For example, the evidence in this paper shows PETH to be a strong correlate of BAC positive tests and IID lockouts. The mean ±SD for PETH of the high-risk group (shown in Table 4) is 1.45 ±1.17 µmol/L. Although this is significantly greater than, and more than twice the level of, the middle risk group, it is less than the 2.47± 2.2 found among alcohol-dependent patients reported by Hartmann et al. [25] or the 3.4 ± 2.6 µmol/L for outpatients reported by Aradottir et al. [40]. The clinical problem and the public roadway safety problem are not identical and the biomarker cutoff levels should not be identical.
PETH is a remarkably strong, general alcohol risk indicator. It is significantly rank correlated with all of the other alcohol biomarkers tested (Table 2), with 16 of 19 psychometric assessments (Table 6) and has an overall F ratio of 35.5 against the three interlock risk groups (Table 4). The strong relationships among the consumption measures (TLFB, AUDIT-C), the direct markers from blood and urine (ETG, ETS, PETH), and the interlock BAC tests makes considerable sense because all reflect actual drinking rather than indirectly reflecting possible disease status as do indirect markers. This constitutes support for using direct markers when assessing public health risk posed by alcohol impaired drivers. ETG, requiring only a urine sample, is appealing on a cost basis. PETH requires whole blood but may be the gold standard. The PETH results in this study are in accord with Wurst and colleagues's conclusion from clinical evidence that this marker is both highly specific and highly sensitive. That conclusion seems to hold as well for this mixed population of DUI offenders who vary widely on both drinking levels and dependence severities.
It is also noteworthy that the more traditional, indirect alcohol markers (GGT, ALT, AST, and MCV) and %CDT related less strongly to the psychometric assessments than did the direct markers. This does not have any particular implication for the adequacy of these markers for assessment of alcohol dependence, but it suggests the sensitivity of indirect markers is less adequate when the target to be estimated is recent consumption. Drinking indicated by the direct markers appears to be compatible with the 30-day TLFB and the 28-day AUDIT.
The salience of the FAEE and ETG markers in hair is more difficult to judge. There were fewer cases for analysis than for other markers, the variance was high, and there was no followup sample to judge within-subject stability. Nonetheless, the group means in Table 4 and Figure 1 for both hair markers formed a sequence from low to high that matched the low to high interlock BAC risk subgroups, and once log transformed, the hair ETG levels were significantly predictive of the interlock BAC groups. Also, from Table 2, the Spearman correlation of 62 pairs of hair FAEE and blood PETH was .521 (P<.0001). This is nearly identical to the r=.527 Spearman correlation of hFAEE and PETH reported by Wurst et al. [45] from 17 alcoholic inpatients.
The hair ETG marker was well associated with the TLFB, AUDIT, and AUDIT-C and had the strongest relationship to DSM dependence and abuse. Hair ETG did less well paired against other markers. The 92 pairs of hair ETG and hair FAEE had a weak but significant Spearman correlation r=.27 (P=.007). Hair ETG and urine ETG/ETS were not related by Spearman correlation.
Psychometric assessments require subject cooperation, yield potentially self-incriminating disclosures, and are expected to perform more poorly as risk indicators in nonresearch situations. In this study, all subjects were given signed confidentiality assurances. The reasonably good relationships between many alcohol self-report measures and the objective biomarker and interlock results might be different without those assurances.
The most widely used markers today are GGT and %CDT. Comment on their performance is warranted. As is evident in Table 4, both GGT and %CDT distinguished the three interlock risk groups well and did so with large F ratios. For the interlock high-risk group, the mean GGT was 91.6 U/L, and the high-risk group %CDT mean was 3.28%, both above widely used clinical cutoffs of 75 U/L and 2.7%, respectively. As expected, these two markers are not correlated with each other (via either Pearson or Spearman) and, therefore, probably discriminate different types of alcohol problems. In a panel of markers, it is beneficial if each test can provide unique rather than redundant information.
The Table 3 biomarker data show little evidence for change within subjects and suggest why IIDs are routinely found to only temporarily suppress re-convictions for impaired driving. It appears that overall drinking does not change while the IIDs are installed. The evidence for IID suppression of DUI convictions from more than 10 studies is very strong, and the evidence is equally strong that the effect is lost when the devices are removed. Because we know that the rate of failed tests declines during the period of installation even while the total number of tests taken holds steady, it seems evident that the offenders learn to separate drinking and driving without reducing drinking per se. Treatment interventions may be needed to change the behavior of some drivers. For DUI samples, motivational approaches [46] have had some success. Marques et al. [47] reported on a motivational approach with interlock using DUI offenders in Texas built around a structured intervention with provider and participant manuals [48; 49]
Finally, when the last driver records were retrieved from Alberta in mid-2008, about 6% of the offenders who provided blood had later been reconvicted for DUI, not enough for conclusive analyses. A usual rate of repeat offense accumulation is 4 to 6% per year. By the end of 2010, 75 or more offenders will likely have been reconvicted, allowing for a more conclusive binary logistic regression and survival analyses.
Implications
Recent technological innovations bring promise that we can do a better job of managing alcohol risks on the roads. Monitoring drivers with ignition interlocks and monitoring alcohol consumption with biomarkers could remove some of the subjectivity in deciding which drivers warrant greater or lesser degrees of control. Few if any states or provinces in North America use alcohol biomarkers as a routine part of this driver license decision-making process, and only a few programs use the alcohol IID record as a criterion in relicensing decisions. Used by skilled treatment providers, and close monitoring by the authority, these tools may help improve public health and safety by bringing more and better science to practice.
ACKNOWLEDGEMENTS
This research was funded by the National Institute on Alcohol Abuse and Alcoholism (NIH/NIAAA 5 R01 AA014206). The authors acknowledge the helpful contributions of Edmonton coordinators, Tina VanderHeide and Patricia Spencer, research colleague Doug Beirness of the Canadian Centre on Substance Abuse in Ottawa, the staff of the Guardian Interlock Systems Office in Edmonton, the leadership at the Alberta Transportation Safety Board and the Alberta Transport Ministry, staff at Dynacare Kasper Medical Laboratories in Edmonton, data processing staff of Alcohol Countermeasures Systems in Toronto, and Eileen Taylor of PIRE who capably managed the IRB reviews and many other tasks over the 6-year project period.
Footnotes
Conflict of Interest: None.
REFERENCES
- 1.National Highway Traffic Safety Administration. Washington, DC: NHTSA's National Center for Statistics and Analysis; 2008. Aug, Traffic Safety Facts: 2007 Traffic Safety Annual Assessment—Alcohol-Impaired Driving Facilities. Report No.: DOT HS 811 016. [Google Scholar]
- 2.Coben JH, Larkin GL. Effectiveness of ignition interlock devices in reducing drunk driving recidivism. Am J Prev Med. 1999;16(1S):81–87. doi: 10.1016/s0749-3797(98)00117-2. [DOI] [PubMed] [Google Scholar]
- 3.Voas RB, Marques PR, Tippetts AS, Beirness DJ. The Alberta Interlock Program: The evaluation of a province-wide program on DUI recidivism. Addiction. 1999;94(12):1849–1859. doi: 10.1046/j.1360-0443.1999.9412184910.x. [DOI] [PubMed] [Google Scholar]
- 4.Beirness D, Marques P. Alcohol Ignition Interlock Programs. Traffic Inj Prev. 2004;5(3):299–308. doi: 10.1080/15389580490465418. [DOI] [PubMed] [Google Scholar]
- 5.Willis C, Lybrand S, Bellamy N. Alcohol ignition interlock programmes for reducing drink driving recidivism. Cochran Database of Systematic Reviews. 2004;18(4) doi: 10.1002/14651858.CD004168.pub2. CD004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marques PR, Voas RB, Tippetts AS, Beirness DJ. Behavioral monitoring of DUI offenders with the alcohol ignition interlock recorder. Addiction. 1999;94(12):1861–1870. doi: 10.1046/j.1360-0443.1999.9412186111.x. [DOI] [PubMed] [Google Scholar]
- 7.Marques PR, Tippetts AS, Voas RB, Beirness DJ. Predicting repeat DUI offenses with the alcohol interlock recorder. Accid Anal Prev. 2001;33(5):609–619. doi: 10.1016/s0001-4575(00)00074-9. [DOI] [PubMed] [Google Scholar]
- 8.Marques PR, Tippetts AS, Voas RB. Comparative and joint prediction of DUI recidivism from alcohol ignition interlock and driver records. J Stud Alcohol. 2003;64(1):83–92. doi: 10.15288/jsa.2003.64.83. [DOI] [PubMed] [Google Scholar]
- 9.Marques PR, Voas RB, Tippetts AS. Behavioral measures of drinking: Patterns in the interlock record. Addiction. 2003;98 Suppl 2:13–19. doi: 10.1046/j.1359-6357.2003.00584.x. [DOI] [PubMed] [Google Scholar]
- 10.Marques PR, Voas RB. Interlock BAC tests, alcohol biomarkers, and motivational interviewing: Methods for detecting and changing high-risk offenders. In: Marques PR, editor. Alcohol Ignition Interlock Devices—Volume II: Research, Policy, and Program Status 2005. Oosterhout, The Netherlands: International Council on Alcohol, Drugs and Traffic Safety; 2005. pp. 25–41. [Google Scholar]
- 11.Allen JP, Litten RZ, Strid N, Sillanaukee P. The role of biomarkers in alcoholism medication trials. Alcohol Clin Exp Res. 2001;25(8):1119–1125. [PubMed] [Google Scholar]
- 12.Smellie WS, Ryder SD. Biochemical "liver function tests". Br Med J. 2006;333(7566):481–483. doi: 10.1136/bmj.333.7566.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korzec ABM, Koeter WJ, De Kieviet W. Diagnosing alcoholism in high-risk drinking drivers: Comparing different diagnostic procedures with estimated prevalence of hazardous alcohol use. Alcohol Alcohol. 2001;36(6):594–602. doi: 10.1093/alcalc/36.6.594. [DOI] [PubMed] [Google Scholar]
- 14.Conigrave KM, Degenhardt LJ, Whitfield JB, Saunders JB, Helander A, Tabakoff B. CDT, GGT, and AST as markers of alcohol use: The WHO/ISBRA collaborative project. Alcohol Clin Exp Res. 2002;26(3):332–339. [PubMed] [Google Scholar]
- 15.Appenzeller BM, Schneider S, Maul A, Wennig R. Relationship between blood alcohol concentration and carbohydrate-deficient transferrin among drivers. Drug Alcohol Depend. 2005;79(2):261–265. doi: 10.1016/j.drugalcdep.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Gilg T, Buchholtz U, Huth O. CDT (carbohydrate deficient transferrin) and other alcohol markers in the MPA (medical and psychological assessment) of alcohol offenders regranting driving licenses - Results of an empirical study in Germany. In: Laurell H, Schlyter F, editors. Proceedings of the 15th International Conference on Alcohol, Drugs and Traffic Safety; 2000. pp. 1183–1184. [Google Scholar]
- 17.Gjerde H, Sakshaug J, Morland J. Heavy drinking among Norwegian male drunken drivers: A study of gamma-glutamyltransferase. Alcohol Clin Exp Res. 1986;10(2):209–212. doi: 10.1111/j.1530-0277.1986.tb05073.x. [DOI] [PubMed] [Google Scholar]
- 18.Bjerre B. Primary and secondary prevention of drink-driving by the use of alcolock device and program. Swedish experiences. Accid Anal Prev. 2005;37:1145–1152. doi: 10.1016/j.aap.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Bjerre B, Kostela J, Selén J. Positive health-care effects of an alcohol ignition interlock programme among driving while impaired (DWI) offenders. Addiction. 2007;102(11) doi: 10.1111/j.1360-0443.2007.02006.x. 1771-1181. [DOI] [PubMed] [Google Scholar]
- 20.Bjerre B, Marques P, Selen J, Thorsson U. A Swedish alcohol ignition interlock programme for drink-drivers: Effect on hospital care utilization and sick leave. Addiction. 2007;102(4):560–570. doi: 10.1111/j.1360-0443.2006.01726.x. [DOI] [PubMed] [Google Scholar]
- 21.Gustavsson L, Alling C. Formation of phosphatidyethanol in rat brain by phospholipase D. Biochemical and Biophysical Research Communications. 1987;142:958–963. doi: 10.1016/0006-291x(87)91507-5. [DOI] [PubMed] [Google Scholar]
- 22.Gunnarsson T, Karlsson A, Hansson P, Johnson G, Alling C, Odham G. Determination of phosphatidylethanol in blood from alcoholic males using high-performance liquid chromatography and evaporative light scattering of electrospray mass spectrometric detection. Journal of Chromatography B. 1998;705:243–249. doi: 10.1016/s0378-4347(97)00541-0. [DOI] [PubMed] [Google Scholar]
- 23.Varga A, Hansson P, Johnson G, Alling C. Normalization rate and cellular localization of phosphatidylethanol in whole blood from chronic alcoholics. Clinica Chimica Acta. 2000;299:141–150. doi: 10.1016/s0009-8981(00)00291-6. [DOI] [PubMed] [Google Scholar]
- 24.Varga A, Hansson P, Lundqvist C, Alling C. Phosphatidylethanol in blood as a marker of ethanol consumption in healthy volunteers: Comparison with other markers. Alcohol Clin Exp Res. 1998;22:1832–1837. [PubMed] [Google Scholar]
- 25.Hartmann S, Aradottir S, Graf M, Wiesbeck G, Lesch O, Ramskogler K, et al. Phosphatidylethanol as a sensitive and specific biomarker—comparison with gamma-glutamyl transpeptidase, mean, corpuscular volume and carbohydrate-deficient transferrin. Addict Biol. 2006;12:81–84. doi: 10.1111/j.1369-1600.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- 26.Høiseth G, Bernard JP, Stephanson N, Normann PT, Christophersen AS, Morland J, et al. Comparison between the urinary alcohol markers EtG, and GTOL/5-HIAA in a controlled drinking experiment. Alcohol Alcohol. 2008;43(2):187–191. doi: 10.1093/alcalc/agm175. [DOI] [PubMed] [Google Scholar]
- 27.Helander A, Boettcher M, Fehr C, Dahmen N, Beck O. Detection times for urinary ethyl glucuronide and ethyl sulfate in heavy drinkers during alcohol detoxification. Alcohol Alcohol. 2009;44(1):55–61. doi: 10.1093/alcalc/agn084. [DOI] [PubMed] [Google Scholar]
- 28.Wurst FM, Vogel R, Jachau K, Varga A, Alling C, Alt A, et al. Ethyl glucuronide discloses recent covert alcohol use not detected by standard testing in forensic psychiatric inpatients. Alcohol Clin Exp Res. 2003;27(3):471–476. doi: 10.1097/01.ALC.0000057942.57330.E2. [DOI] [PubMed] [Google Scholar]
- 29.Dresen S, Weinmann W. Forensic confirmatory analysis of ethyl sulfate - A new marker for alcohol consumption - by liquid-chromatography/electrospray ionization/tandem mass spectrometry. American Society for Mass Spectrometry. 2004;15:1644–1648. doi: 10.1016/j.jasms.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Helander A, Dahl H. Urinary tract infection: A risk factor for false negative urinary ethyl glucuronide but not ethyl sulfate in the detection of recent alcohol consumption. Clinical Chemistry. 2005;51(9):1728–1730. doi: 10.1373/clinchem.2005.051565. [DOI] [PubMed] [Google Scholar]
- 31.Kip MJ, Spies CD, Neumann T, Nachbar Y, Alling C, Aradottir S, et al. The usefulness of direct ethanol metabolites in assessing alcohol intake in nonintoxicated male patients in an emergency room setting. Alcohol Clin Exp Res. 2008;32(7):1–8. doi: 10.1111/j.1530-0277.2008.00696.x. [DOI] [PubMed] [Google Scholar]
- 32.Pragst F, Yegles M. Chapter 14: Alcohol markers in hair. In: Kintz P, editor. Analytical and Practical Aspects of Drug Testing in Hair. Boca Raton, FL: CRC Press, Taylor and Francis Group; 2007. pp. 287–323. [Google Scholar]
- 33.Yegles M, Labarthe A, Auwärter V, Hartwig S, Vater H, Wennig R, et al. Comparison of ethyl glucuronide and fatty acid ethyl ester concentrations in hair of alcoholics, social drinkers and teetotallers. Forensic Science International. 2004;145(2–3):167–173. doi: 10.1016/j.forsciint.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 34.Kintz P, Villain M, Vallet E, Etter M, Salquebre G, Cirimele V. Ethyl glucuronide: Unusual distribution between head hair and pubic hair. Forensic Science International. 2008;176(1):87–90. doi: 10.1016/j.forsciint.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 36.Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule: Its history, characteristics, and validity. Arch Gen Psychiat. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 37.Babor TF, de la Fuente JR, Saunders J, Grant M. The Alcohol Use Disorders Identification Test: Guidelines for use in primary health care. Geneva: World Health Organization; 1992. [Google Scholar]
- 38.Miller WK, Tonigan S, Longabaugh R. The drinker inventory of consequences. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1995 Report No.: NIH Publication No. 95–3911.
- 39.Collins RL, Lapp WM. The temptation and restraint inventory for measuring drinking restraint. Brit J Addict. 1992;87:625–633. doi: 10.1111/j.1360-0443.1992.tb01964.x. [DOI] [PubMed] [Google Scholar]
- 40.Aradottir S, Asanovska G, Gjerss S, Hansson P, Alling C. Phospoatidylethanol (PEth) concentrations in blood are correlated to reported alcohol intake in alcohol-dependent patients. Alcohol Alcohol. 2006;41(4):431–437. doi: 10.1093/alcalc/agl027. [DOI] [PubMed] [Google Scholar]
- 41.Alling C, Gustavsson L, Anggard E. An abnormal phospholipid in rat organs after ethanol. FEBS Letters. 1983;152:24–28. doi: 10.1016/0014-5793(83)80474-8. [DOI] [PubMed] [Google Scholar]
- 42.Javors MA, Koek W, Johnson BJ, Marques PR, Anthenelli RM, Tian PY. Test retest reliability of %CDT. Alcohol Clin Exp Res. 2008;32 Suppl. 6 66A. [Google Scholar]
- 43.Pragst F, Auwaerter V, Sporkert F, Spiegel K. Analysis of fatty acid ethyl esters in hair as possible markers of chronically elevated alcohol consumption by headspace solid-phase microextracion (HS-SPME) and gas chromatography-mass spectrometry (GC-MS) Forensic Science International. 2001;121:76–88. doi: 10.1016/s0379-0738(01)00456-x. [DOI] [PubMed] [Google Scholar]
- 44.Mothers Against Drunk Driving. Campaign to Eliminate Drunk Driving: State Progress Report: Mothers Against Drunk Driving. [Last accessed on December 19, 2008];2008 Available online: http://www.madd.org/getfile/3fd6fa65-c8fb-4715-a5ba-67b87e12c943/State_Progress_Report-2008.aspx.
- 45.Wurst FM, Alexson S, Wolfersdorf M, Bechtel G, Forster S, Alling C, et al. Concentration of fatty acid ethyl esters in hair of alcoholics: Comparison to other biological state markers and self-reported ethanol intake. Alcohol Alcohol. 2004;39(1):33–38. doi: 10.1093/alcalc/agh005. [DOI] [PubMed] [Google Scholar]
- 46.Miller WR, Rollnick S. Motivational interviewing. Preparing people to change addictive behavior. New York: Guilford; 1991. [Google Scholar]
- 47.Marques PR, Voas RB, Timken DS. Preliminary outcomes from a Texas manual-based group motivational intervention supplement for court-stipulated interlock DUI offenders. In: Oliver J, Williams P, Clayton A, editors. Proceedings of the 17th International Conference on Alcohol, Drugs and Traffic Safety; August 8–13, 2004; Glasgow, UK. X-CD Technologies; 2004. [Google Scholar]
- 48.Timken D, Marques PR. Support for Interlock Planning (SIP): Providers Manual: Pacific Institute for Research and Evaluation. [Last accessed on April 20, 2009];2001 Available online: www.pire.org/sip/sipmanuals.htm.
- 49.Timken D, Marques PR. Support for Interlock Planning (SIP): Participants Workbook: Pacific Institute for Research and Evaluation. [Last accessed on April 20, 2009];2001 Available online: www.pire.org/sip/sipmanuals.htm.