Abstract
Background
Adolescents with alcohol use disorders (AUD) have shown smaller prefrontal cortex (PFC) volumes compared with healthy controls; however, differences may have been due to comorbid disorders. This study examined PFC volumes in male and female adolescents with AUD who did not meet criteria for comorbid mood or attention disorders.
Methods
Participants were adolescents aged 15 to 17 who met criteria for AUD (n = 14), and demographically similar healthy controls (n = 17). Exclusions included any history of a psychiatric or neurologic disorder other than AUD or conduct disorder. Magnetic resonance imaging scans occurred after at least 5 days of abstinence from alcohol or drugs. Overall PFC volumes and white matter PFC volumes were compared between groups.
Results
After controlling for conduct disorder, gender, and intracranial volume, AUD teens demonstrated marginally smaller anterior ventral PFC volumes (p = 0.09) than controls, and significant interactions between group and gender were observed (p < 0.001 to p < 0.03). Compared with same-gender controls, females with AUD demonstrated smaller PFC volumes, while males with AUD had larger PFC volumes. The same pattern was observed for PFC white matter volumes.
Conclusions
Consistent with adult literature, alcohol use during adolescence is associated with prefrontal volume abnormalities, including white matter differences. However, adolescents with AUD demonstrated gender-specific morphometric patterns. Thus, it is possible that gender may moderate the impact of adolescent alcohol use on prefrontal neurodevelopment, and the neurodevelopmental trajectories of heavy drinking boys and girls should be evaluated separately in longitudinal studies.
Keywords: Adolescence, Brain Imaging, Magnetic Resonance Imaging, Alcohol Abuse, White Matter, Prefrontal Cortex
In the United States, alcohol is the most widely consumed intoxicant among adolescents, with 75% of 12th graders having drank in their lifetime. More startling is that 30% of seniors reported getting drunk in the previous month (Johnston et al., 2005). Given the prevalence of alcohol use during adolescence, its effects on neuromaturation are of great interest.
In adults, the prefrontal cortex (PFC) appears to be particularly vulnerable to the neurotoxic effects of alcohol use (Desmond et al., 2003; George et al., 2004; Pfefferbaum et al., 1997, 2001; Schweinsburg et al., 2001; Sullivan et al., 2003). Postmortem studies have found reduced glial and neuronal cell densities in the PFC of adults with alcohol use disorders (AUD) (Miguel-Hidalgo et al., 2006). Morphometric studies have revealed smaller PFC total volume (Kubota et al., 2001; Liu et al., 1998), gray matter (Chanraud et al., 2007; Liu et al., 1998; Pfefferbaum et al., 1997), and white matter volume (Kril et al., 1997; Pfefferbaum et al., 1997) among adults with AUD compared with controls. The more anterior portions of the PFC appear most vulnerable to alcohol-induced neurotoxic effects (Kril et al., 1997). Gender may moderate the effects of alcohol on PFC morphometry. It appears that women with AUD may be more sensitive to the neurotoxic effects of alcohol than men (Agartz et al., 1999; Hommer et al., 2001; Mann et al., 2005; Schweinsburg et al., 2003), although some studies have pointed to relative vulnerabilities in males (Pfefferbaum et al., 2001) and others found no evidence of brain abnormalities among alcoholic females (Kroft et al., 1991; Pfefferbaum et al., 2002).
Because neuromaturation continues into late adolescence (Giedd et al., 1996a; Lenroot and Giedd, 2006; Sowell et al., 2004) studies based on adults cannot necessarily generalize to adolescents with AUD, particularly findings in the PFC, a late region to undergo gray matter pruning (Gogtay et al., 2004; Lenroot and Giedd, 2006; Sowell et al., 2004). Maturation of white matter appears to continue into the twenties, with white matter volume typically increasing (Giedd et al., 1999; Jernigan and Gamst, 2005; Paus et al., 2001) and PFC white matter integrity improving (Ashtari et al., 2007; Barnea-Goraly et al., 2005; Watts et al., 2003) from childhood to adulthood, although 1 study found decreased white matter density from ages 16 to 17 (Paus et al., 1999). Gender differences in neuromaturation also exist. Females' frontal gray matter volumes peaks at age 11.0 while males' peak at age 12.1 years on average, and male brains are about 9% larger than females' (Giedd et al., 1996b). Furthermore, in contrast with most prior studies of white matter neuromaturation (e.g., Giedd et al., 1999; Reiss et al., 1996), our laboratory found that among healthy adolescent females ages 12 to 18, PFC white matter volume actually decreased from age 15 to 18, while males' PFC white matter remained relatively stable (Nagel et al., 2006). Thus, as male and female brains mature with somewhat different time courses during adolescence, neurotoxic exposures may have disparate effects.
Animal models indicate that adolescence is a period of particular vulnerability to the neurotoxic effects of alcohol (Barron et al., 2005; Carpenter-Hyland and Chandler, 2007; Crews et al., 2000; Spear, 2000). Compared with adults, adolescent animals exposed to alcohol are more likely to demonstrate cognitive, social, and neuronal damage (Crews et al., 2000; Little et al., 1996; Pyapali et al., 1999; Silveri and Spear, 1998; Swartzwelder et al., 1995, 1998; Varlinskaya and Spear, 2006; White et al., 2002; Yttri et al., 2004). Despite that many of these abnormalities are subserved by frontal systems, few studies have examined the PFC morphometric consequences of heavy alcohol use during adolescence in humans.
Thus far, human adolescent studies examining the neural effects of heavy drinking have suggested alcohol-related structural (De Bellis et al., 2000, 2005; Medina et al., 2007a; Nagel et al., 2005), and functional (Tapert et al., 2004) abnormalities. Specifically, despite similar performance during a spatial working memory task, adolescents with AUD showed less PFC and cerebellar activation to complete the task with controls, with a greater degree of abnormality linked to lifetime alcohol withdrawal symptoms (Tapert et al., 2004). These results may indicate that the brain compensates for subtle alcohol-induced neuronal insult by relying on other areas (e.g., parietal cortex). A follow-up study revealed that gender moderated the relationship between alcohol use and brain response to this spatial working memory task (Caldwell et al., 2005). That is, after controlling for typical blood alcohol concentrations, girls with AUD demonstrated less frontal cortex response than female controls, while the males showed the opposite pattern. Overall, females demonstrated more alcohol-related abnormalities than males.
The only study to date examining PFC morphometry (defined as anterior to the genu) in adolescents with AUD compared a treatment sample of 13- to 21-year olds with AUD and other Axis I disorders (including conduct, depressive, attention deficit hyperactivity, post-traumatic stress, generalized anxiety, bipolar, and cannabis and hallucinogen use disorders) to controls without a history of AUD (De Bellis et al., 2005). Even after controlling for comorbid disorders, males and females with AUD demonstrated smaller PFC total and white matter volumes than controls, although psychiatric contributions cannot entirely be ruled out, and gender-by-group interactions were not found.
Therefore, no studies to date have examined PFC volume among adolescents with AUD without comorbid psychiatric disorders. As comorbid psychiatric and other premorbid risk factors for substance use disorders (e.g., familial AUD) are also linked to neurocognitive abnormalities (Kamarajan et al., 2006; Kim et al., 2001; Kruesi et al., 2004; Meyerhoff et al., 2004; Tapert and Brown, 2000), the current study sought to examine the relationship between AUD and PFC structure among adolescents without comorbid mood, attention, or anxiety disorders. Conduct disorder, which is highly prevalent among adolescents with AUD, was statistically controlled for, and family history of substance use disorders was equivalent between the groups. A secondary goal of the study was to examine whether gender moderated the relationship between alcohol use and PFC morphometry.
Methods
Participants
Adolescent participants ages 15 to 17 were recruited from San Diego area high schools (Medina et al., 2007b; Tapert et al., 2003, 2004). Written informed consent and assent were obtained from each participant and parent in accordance with the University of California San Diego Human Research Protections Program. Participants and their parents were screened for eligibility with telephone interviews. Exclusion criteria included history of head injury with loss of consciousness >2 minutes, neurological or medical problems, learning disabilities, Diagnostic and Statistical Manual (DSM)-IV psychiatric disorder other than conduct disorder as ascertained by the Computerized Diagnostic Interview Schedule for Children 4.0 (Shaffer et al., 2000) parent and youth versions, current psychotropic medication use, significant maternal drinking (≥4 drinks per occasion or ≥7 drinks per week) or drug use during pregnancy, family history of bipolar I or psychotic disorder as assessed by the Family History Assessment Module screener (Rice et al., 1995), left handedness, and magnetic resonance imaging contraindications. Teens meeting criteria for mild or moderate conduct disorder (n = 5) were not excluded due to high comorbidity with substance use disorders (Button et al., 2006). Eligible participants in the current study were 14 teens (9 male) who met current criteria for DSM-IV AUD and 17 (10 male) demographically similar nonabusing controls with limited alcohol experience.
Measures
Demographic and Behavioral Assessment
Screening interviews were conducted separately with each teen and their parent to obtain information on personal and family background. Adolescents and parents were administered parallel versions of the Computerized NIMH Diagnostic Interview Schedule for Children 4.0 (Shaffer et al., 2000) to ascertain current and past psychiatric disorders for adolescent participants, the Family History Assessment Module screener (Rice et al., 1995) to determine family history of substance use and other psychiatric disorders, and a structured clinical interview (Brown et al., 1989) to collect information on demographic characteristics, medical and developmental history, and social and academic functioning.
Substance Involvement
Substance involvement was determined with the Customary Drinking and Drug Use Record (Brown et al., 1998), which collects lifetime and past 3-month information on alcohol, nicotine, and other drug use, and assesses DSM-IV abuse and dependence criteria, withdrawal symptoms, and other consequences of substance use. Good internal consistency, test–retest, and inter-rater reliability have been demonstrated with adolescents (Brown et al., 1998; Stewart and Brown, 1995). The Timeline Follow-back (Sobell and Sobell, 1992) obtained substance use patterns for the 30 days prior to scanning. Participants provided samples for Breathalyzer (Intoximeter, St Louis, MO) and urine drug toxicology on the day of scanning.
Procedures
Participants were asked to abstain from alcohol and other drugs for at least 48 hours before scanning. Imaging sessions were held on Thursday evenings to maximize recovery from weekend drinking and to minimize possible circadian influences. All participants submitted samples for Breathalyzer and urine drug toxicology analyses prior to scanning.
Anatomical imaging was acquired on a 1.5 Tesla General Electric Signa LX system (Waukesha, WI). The high-resolution imaging protocol used a sagittally acquired inversion recovery prepared T1-weighted 3D spiral fast spin echo sequence (TR = 2,000 ms, TE = 16 ms, FOV = 240 mm, voxel dimensions = 0.9375 × 0.9375 × 1.328 mm, 128 continuous slices, and acquisition time = 8:36) (Wong et al., 2000).
Image Processing
Each participant's high resolution anatomical image was AC–PC aligned and skull-stripped using a combination of a hybrid watershed and deformable surface semi-automated skull-stripping program (Segonne et al., 2004) and manual editing. Intracranial volume (ICV) was determined from the skull-stripped brain. PFC regions of interest (ROI) were defined manually in AFNI (Cox, 1996) by raters blind to participant characteristics who attained high levels of inter-rater reliability (intraclass correlation coefficients >0.90) prior to data collection.
All ROIs were manually delineated on coronal 3D images (perpendicular to the AC–PC plane). The PFC ROI protocol was a modified version based on Nagel et al. (2006). The posterior PFC ROI included all cortical area anterior to the anterior commissure and posterior to the anterior edge of the genu. This ROI excluded the corpus callosum, subcortical regions (caudate, putamen, globus palladus, and internal capsule), optic tracts, insula, and lateral ventricles. The anterior PFC ROIs (dorsal and ventral) included all cortical areas anterior to the anterior edge of the genu. The anterior dorsal PFC ROI included all cortex superior to the midline of the most anterior portion of the genu, while the anterior ventral PFC ROI included cortex inferior to the midline of the genu. Tracing continued until the most anterior slice on which cortex was still visible. These ROIs excluded the lateral ventricles and the corpus callosum. The corpus callosum was excluded from the mid-sagittal slice and then from each left and right lateral slice until the edge could no longer be detected (using AFNIs edge detection and sharpen features; Fig. 1).
Fig. 1.
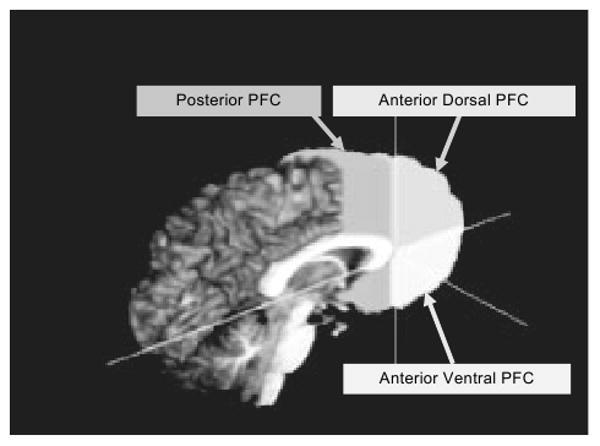
3D sagittal view of prefrontal cortex (PFC) boundary delineation.
White matter was segmented from gray and CSF compartments by processing skull-stripped T1 images with the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain's FAST automated segmentation tool (Zhang et al., 2001). This process uses a hidden Markov random field model and an associated expectation-maximization algorithm that corrects for spatial intensity variations. The segmentation process did not reliably separate gray matter from CSF, but reliably identified white matter. Each PFC ROI mask was applied to the white matter compartment to calculate the white matter volume for each region. All total volume and white matter ROIs were analyzed as a ratio to overall ICV to control for individual variability in brain size (Giedd et al., 1996a).
Statistical Analysis
Sample Characterization
ANOVAs and chi-square tests compared groups on the potentially confounding or moderating variables of gender, ethnic category, family history of substance use disorders, conduct disorder, age, parental socioeconomic status (SES), verbal intellect, and reading ability. Differences between groups in frequency, duration, and severity of substance use and ICV were also analyzed. A post hoc analysis (due to the significant gender-by-group interactions on PFC morphometry) examined whether group and gender differences (utilizing ANOVAs) existed in the aforementioned demographic variables. If necessary, post hoc Tukey's HSD tests were conducted. Interpretations about statistical significance were made if p < 0.05.
Prefrontal Cortex Volume
To examine whether group status or group-by-gender interactions predicted PFC volume after controlling for potentially confounding variables that significantly differentiated the groups (conduct disorder), univariate ANOVAs were run (n = 31) with each of the 8 PFC volume/ICV measures (total volume, posterior, anterior dorsal, and anterior ventral; white matter volume: total, posterior, anterior dorsal, and anterior ventral) as dependent variables. Factors included group status (AUD vs. control), conduct disorder status, and gender. An interaction between group and gender was also examined. To ensure family history of substance use disorders did not influence results, the ANOVAs were re-examined with family history status included. Interpretations of statistical significance were made if p < 0.05. No correction for multiple tests was made to manage low power due to small sample size (Jennions and Møller, 2003; Nakagawa, 2004). To help interpret the magnitude of findings, partial eta squares (ηp2) are reported.
Results
Group Characterization
Demographics/Intracranial Volume
The teens with AUD did not significantly differ from controls on age [F(1,30) = 0.42, p < 0.52], ethnic category [χ2(2) = 2.74, p < 0.26], gender composition [χ2(1) = 0.09, p < 0.76], family history of substance use disorders (negative vs. positive) [χ2(1) = 1.1, p < 0.74], parental SES (based on Hollingshead) [F(1,31) = 0.02, p < 0.89], reading ability [F(1,30) = 0.02, p < 0.88], verbal intellectual functioning [F(1,30) = 0.09, p < 0.77], or ICV [F(1,30) = 0.04, p < 0.85] (See Tables 1 and 2). Male adolescents with AUD were more likely than control males to meet criteria for conduct disorder [χ2(1) = 7.24, p < 0.01].
Table 1.
Demographics, Cognitive Function, and Substance Use Characteristics by Alcohol Use Group and Gender
| Female controls (n = 7) mean (SD) or percentage [range] | AUD females (n = 5) mean (SD) or percentage [range] | Male controls (n = 10) mean (SD) or percentage [range] | AUD male (n = 9) mean (SD) or percentage [range] | |
|---|---|---|---|---|
| Age | 16.5 (1.0) [15.3–17.75] | 17.1 (0.6) [16.4–17.7] | 16.6 (0.7) [15.7–17.9] | 16.6 (0.7) [15.2–17.5] |
| % Caucasian | 86% | 100% | 80% | 100% |
| % Family history negativea | 43% | 20% | 44% | 44% |
| % Conduct disorder positive* | 0% | 0% | 0% | 56% |
| Hollingshead socioeconomic index | 26.0 (10.1) [18–47] | 17.0 (6.6) [11–27] | 21.4 (11.9) [11–47] | 26.1 (16.1) [11–53] |
| WRAT-3 reading standard score | 109.6 (6.9) [103–123] | 110.2 (4.4) [105–115] | 104.9 (8.2) [93–115] | 104.4 (9.4) [90–120] |
| Vocabulary scaled score | 13.6 (3.7) [9–18] | 13.6 (1.8) [12–16] | 11.7 (2.2) [8–14] | 12.3 (1.3) [11–14] |
| Days since last drinkb,e | 652.8 (473.8) [90–998] | 9.0 (4.6) [5–14] | 356.7 (483.0) [5–998] | 21.0 (17.3) [5–60] |
| Lifetime alcohol use episodesc,f,g | (4.1) [0–11] | 164.2 (131.6) [45–505] | 5.8 (7.7) [0–20] | 137.8 (160.1) [45–505] |
| Drinks/month, past 3 monthsh | 0.0 | 49.0 (42.2) [3–108] | 4.67 (7.2) [0–18] | 43.5 (26.1) [17–98] |
| Regular drinking age of onset | n/a | 15.2 (1.3) [14–17] | n/a | 14.6 (0.9) [14–16] |
| Lifetime alcohol withdrawal symptoms | 0 (0) [0–0] | 2.0 (1.6) [0–4] | 1.3 (3.8) [0–12] | 2.5 (2.1) [0–6] |
| Alcohol abuse and dependence criteria, past 3 monthsd,f | 0.0 | 2.0 (1.0) [1–3] | 0.3 (0.5) [0–1] | 3.0 (2.3) [0–6] |
| Lifetime marijuana use episodesc,g | (0.4) [0–1] | 17.0 (17.2) [0–40] | 3.2 (7.8) [0–25] | 7.7 (8.5) [0–20] |
| Marijuana use/month, past 3 months | 0.0 | 0.8 (1.3) [0–3] | 0.6 (1.1) [0–3] | 0.8 (1.6) [0–5] |
| Days/month smoked cigarettesi | 0.0 | 6.4 (6.0) [0–15] | 0.8 (2.3) [0–7] | 0.9 (2.0) [0–6] |
| Cigarettes per dayb | 0.0 | 1.2 (1.6) [0–4] | 0.3 (1.0) [0–3] | 0.0 |
| Lifetime other drug use episodes | 0.0 | 2.2 (4.4) [0–10] | 0.0 | 0.0 |
Notes: Chi-square (p < 0.05), males with AUD more likely than other groups;
Family history negative, no biological first or second degree relatives with substance use disorder;
Figures include only those who reported use;
Maximum allowed was 998;
Number of abuse/dependent symptoms do not necessarily indicate a positive DSM-IV diagnosis;
Female controls different than users (p < 0.05);
Male AUD different than male controls (p < 0.05);
Female AUD different than controls (p < 0.05);
Controls less than users (p < 0.05);
Female AUD different than all others (p < 0.05). AUD, alcohol use disorders.
Table 2.
Prefrontal Cortex Morphometry and Composition by Alcohol Use Group and Gender
| Female controls (n = 7) mean (SD) [range] | AUD females (n = 5) mean (SD) [range] | Male controls (n = 10) mean (SD) [range] | AUD males (n = 9) mean (SD) [range] | |
|---|---|---|---|---|
| Intracranial volume (ICV) (cc3)a | 1,519.1 (117.1) [1,374.0–1,703.1] | 1,549.0 (86.0) [1,430.0–1,636.6] | 1,674.2 (158.0) [1,438.4–2,015.9] | 1,658.9 (80.2) [1,551.7–1,842.1] |
| Posterior PFC/ICV (cc3) | 0.1040 (0.0071) [0.0908–0.1135] | 0.1007 (0.0084) [0.0939–0.1149] | 0.1026 (0.0112) [0.0843–0.1178] | 0.1039 (0.0134) [0.0809–0.1248] |
| Anterior dorsal PFC/ICV (cc3) | 0.0663 (0.0058) [0.0577–0.0749] | 0.0643 (0.0064) [0.0568–0.0725] | 0.0656 (0.0095) [0.0482–0.0784] | 0.0709 (0.0093) [0.0585–0.0894] |
| Anterior ventral PFC/ICV (cc3)b | 0.0554 (0.0064) [0.0477–0.0678] | 0.0473 (0.0040) [0.0446–0.0541] | 0.0531 (0.0071) [0.0378–0.0642] | 0.0586 (0.0063) [0.0515–0.0684] |
| Posterior PFC WM/ICV (cc3) | 0.0364 (0.0031) [0.0321–0.0405] | 0.0354 (0.0032) [0.0327–0.0408] | 0.0372 (0.0049) [0.0302–0.0437] | 0.0373 (0.0053) [0.0282–0.0463] |
| Anterior dorsal PFC WM/ICV (cc3) | 0.0164 (0.0023) [0.0140–0.0197] | 0.0152 (0.0029) [0.0116–0.0185] | 0.0165 (0.0031) [0.0117–0.0210] | 0.0182 (0.0033) [0.0124–0.0231] |
| Anterior ventral PFC WM/ICV (cc3)c | 0.0140 (0.0024) [0.0113–0.0172] | 0.0113 (0.0010) [0.0100–0.0125] | 0.0139 (0.0020) [0.0093–0.0163] | 0.0149 (0.0019) [0.0123–0.0170] |
Notes: Females significantly smaller than males (p < 0.05);
Female AUD different than controls (p < 0.05);
Male AUD different than female AUD (p < 0.05). AUD, alcohol use disorders; WM, white matter volume; PFC = prefrontal cortex volume.
Substance Use
All participants were abstinent from alcohol and drugs for at least 5 days before scanning. As shown in Table 1, user groups reported more recent alcohol use than controls, as well as more lifetime alcohol use, drinks per month, alcohol abuse/dependence symptoms, lifetime marijuana use, and cigarettes smoked per day (p < 0.05). Teens with AUD did not differ from controls in lifetime use of other drugs, recent marijuana use, or marijuana abuse/dependence criteria.
Gender × Group Interactions
When groups were examined by gender, males with AUD were more likely to be diagnosed with conduct disorder than other groups, and females with AUD smoked more cigarettes than other groups. Otherwise, males and females with AUD had similar substance use patterns (see Table 1).
Primary Results: PFC Volumes
All PFC volumes were analyzed as ratios to ICV to control for individual differences (see Table 2 for means and SD).
Group Status
After controlling for ICV, conduct disorder status, and gender, AUD teens demonstrated marginally smaller anterior dorsal PFC volumes [F(4,31) = 2.97, p < 0.09, ηp2 = 0.10], but no differences in other PFC volumes.
Conduct Disorder Status
Controlling for ICV, group, and gender, youths with conduct disorder had significantly smaller total PFC volumes than participants without conduct disorder [F(4,31) = 5.67, p < 0.03, ηp2 = 0.18] [conduct disorder PFC volume mean = 0.224 (±0.005); no conduct disorder PFC volume mean = 0.225 (±0.017)]. This difference was driven primarily by smaller anterior dorsal [F(4,31) = 7.10, p < 0.01, ηp2 = 0.22] and anterior ventral [F(4,31) = 5.41, p < 0.03, ηp2 = 0.17] regions of the PFC. Conduct disorder was also associated with significantly smaller volume of the anterior dorsal PFC white matter volume [F(4,31) = 5.71, p < 0.03, ηp2 = 0.18].
Gender
Controlling for ICV, group, and conduct disorder, females demonstrated significantly smaller total PFC volume than males [F(4,31) = 6.32, p < 0.02, ηp2 = 0.20], due primarily to smaller anterior ventral [F(4,31) = 8.35, p < 0.008, ηp2 = 0.24] and dorsal [F(4,31) = 4.57, p < 0.04, ηp2 = 0.15] PFC volumes. Females also had smaller total PFC white matter size [F(4,31) = 7.40, p < 0.01, ηp2 = 0.22], driven by smaller anterior ventral [F(4,31) = 10.09, p < 0.004, ηp2 = 0.28] and dorsal [F(4,31) = 6.08, p < 0.02, ηp2 = 0.19] PFC white matter volumes.
Gender-by-Group Interaction
Controlling for ICV and conduct disorder, significant group-by-gender interactions were observed in total PFC volume [F(4,31) = 10.63, p < 0.003, ηp2 = 0.29], due to differences in both anterior ventral [F(4,31) = 14.71, p < 0.001, ηp2 = 0.36; see Fig. 2] and dorsal [F(4,31) = 5.58, p < 0.03, ηp2 = 0.18] regions. Compared with female controls, females with AUD demonstrated smaller PFC volumes, while males with AUD had larger PFC volumes than male controls. Group-by-gender interactions were again observed in total white matter PFC volume [F(4,31) = 5.64, p < 0.03, ηp2 = 0.10], specifically in anterior ventral [F(4,31) = 11.28, p < 0.002, ηp2 = 0.30] and dorsal [F(4,31) = 5.37, p < 0.03, ηp2 = 0.17] PFC white matter volumes. As with total PFC volumes (Fig. 2), AUD females demonstrated smaller PFC white matter volumes compared with female controls, while males with AUD had larger PFC white matter volumes than male controls.
Fig. 2.
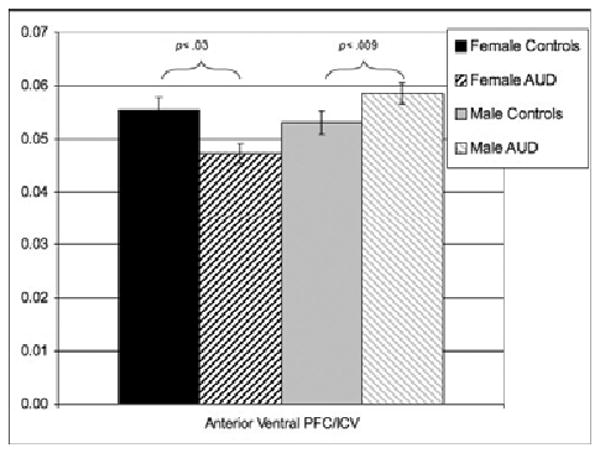
Mean (±1 SE) of the anterior ventral prefrontal cortex volume/intracranial volume by alcohol use disorder (AUD) group and gender.
Family History of Substance Use Disorders, Alcohol Use, and PFC Volumes
To determine if family history of substance use disorders might account for the primary results, the aforementioned ANOVAs were rerun with family history included. Family history was not associated with PFC volume (p > 0.10), and the relationships between alcohol use group and PFC volumes remained significant.
To examine the relationship between alcohol use indicators and PFC volumes, correlations were run among the males and females with AUD separately. No significant correlations between lifetime alcohol use, age of onset of regular alcohol use, alcohol withdrawal symptoms, or alcohol dependence symptoms and PFC volume variables were found (p > 0.10).
Discussion
Alcohol use is highly prevalent during adolescence (Johnston et al., 2005), and adolescence is a period of increased risk for neurocognitive consequences of alcohol use (Spear, 2000). Further, adult neuroimaging data suggest that the PFC is particularly sensitive to alcohol neurotoxicity (Desmond et al., 2003; Harper, 1989; Kril et al., 1997; Pfefferbaum et al., 1997; Schweinsburg et al., 2001). The present study was designed to examine PFC morphometry among adolescents with AUD. The primary finding was that even after controlling for conduct disorder and family history, gender significantly moderated the relationship between alcohol use and PFC morphometry. Despite similar alcohol use patterns, the males and females with AUD demonstrated opposite PFC morphometry patterns: girls with AUD showed smaller volumes while boys had larger volumes compared with same-gender controls. These findings parallel functional neuroimaging findings, which reported that males with AUD had increased frontal activation while female drinkers had limited frontal activation in response to a spatial working memory task (Caldwell et al., 2005).
This gender by drinking status interaction is interesting to consider in the context of adolescent neurodevelopment. Boys with AUD had significantly larger PFC anterior (including the dorsolateral and orbitofrontal regions), total, and PFC white matter volumes compared with control boys. When compared with girls, boys generally demonstrate pruning later in adolescence (Lenroot and Giedd, 2006), and the introduction of heavy drinking early in adolescence may inhibit healthy pruning. However, because the CSF and gray matter could not be reliably parsed, this hypothesis could not be tested. Alternatively, differences in overall PFC volume may have been primarily driven by white matter, as the boys with AUD demonstrated larger overall white matter volumes than controls. One possible explanation of these findings is that males are less sensitive than females to the neurotoxic effects of alcohol, so the PFC continues to myelinate normally. Studies examining the functional relationships between white matter integrity (measured by diffusion tensor imaging) and neuropsychological functioning are needed to help clarify PFC white matter functioning among boys with AUD.
These findings partially conflict with De Bellis et al. (2005), who reported smaller PFC total and white matter volumes among both males and females with AUD. However, compared with the current study, their sample was older (ages 13 to 21; so pruning may have occurred already in some males) and had a greater prevalence of comorbid Axis I disorders among the males with AUD (including attention deficit hyperactivity, major depressive, post-traumatic stress, and cannabis and hallucinogen use disorders). These comorbid disorders have also been associated with brain abnormalities (Bauer and Hesselbrock, 1999; Medina et al., 2007a; Mostofsky et al., 2002; Steingard et al., 2002). Therefore, it is difficult to disentangle the interactive effects of these conditions and AUD on PFC structure. For example, we found that conduct disorder was independently associated with smaller PFC volumes in the males, while AUD was associated with larger volumes among the boys. In De Bellis et al. (2005) sample, 75% of the males with AUD met criteria for conduct disorder, when compared with 56% in our sample, so increased incidence of conduct disorder in an adolescent sample may result in smaller overall PFC volumes.
Consistent with De Bellis et al. (2005), girls with AUD had significantly smaller PFC total and white matter volumes in the anterior regions compared with female controls. Interestingly, the current results also parallel previous pathology findings that demonstrated cell loss in superior, but not posterior, PFC areas (Harper, 1989). This preliminary evidence may indicate pathological processes such as neuronal death or atrophy (Pascual et al., 2007), or altered synaptic refinement. More specifically, rich concentrations of excitatory amino acid pathways in the PFC continue to develop during adolescence and alcohol consumption during adolescence may lead glutamate-mediated excitotoxicity, resulting in cell shrinkage and axonal loss (Carpenter-Hyland and Chandler, 2007; Freund and Anderson, 1996; Rossetti et al., 1999; Samnick et al., 1998). Alcohol-related brain effects may also be due to an upregulation of inflammatory mediators (e.g., COX-2 and inducible nitric oxide synthase [iNOS]) that ultimately lead to neural cell death or injury (Blanco et al., 2005; Pascual et al., 2007; Valles et al., 2004). Alternatively, the observed reduced PFC volumes among the females may have been driven exclusively by white matter pathology (Harper et al., 1990; Okamoto et al., 2006). Larger studies examining PFC morphometry among adolescents with AUD are necessary to determine whether the observed volume abnormalities are primarily due to white or gray matter pathology and to assess whether these structural differences are associated with altered cognitive functioning.
Gender differences in the brain-related effects of adolescent alcohol exposure may be due to several factors, including underlying differences in PFC development rate and timing (e.g., Giedd et al., 1999; Lenroot and Giedd, 2006), differential gene expression linked to enhanced alcohol-related neurotoxicity in females (Hashimoto and Wiren, 2007), blood glucose levels (Sumida et al., 2004), increased blood alcohol concentration among girls despite similar drinking patterns (National Institute on Alcohol Abuse and Alcoholism, 1993), alcohol dehydrogenase levels (Lieber, 2000), and hormone and receptor distributions (Emanuele et al., 2001; Kim et al., 2003; Ogilvie and Rivier, 1996). Comorbid disorders that affect boys and girls differently may also interact with alcohol use and PFC development during adolescence. For example, in our sample, males with AUD were more likely to be diagnosed with conduct disorder, while girls with AUD smoked more cigarettes (although this was relatively low, at an average of 1 cigarette per day). Both factors may affect frontal lobe function (Jacobsen et al., 2006; Kim et al., 2001; Kruesi et al., 2004). Longitudinal studies examining PFC morphometry, hormone levels, and alcohol use among male and female adolescents are necessary to test these hypotheses.
Premorbid differences may, in part, explain the observed PFC morphometric differences. For example, executive dysfunction is a risk factor for substance use disorders (Aytaclar et al., 1999; Nigg et al., 2004). However, the current study statistically controlled for conduct disorder, had no group differences in family history of substance use disorders (which was not associated with PFC volume in this sample), and excluded individuals with premorbid psychiatric disorders such as attention deficit hyperactivity disorder. Furthermore, there were no group differences in other risk factors potentially associated with substance use such as parental SES, verbal intellectual functioning, reading ability, and education. Still, it remains possible that the observed PFC differences may be due to pre-existing individual differences. For example, statistical control may be insufficient to eliminate the effects of conduct disorder on PFC morphometry, especially among the male adolescents with AUD. Future studies designed to disentangle the unique effects of alcohol use and conduct disorder on PFC structure in males are necessary, and longitudinal studies of at-risk adolescents are needed to rule out premorbid influences.
As with any study, some limitations warrant consideration. First, although comparable with other published neuroimaging studies, the current study had a low sample size. Additional studies with larger numbers of males and females with AUD are necessary. Second, reliable parcellation of gray matter from CSF using automated segmentation methods was not possible, so the ratio of gray matter to ICV could not be established in the current study. Third, given the relatively low comorbid drug use and extensive exclusionary criteria, this sample reflects a fairly high functioning sample within the population of adolescents with AUD. Therefore, results may not generalize to adolescents with significant comorbid polydrug use or psychiatric disorders. Finally, due to the cross-sectional nature of this study, the directional and developmental relationship of PFC morphometry and alcohol use cannot be clearly ascertained. Longitudinal studies examining the developmental trajectories of male and female adolescents will help ascertain these associations.
In summary, the present study found that adolescents with AUD demonstrated PFC structural abnormalities, especially in the white matter anterior to the genu of the corpus callosum. Gender significantly moderated the relationship between alcohol use and PFC morphometry. These results highlight the need for additional animal and human research examining the interaction between gender and alcohol use on PFC neurodevelopment during adolescence.
Acknowledgments
We would like to express our appreciation to the research participants and their families, the research associates in the Laboratory of Cognitive Imaging (LOCI) in the Department of Psychiatry, UCSD, and the LOCI Information Technology team.
Funding for this study provided by Grants from the National Institute on Alcohol Abuse and Alcoholism (PI: Tapert, R21 AA12519 and R01 AA13419), National Institute on Drug Abuse (PI: Medina, F32 DA020206, PI: Tapert R01 DA021182), the National Institute of Neurological Disorders and Stroke (PI: Nagel, 7K08 NS052147), and a National Institute on Alcohol Abuse and Alcoholism fellowship (Hanson; PI: Riley, 5T32 AA1352505).
References
- Agartz I, Momenan R, Rawlings R, Kerich M, Hommer D. Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry. 1999;56:356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Cervellione KL, Hasan KM, Wu J, McIlree C, Kester H, Ardekani BA, Roofeh D, Szeszko PR, Kumra S. White matter development during late adolescence in healthy males: a cross-sectional diffusion tensor imaging study. Neuroimage. 2007;35:501–510. doi: 10.1016/j.neuroimage.2006.10.047. [DOI] [PubMed] [Google Scholar]
- Aytaclar S, Tarter RE, Kirisci L, Lu S. Association between hyperactivity and executive cognitive functioning in childhood and substance use in early adolescence. J Am Acad Child Adolesc Psychiatry. 1999;38:172–178. doi: 10.1097/00004583-199902000-00016. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Barron S, White A, Swartzwelder HS, Bell RL, Rodd ZA, Slawecki CJ, Ehlers CL, Levin ED, Rezvani AH, Spear LP. Adolescent vulnerabilities to chronic alcohol or nicotine exposure: findings from rodent models. Alcohol Clin Exp Res. 2005;29:1720–1725. doi: 10.1097/01.alc.0000179220.79356.e5. [DOI] [PubMed] [Google Scholar]
- Bauer L, Hesselbrock V. P300 decrements in teenagers with conduct problems: implications for substance abuse risk and brain development. Biol Psychiatry. 1999;46:263–272. doi: 10.1016/s0006-3223(98)00335-7. [DOI] [PubMed] [Google Scholar]
- Blanco AM, Valles SL, Pascual M, Guerri C. Involvement of TLR4/type I IL-1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. J Immunol. 2005;175:6893–6899. doi: 10.4049/jimmunol.175.10.6893. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Creamer VA. Characteristics of relapse following adolescent substance abuse treatment. Addict Behav. 1989;14:291–300. doi: 10.1016/0306-4603(89)90060-9. [DOI] [PubMed] [Google Scholar]
- Button TMM, Rhee SH, Hewitt JK, Young SE, Corley RP, Stallings MC. The role of conduct disorder in explaining the comorbidity between alcohol and illicit drug dependence in adolescence. Drug Alcohol Depend. 2007;87:46–53. doi: 10.1016/j.drugalcdep.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Caldwell LC, Schweinsburg AD, Nagel BJ, Barlett VC, Brown SA, Tapert SF. Gender and adolescent alcohol use disorders on bold (blood oxygen level dependent) response to spatial working memory. Alcohol Alcohol. 2005;40:194–200. doi: 10.1093/alcalc/agh134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Chandler LJ. Adaptive plasticity of NMDA receptors and dendritic pines: implications for enhanced vulnerability of the adolescent brain to alcohol addiction. Pharmacol Biochem Behav. 2007;86:200–208. doi: 10.1016/j.pbb.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus and cerebellar volumes in adolescents and young adults with adolescent onset alcohol use disorders and co-morbid mental disorders. Alcohol Clin Exp Res. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- Desmond J, Chen S, DeRosa E, Pryor M, Pfefferbaum A, Sullivan E. Increased frontocerebellar activation in alcoholics during verbal working memory: an fMRI study. Neuroimage. 2003;19:1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Emanuele NV, LaPaglia N, Steiner J, Kirsteins L, Emanuele MA. Effect of chronic ethanol exposure on female rat reproductive cyclicity and hormone secretion. Alcohol Clin Exp Res. 2001;25:1025–1029. [PubMed] [Google Scholar]
- Freund G, Anderson K. Glutamate receptors in the frontal cortex of alcoholics. Alcohol Clin Exp Res. 1996;20:1165–1172. doi: 10.1111/j.1530-0277.1996.tb01106.x. [DOI] [PubMed] [Google Scholar]
- George MR, Potts G, Kothman D, Martin L, Mukundan CR. Frontal deficits in alcoholism: an ERP study. Brain Cogn. 2004;54:245–247. doi: 10.1016/j.bandc.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapaport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapaport JL. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cereb Cortex. 1996a;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. J Comp Neurol. 1996b;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;17:17. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C. Patterns of neuronal loss in the cerebral cortex in chronic alcoholic patients. J Neurol Sci. 1989;92:81–89. doi: 10.1016/0022-510x(89)90177-9. [DOI] [PubMed] [Google Scholar]
- Harper C, Smith NA, Kril JJ. The effects of alcohol on the female brain: a neuropathological study. Alcohol Alcohol. 1990;25:445–448. [PubMed] [Google Scholar]
- Hashimoto JG, Wiren KM. Neurotoxic consequences of chronic alcohol withdrawal: expression profiling reveals importance of gender over withdrawal severity. Neuropsychopharmacology (Epub ahead of print) 2007 doi: 10.1038/sj.npp.1301494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Kaiser E, Rawlings R. Evidence for a gender-related effect of alcoholism on brain volumes. Am J Psychiatry. 2001;158:198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biol Psychiatry. 2006;61:31–40. doi: 10.1016/j.biopsych.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Jennions MD, Møller AP. A survey of the statistical power of research in behavioral ecology and animal behavior. Behav Ecol. 2003;14:438–445. [Google Scholar]
- Jernigan T, Gamst A. Changes in volume with age: consistency and interpretation of observed effects. Neurobiol Aging. 2005;26:1271–1274. doi: 10.1016/j.neurobiolaging.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Secondary School Students. Vol. 1. National Institute on Drug Abuse; Bethesda, MD: 2005. Monitoring the Future National Results on Adolescent Drug use, 1975-2004. NIH Publication No 05-5727. [Google Scholar]
- Kamarajan C, Porjesz B, Jones K, Chorlian D, Padmanabhapillai A, Rangaswamy M, Stimus A, Begleiter H. Event-related oscillations in offspring of alcoholics: neurocognitive disinhibition as a risk for alcoholism. Biol Psychiatry. 2006;59:625–634. doi: 10.1016/j.biopsych.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Kim JJ, Kwon JS. Frontal P300 decrement and executive dysfunction in adolescents with conduct problems. Child Psychiatry Hum Dev. 2001;32:93–106. doi: 10.1023/a:1012299822274. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim HJ, Noh HS, Roh GS, Kang SS, Cho GJ, Park SK, Lee BJ, Choi WS. Suppression by ethanol of male reproductive activity. Brain Res. 2003;989:91–98. doi: 10.1016/s0006-8993(03)03372-9. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Kroft CL, Gescuk B, Woods BT, Mello NK, Weiss RD, Mendelson JH. Brain ventricular size in female alcoholics: an MRI study. Alcohol. 1991;8:31–34. doi: 10.1016/0741-8329(91)91200-l. [DOI] [PubMed] [Google Scholar]
- Kruesi MJ, Casanova MF, Mannheim G, Johnson-Bilder A. Reduced temporal lobe volume in early onset conduct disorder. Psychiatry Res Neuroimaging. 2004;132:1–11. doi: 10.1016/j.pscychresns.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Kubota M, Nakazaki S, Hirai S, Saeki N, Yamaura A, Kusaka T. Alcohol consumption and frontal lobe shrinkage: study of 1432 non-alcoholic subjects. J Neurol Neurosurg Psychiatry. 2001;71:104–106. doi: 10.1136/jnnp.71.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Ethnic and gender differences in ethanol metabolism. Alcohol Clin Exp Res. 2000;24:417–418. [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Matochik J, Cadet JL, London E. Smaller volume of prefrontal lobe in polysubstance abusers: a magnetic resonance imaging study. Neuropsychopharmacology. 1998;18:243–252. doi: 10.1016/S0893-133X(97)00143-7. [DOI] [PubMed] [Google Scholar]
- Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A. Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcohol Clin Exp Res. 2005;29:896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, McQueeny T, Park A, Tapert SF. Depressive symptoms in adolescents: associations with white matter volume and marijuana use. J Child Psychol Psychiatry. 2007a;48:592–600. doi: 10.1111/j.1469-7610.2007.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal asymmetry. Exposures during adolescent development: are neurotoxic risks increased? Neurotoxicol Teratol. 2007b;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff DJ, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, Chao LL, Rothlind J, Studholme C, Weiner MW. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcohol Clin Exp Res. 2004;28:650–661. doi: 10.1097/01.ALC.0000121805.12350.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G. Reduced glial and neuronal packing density in the orbitofrontal cortex in alcohol dependence and its relationship with suicide and duration of alcohol dependence. Alcohol Clin Exp Res. 2006;30:1845–1855. doi: 10.1111/j.1530-0277.2006.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Cooper KL, Kates WR, Denckla MB, Kaufmann WE. Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2002;52:785–794. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Medina KL, Yoshii J, Schweinsburg AD, Moadab I, Tapert SF. Age related changes in prefrontal white matter volume across adolescence. NeuroReport. 2006;17:1427–1431. doi: 10.1097/01.wnr.0000233099.97784.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behavioral Ecology. 2004;15:1044–1045. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Biochemical effects of alcohol metabolism, in Eight Special Report to the Congress on Alcohol and Health. National Institutes of Health; Bethesda, MD: 1993. pp. 148–164. [Google Scholar]
- Nigg JT, Glass JM, Wong MM, Poon E, Jester JM, Fitzgerald HE, Puttler LI, Adams KM, Zucker RA. Neuropsychological executive functioning in children at elevated risk for alcoholism: findings in early adolescence. J Abnorm Psychol. 2004;113:302–314. doi: 10.1037/0021-843X.113.2.302. [DOI] [PubMed] [Google Scholar]
- Ogilvie K, Rivier C. Gender differences in alcohol-evoked hypothalamic-pituitary-adrenal activity in the rat: ontogeny and role of neonatal steroids. Alcohol Clin Exp Res. 1996;20:255–261. doi: 10.1111/j.1530-0277.1996.tb01637.x. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Miki T, Lee KY, Yokoyama T, Kuma H, Wang ZY, Gu H, Li HP, Matsumoto Y, Irawan S, Bedi KS, Nakamura Y, Takeuchi Y. Oligodendrocyte myelin glycoprotein (OMgp) in rat hippocampus is depleted by chronic ethanol consumption. Neurosci Lett. 2006;406:76–80. doi: 10.1016/j.neulet.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Minarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur J Neurosci. 2007;25:541–550. doi: 10.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond J, Galloway C, Menon V, Glover G, Sullivan E. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage. 2001;14:7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Serventi KL, Sullivan EV. Corpus callosum, pons, and cortical white matter in alcoholic women. Alcohol Clin Exp Res. 2002;26:400–406. [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA, Wilson WA, Swartzwelder HS. Age and dose dependent effects of ethanol on the induction of hippocampal long term potentiation. Alcohol. 1999;19:107–111. doi: 10.1016/s0741-8329(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children: a volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S, Fadda F. Glutamate-induced increase of extracellular glutamate through N-methyl-D-aspartate receptors in ethanol withdrawal. Neuroscience. 1999;93:1135–1140. doi: 10.1016/s0306-4522(99)00250-x. [DOI] [PubMed] [Google Scholar]
- Samnick S, Ametamey S, Leenders KL, Vontobel P, Quack G, Parsons CG, Neu H, Schubiger PA. Electrophysiological study, biodistribution in mice, and preliminary PET evaluation in a rhesus monkey of 1-amino-3-[18F]fluoromethyl-5-methyl-adamantane (18F-MEM): a potential radioligand for mapping the NMDA-receptor complex. Nucl Med Biol. 1998;25:323–330. doi: 10.1016/s0969-8051(98)00003-1. [DOI] [PubMed] [Google Scholar]
- Schweinsburg BC, Alhassoon OM, Taylor MJ, Gonzalez R, Videen JS, Brown GG, Patterson TL, Grant I. Effects of alcoholism and gender on brain metabolism. Am J Psychiatry. 2003;160:1180–1183. doi: 10.1176/appi.ajp.160.6.1180. [DOI] [PubMed] [Google Scholar]
- Schweinsburg BC, Taylor MJ, Alhassoon OM, Videen JS, Brown GG, Patterson TL, Berger F, Grant I. Chemical pathology in brain white matter of recently detoxified alcoholics: a 1H magnetic resonance spectroscopy investigation of alcohol-associated frontal lobe injury. Alcohol Clin Exp Res. 2001;25:924–934. [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliablility of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Raye Z, Litten JPA, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press Inc.; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steingard RJ, Renshaw PF, Hennen J, Lenox M, Cintron CB, Young AD, Connor DF, Au TH, Yurgelun-Todd DA. Smaller frontal lobe white matter volumes in depressed adolescents. Biol Psychiatry. 2002;52:413–417. doi: 10.1016/s0006-3223(02)01393-8. [DOI] [PubMed] [Google Scholar]
- Stewart DG, Brown SA. Withdrawal and dependency symptoms among adolescent alcohol and drug abusers. Addiction. 1995;90:627–635. doi: 10.1046/j.1360-0443.1995.9056274.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Harding AJ, Pentney R, Dlugos C, Martin PR, Parks MH, Desmond JE, Chen SHA, Pryor MR, De Rosa E, Pfefferbaum A. Disruption of frontocerebellar circuitry and function in alcoholism. Alcohol Clin Exp Res. 2003;27:301–309. doi: 10.1097/01.ALC.0000052584.05305.98. [DOI] [PubMed] [Google Scholar]
- Sumida KD, Qureshi T, Catanzaro MJ, Arimoto SM, Hill JM. Chronic alcohol consumption yields sex differences in whole-body glucose production in rats. Alcohol Alcohol. 2004;39:418–426. doi: 10.1093/alcalc/agh082. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Richardson RC, Markwiese-Foerch B, Wilson WA, Little PJ. Developmental differences in the acquisition of tolerance to ethanol. Alcohol. 1998;15:311–314. doi: 10.1016/s0741-8329(97)00135-3. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res. 1995;19:1480–1485. doi: 10.1111/j.1530-0277.1995.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Substance dependence, family history of alcohol dependence, and neuropsychological functioning in adolescence. Addiction. 2000;95:1043–1053. doi: 10.1046/j.1360-0443.2000.95710436.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch Gen Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol Clin Exp Res. 2004;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Valles SL, Blanco AM, Pascual M, Guerri C. Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain Pathol. 2004;14:365–371. doi: 10.1111/j.1750-3639.2004.tb00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Dev Psychobiol. 2006;48:146–161. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- Watts R, Liston C, Niogi S, Ulug AM. Fiber tracking using magnetic resonance diffusion tensor imaging and its applications to human brain development. Ment Retard Dev Disabil Res Rev. 2003;9:168–177. doi: 10.1002/mrdd.10077. [DOI] [PubMed] [Google Scholar]
- White AM, Bae JG, Truesdale MC, Ahmad S, Wilson WA, Swartzwelder HS. Chronic-intermittent ethanol exposure during adolescence prevents normal developmental changes in sensitivity to ethanol-induced motor impairments. Alcohol Clin Exp Res. 2002;26:960–968. doi: 10.1097/01.ALC.0000021334.47130.F9. [DOI] [PubMed] [Google Scholar]
- Wong EC, Luh WM, Buxton RB, Frank RL. Single slab high resolution 3D whole brain imaging using spiral FSE. Proc Int Soc Magn Reson Med. 2000;8:683. [Google Scholar]
- Yttri EA, Burk JA, Hunt PS. Intermittent ethanol exposure in adolescent rats: dose dependent impairments in trace conditioning. Alcohol Clin Exp Res. 2004;28:1433–1436. doi: 10.1097/01.alc.0000147657.51745.a7. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]


