Abstract
Background
Major depression (MDD) is characterized by altered emotion processing and deficits in cognitive control. In cognitive interference tasks, patients with MDD have shown excessive amygdala activity and under-recruitment of dorsolateral prefrontal cortex (DLPFC). The purpose of this study was to examine the effects of antidepressant treatment on anomalous neural activity in cognitive-control and emotion-processing circuitry.
Methods
Functional magnetic resonance imaging was conducted on depressed patients (n=23) (both before and after antidepressant treatment) compared with matched controls (n= 18) while they performed a cognitive task involving attended and unattended fear-related stimuli.
Results
After eight weeks of SSRI antidepressant treatment, patients with depression showed significantly increased DLPFC activity to unattended fear-related stimuli and no longer differed from controls in either DLPFC or amygdala activity.
Conclusions
These results suggest that antidepressant treatment increases DLPFC under-activity during cognitive tasks that include emotional interference.
Limitations
The sample was fairly homogeneous and this may limit generalizability.
Introduction
Major depression (MDD) involves abnormal emotion processing and altered cognitive function, including executive dysfunction. In particular, MDD patients show deficits in managing cognitive interference when distracters have negative emotional valence Tasks requiring control over interference usually recruit dorsolateral prefrontal cortex (DLPFC), and cognitive control of emotional responses has also been shown to activate lateral prefrontal (PFC) (Ochsner and Gross, 2005). Recently we demonstrated that when performing cognitive tasks that required ignoring negatively-valenced distracters, MDD patients showed under-activity in right DLPFC, and increased activation in the amygdala (Fales et al., 2008). We interpreted this finding as demonstrating simultaneous amygdala over-activation and reduced cognitive control over emotional responsiveness in MDD consistent with reports showing decreased task-related connectivity between DLPFC and amygdala (Siegle et al., 2007).
In the current study, we investigated the effects of antidepressant treatment on functional brain activation in MDD patients while performing the emotional interference task described above. Antidepressants have been found to normalize anomalies in resting activity in the amygdala and to reduce amygdala responsiveness to negative stimuli when presented outside of conscious awareness (Sheline et al., 2001, Drevets, 2001). However, when stimuli are presented overtly, but unattended, cognitive control is needed to resolve or prevent interference. It is not yet clear whether antidepressant therapy normalizes functional activity of prefrontal cortex during cognitive control of amygdala responses. To examine this question, we conducted a functional magnetic resonance imaging study of MDD patients both before and after antidepressant treatment while they performed a cognitive task involving attended and unattended fear-related stimuli. We predicted that, relative to their premedicated state, MDD patients after medication would show increased lateral prefrontal response to emotional distracters and reduced amygdala response.
Method
Participants
Depressed patients (n = 23, males/females: 10/13, with mean age: 36.4 years (SD 9.4)) all sought treatment for depression, fulfilled DSM-IV criteria for major depression as diagnosed by structured clinical interview (SCID), and were matched with 18 demographically similar healthy controls (M/F: 9/9, mean age: 33.4 years (SD 8.2). Exclusionary criteria for all participants were use of any psychotropic drugs within the last four weeks, or the presence of any comorbid psychiatric illness or complicating medical illness (Fales et al., 2008). Sixteen patients had a current duration of depression less than one year and seven patients had a depression duration greater than one year. Mean age of depression onset was 29.3 years. Before antidepressant treatment, the patients had mean scores on the 17-item Hamilton Rating Scale for Depression (HRSD) (Hamilton, 1960) of 20 (SD 2.3) while control participants had a mean score of 0.3 (SD .6). Depressed subjects' treatment histories consisted of 17 with recurrent depression and 6 with first episode, never treated. Of the 17 recurrent subjects, eleven had a prior history of antidepressant treatment. Eight had previously responded to treatment; one was a partial responder; two were non-responders. Five patients had never been treated and one subject had an inadequate duration of treatment to determine response. Depressed subjects were started on escitalopram 10 mg/day, initiated immediately following the first fMRI scan and subsequent dose adjustment was determined by clinical response. Ending doses of escitalopram were 20 mg (n=9) and 10 mg (n=8). Five participants were treated with other antidepressants due to patient preference: sertraline 150 mg (n=3) sertraline 100 mg (n=1) and paroxetine 20 mg. (n=2). Following eight weeks of anti-depressant treatment, the participants received a second fMRI scan. At that time the patients' mean HRSD score was 3.5 (SD 3.3), representing a mean reduction in symptoms of 82 percent. After complete description of the study to the participants, written informed consent was obtained in accordance with criteria established by the Washington University Human Subjects Committee.
Procedure
The emotion-interference task (Vuilleumier et al., 2001, Bishop et al., 2004) presented participants with a pair of houses and a pair of faces in each trial, with one pair arranged horizontally and the other vertically around a central fixation cross. Participants were instructed to attend to the horizontal or vertical axis for a given block, and for each trial, the task was to tell whether the two items in the target axis were the same or different. There were four trial types: attend-fearful-faces, attend-neutral-faces, ignore-fearful-faces (attend to houses), and ignore-neutral-faces.
fMRI imaging and analysis
Image acquisition and analysis
fMRI images were collected on a Siemens 3T Allegra MRI scanner. The functional data were preprocessed and analyzed using in-house software. A General Linear Model (GLM) was defined for each participant, with separate regressors to estimate hemodynamic response to each combination of trial type (attention × emotion) and trial answer (same or different), yielding a total of 8 response types (see (Fales et al., 2008) for further details).
ROI identification
Our previous study (Fales et al., 2008) focused specifically on regions linked to emotion processing (rostral, pregenual and subgenual cingulate and amygdala) or cognitive control (dorsal anterior cingulate and dorsolateral prefrontal cortex) (Drevets and Raichle, 1998, Drevets et al., 1992, Botvinick et al., 2004, Ochsner and Gross, 2005). We devised hand-drawn a priori ROIs using published neuroanatomical guides (Talairach and Tournoux, 1988, Rajkowska and Goldman-Rakic, 1995) to constrain our search for task-related activity. Within this a priori focus, we then identified regions that showed anomalous functional activation patterns in unmedicated patients during their first scanning session (time-1). For the current paper we report on activity in these same regions at the time of the second scan (time-2), after eight weeks of antidepressant treatment for the patients.
Results
Of the initial 51 participants (24 controls and 27 depressed at time-1) (Fales et al., 2008), 41 returned at time-2 (18 controls and 23 depressed). All time-1 effects were significant both for the original sample (n=51) and for the reduced sample (n=41). There were no significant differences in depression or anxiety scores between the 18 controls who returned at time-2 and the six controls who did not, nor between the 23 patients who returned and the four patients who did not (independent t-tests, all ps>.1).
Clinical Change
After eight weeks of antidepressant therapy, 14 out of the 23 depressed subjects achieved 89% reduction or better in depressive symptoms, as measured on the HDRS scale. Seven showed 50-88% improvement, and the remaining two showed 43-49% reductions. Thus in our sample, approximately half the sample showed a very significant improvement in symptoms, and all patients responded at least partially to treatment.
Behavioral results
At time-1, depressed patients were significantly slower than controls, but no less accurate. At time-2, the patients showed a modest but non-significant improvement in response time. (See Supplementary Information for details of behavioral data.)
Neuroimaging results: DLPFC
At time-1, a right DLPFC region showed a 3-way interaction between group, attention (attended, ignored) and emotion (fear, neutral) F(1,39) = 5.44, p=.03, η2=.122. When ignoring fearful faces (versus ignore-neutral), depressed patients were significantly less activated than controls, t(39)=2.28, p=.028, η2=.12 (uncorrected) but not in the attend condition, p=.17. To assess change after treatment, we conducted a four-way ANOVA with time (pre-treatment, post-treatment) as an additional factor. The right DLPFC did not show a significant four-way interaction, p=.17. However the depressed patients showed a significant increase in DLPFC activity in the ignore-fear versus ignore-neutral comparison from time 1 to time-2, F(1,22)=6.21, p=.02, η2=.220. In contrast, the controls' DLPFC activity for ignore-fear minus ignore-neutral did not change significantly from time-1 to time-2, F(1,17)=2.10, p>.16, η2=.110.
Neuroimaging results: Amygdala
At time-1, a left amygdala region had also showed a 3-way interaction between group, attention and emotion, F(1,39) = 7.20, p=.011, η2=.156. When ignoring fearful faces (versus ignore-neutral), depressed patients were significantly more activated than controls, F(1,39) = 4.31, p=.045, η2=.099, but significantly less activated for the same contrast in the attend condition, F(1,39) = 4.94, p=.03, η2=.112. Incorporating time-2 data into the ANOVA, the left amygdala (Figure 1) showed a significant four-way interaction, F(1,39) = 4.056, p=.05, η2=.094, because the significant three-way interaction at time-1 disappeared at time-2, p>.3. By time-2, amygdala activation for the fear-minus-neutral contrast in the depressed was no longer different from that of controls at time-2 (both ps>.4), nor did they differ from control values at time-1, (ps>.7). However paired t-tests for the depressed patients alone did not reveal significant reductions in amygdala activity at time-2 in response to ignore-fear trials (versus ignore-neutral), F(1,22)=6.21, p=.02, η2=.220.
Figure 1.
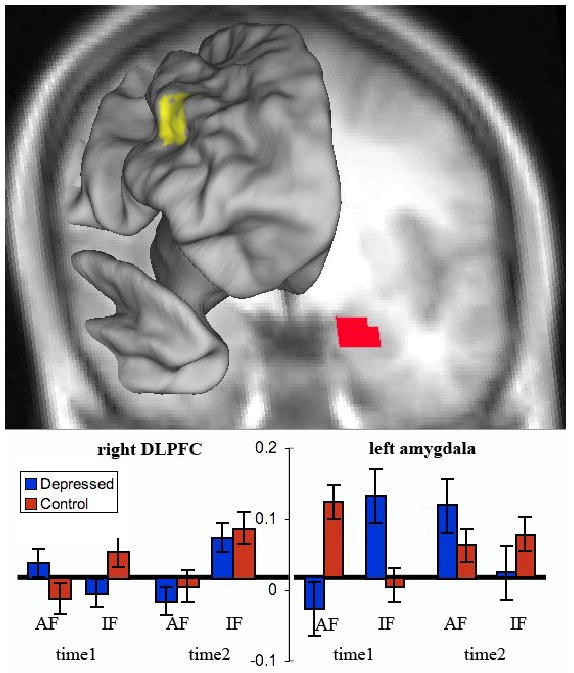
Fear-minus-neutral activity in the right dorsolateral prefrontal cortex and left amygdala. These regions showed a significant three-way interaction of attention × emotion × group at time-1. The graphs show percent change in signal magnitude. Error bars indicate standard errors. Time 1 indicates pre-treatment activations. Time 2 indicates post-antidepressant treatment activations. AF is shorthand for attend fearful faces minus attend neutral faces (see Methods). IF indicates ignore fearful faces minus ignore neutral faces.
Four other regions showed significant group-related effects at time-1, but these effects were not normalized at time-2. These regions were the 1) subgenual cingulate, 2) pregenual cingulate, 3) an area spanning dorsal/rostral cingulate, and 4) the dorsal cingulate. These effects (summarized in Table 1) are described in more detail in the Supplemental Information.
Table 1.
| Attention × Emotion × Group | ||||||||
|---|---|---|---|---|---|---|---|---|
| Talairach coordinates | ||||||||
| Brain region | BA | Voxel | Side | x | y | z | Time-1 effect | Time-2 changes in the Depressed |
| Middle frontal G. | 9/46 | 41 | R | 36 | 27 | 29 | (see text) | Under-activity is normalized. (See text.) |
| Amygdala | 28 | L | -18 | -5 | -19 | (see text) | No significant changes over time. | |
| Attention × Group | ||||||||
| Talairach coordinates | ||||||||
| Brain region | BA | Voxel | Side | x | y | z | Time-1 effect | Time-2 changes in the Depressed |
| Pregenual cing. | 24 | 33 | L | -10 | 35 | -2 | Cont: Attend>Ignore | No significant changes over time. |
| Depr: Ignore>Attend | ||||||||
| Emotion × Group | ||||||||
| Talairach coordinates | ||||||||
| Brain region | BA | Voxel | Side | x | y | z | Time-1 effect | Time-2 changes in the Depressed |
| Dorsal cingulate | 32 | 57 | R | 4 | 17 | 41 | Cont: Neutral>Fear | No significant changes over time. |
| Depr: Fear>Neutral | ||||||||
| Main effect of group | ||||||||
| Talairach coordinates | ||||||||
| Brain region | BA | Voxel | Side | x | y | z | Time-1 effect | Time-2 changes in the Depressed |
| Subgenual cing | 25 | 40 | L | -6 | 13 | -13 | Depr > Cont | No significant changes over time. |
| Sup.-rostral cing. | 24 | 25 | 0 | 13 | 29 | Cont > Depr | No significant changes over time. | |
| Dorsal cingulate | 24 | 29 | 0 | 13 | 34 | Cont > Depr | No significant changes over time. | |
We tested whether HDRS scores in the patients at time-2 were related to their time-2 activation values for the ignore-fear-minus-ignore-neutral contrast, or whether the percent change in HDRS scores across time was related to activation changes in the ignore-fear-minus-ignore-neutral contrast across time. Similar to time-1, there was no relationship between HDRS scores at time-2 and activation values at time-2, nor between the improvement in HDRS scores across time and changes in ignore-fear contrasts for left amygdala or right DLPFC, all ps >.1.
Discussion
This study examined the effects of antidepressant therapy on activity in dorsolateral PFC and the amygdala in response to emotional interference during a cognitive task. Patients showed a significant increase in recruitment of dorsolateral PFC following treatment, as well as some reduction (non-significant) of amygdala overactivity in response to unattended fearful faces. These results suggest that antidepressant therapy may improve recruitment of the DLPFC during performance of emotional interference tasks. Resting hypoactivation in DLPFC has long been a recognized concomitant of depression, and this resting under-activity has been seen to increase toward normal levels with antidepressant treatment. The current study found enhanced task-related activation of DLPFC following antidepressant treatment. Enhanced recruitment in DLPFC has been found to facilitate selective attention to target stimuli, and also to help individuals regulate emotional responses. Our patients showed no behavioral improvements over time, but they were not impaired in the task even at time-1. However, their reports of improved mood at time-2 is consistent with enhanced emotion regulation, and also with FDG-PET studies showing that antidepressant treatment lowers resting levels of amygdala hypermetabolism.
Some previous fMRI studies, including our own, reported that antidepressants normalize amygdala over-activation in response to negative stimuli (Harmer et al., 2006, Sheline et al., 2001). In the current study, we found that depressed individuals showed reduced amygdala activity following antidepressant treatment, though this change was not significant. Several of these previous studies focused on subliminal presentation of negative stimuli, and the current results may differ because negative stimuli were unattended but available to conscious awareness. Several studies have now posited a reciprocal relationship between the amygdala and lateral PFC in healthy participants during cognitive or emotion processing, initially in metabolic studies and more recently during a cognitive task with emotional distracters. In such studies, it has been suggested that either increased amygdala activity suppresses DLPFC activity, or increased DLPFC activity serves to suppress amygdala activity. Given the patients' improved mood in the current study, it is possible that reductions in resting amygdala did take place, and this reduction may have prevented bottom-up suppression of DLPFC by amygdala activity, thus enabling patients to recruit DLPFC normally during task performance. Potentially, changes in resting amygdala activity could occur without significant reductions in task-evoked amygdala response.
We did not find that antidepressant therapy normalized activation in subgenual or dorsal/rostral cingulate cortex. Elevated activity in subgenual cortex is a common finding in studies of major depression, and metabolic hyperactivity in this region has been seen to resolve with antidepressant therapy (Mayberg et al., 1999). In contrast, in the current study, only the DLPFC showed significant normalization of activity following antidepressant treatment, suggesting that subgenual PFC hyperactivity and reduced DLPFC activity in major depression may be dissociable phenomena that reflect different mechanisms.
Supplementary Material
Footnotes
Previous presentation: Part of the contents of this paper were initially reported in preliminary form in a poster presented at the 36th meeting of the Society for Neuroscience, October 2006, Atlanta, Georgia.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baxter LR, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, Gerner RH, Sumida RM. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Archives of General Psychiatry. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. Journal of Neuroscience. 2004;24:10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Science. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- D'esposito M, Postle BR, Jonides J, Smith EE. The neural substrate and temporal dynamics of interference effects in working memory as revealed by event-related functional MRI. Proceedings of the National Academy of Sciences of the USA. 1999;96:7514–7519. doi: 10.1073/pnas.96.13.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, Mccarthy G. Brain systems mediating cognitive interference by emotional distraction. The Journal of Neuroscience. 2006;26:2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: Implications for the cognitive-emotional features of mood disorders. Current Opinion in Neurobiology. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implication for interactions between emotion and cognition. Cognition and Emotion. 1998;12:353–385. [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. Journal of Neuroscience. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales CL, Barch DB, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, Mathews J, Sheline YI. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biological Psychiatry. 2008;63:377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biological Psychiatry. 2006;59:816–820. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Kruger S, Mayberg HS, Meyer JH, Mccann S, Arifuzzman AI, Houle S, Vaccarino FJ. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. American Journal of Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, Mcginnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. American Journal of Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Williams R. Attentional bias in anxiety and depression: The role of awareness. British Journal of Clinical Psychology. 1995;34:17–36. doi: 10.1111/j.2044-8260.1995.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Ochsner K, Gross JJ. The cognitive control of emotion. Trends in Cognitive Science. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach coordinate system. Cerebral Cortex. 1995;5:323–337. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DB, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biological Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biological Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Stuttgart, Germany: Georg Thiem Verlag; 1988. [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: An event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


